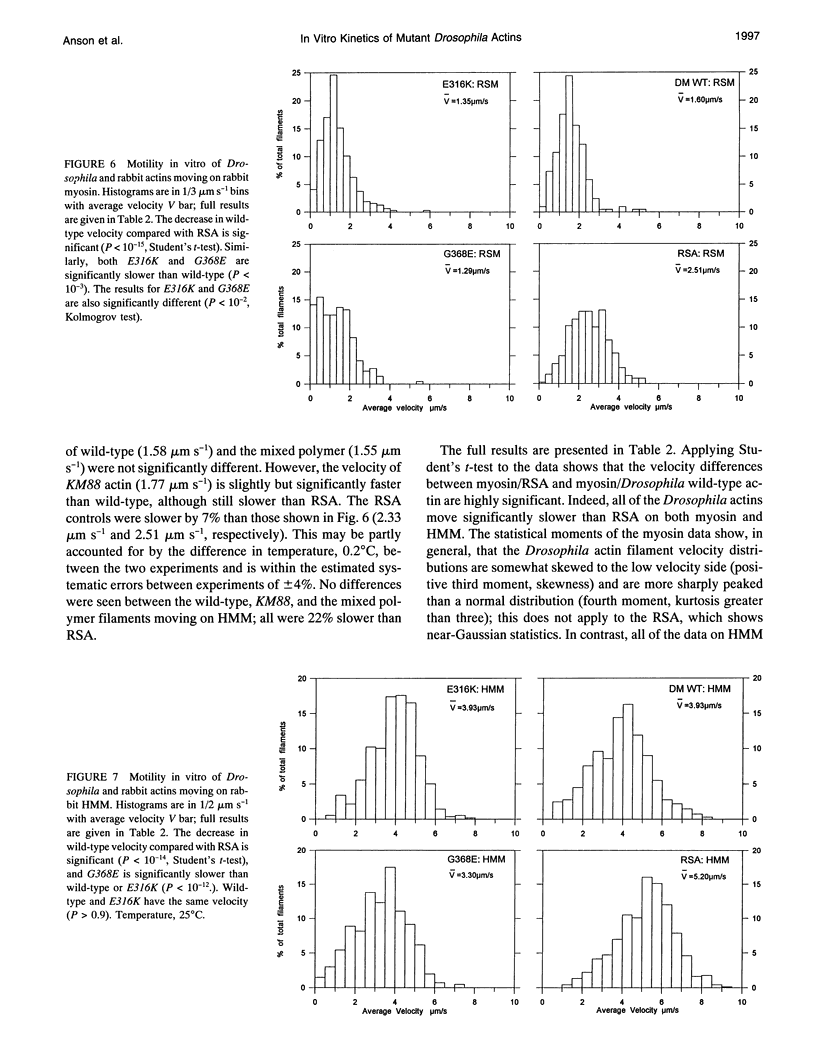
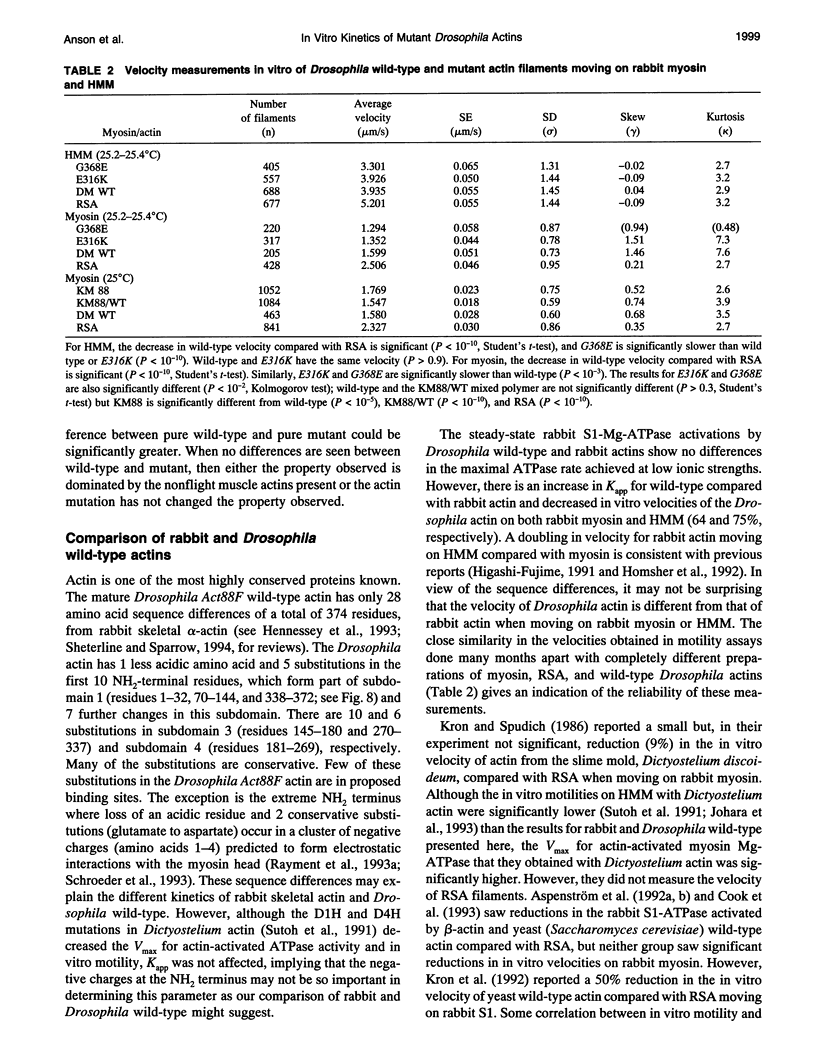
Abstract
Two missense mutations of the flight muscle-specific actin gene of Drosophila melanogaster, Act88F, assemble into normally structured myofibrils but affect the flight ability of flies and the mechanical kinetics of isolated muscle fibers. We describe the isolation of actin from different homozygous Act88F strains, including wild-type, an Act88F null mutant (KM88), and two Act88F single point mutations (E316K and G368E), their biochemical interactions with rabbit myosin subfragment 1 (S1), and behavior with rabbit myosin and heavy meromyosin in in vitro motility assays. The rabbit and wild-type Drosophila actins have different association rate constants with S1 (2.64 and 1.77 microM-1 s-1, respectively) and in vitro motilities (2.51, 1.60 microns s-1) clearly demonstrating an isoform-specific difference. The G368E mutation shows a reduced affinity for rabbit S1 compared with the wild type (increasing from 0.11 to 0.17 microM) and a reduced velocity in vitro (reduced by 19%). The E316K mutant actin has no change in affinity for myosin S1 or in vitro motility with heavy meromyosin but does have a reduced in vitro motility (15%) with myosin. These results are discussed with respect to the recently published atomic models for the actomyosin structure and our findings that G368E fibers show a reduced rate constant for delayed tension development and increased fiber stiffness. We interpret these results as possibly caused either by effects on A1 myosin light chain binding or conformational changes within the subdomain 1 of actin, which contains the myosin binding site. E316K is discussed with respect to its likely position within the tropomyosin binding site of actin.
Full text
PDF










Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anson M. Temperature dependence and Arrhenius activation energy of F-actin velocity generated in vitro by skeletal myosin. J Mol Biol. 1992 Apr 20;224(4):1029–1038. doi: 10.1016/0022-2836(92)90467-x. [DOI] [PubMed] [Google Scholar]
- Aspenström P., Engkvist H., Lindberg U., Karlsson R. Characterization of yeast-expressed beta-actins, site-specifically mutated at the tumor-related residue Gly245. Eur J Biochem. 1992 Jul 1;207(1):315–320. doi: 10.1111/j.1432-1033.1992.tb17052.x. [DOI] [PubMed] [Google Scholar]
- Aspenström P., Karlsson R. Interference with myosin subfragment-1 binding by site-directed mutagenesis of actin. Eur J Biochem. 1991 Aug 15;200(1):35–41. doi: 10.1111/j.1432-1033.1991.tb21045.x. [DOI] [PubMed] [Google Scholar]
- Aspenström P., Lindberg U., Karlsson R. Site-specific amino-terminal mutants of yeast-expressed beta-actin. Characterization of the interaction with myosin and tropomyosin. FEBS Lett. 1992 May 25;303(1):59–63. doi: 10.1016/0014-5793(92)80477-x. [DOI] [PubMed] [Google Scholar]
- Ball E., Karlik C. C., Beall C. J., Saville D. L., Sparrow J. C., Bullard B., Fyrberg E. A. Arthrin, a myofibrillar protein of insect flight muscle, is an actin-ubiquitin conjugate. Cell. 1987 Oct 23;51(2):221–228. doi: 10.1016/0092-8674(87)90149-8. [DOI] [PubMed] [Google Scholar]
- Bullard B., Dabrowska R., Winkelman L. The contractile and regulatory proteins of insect flight muscle. Biochem J. 1973 Oct;135(2):277–286. doi: 10.1042/bj1350277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook R. K., Root D., Miller C., Reisler E., Rubenstein P. A. Enhanced stimulation of myosin subfragment 1 ATPase activity by addition of negatively charged residues to the yeast actin NH2 terminus. J Biol Chem. 1993 Feb 5;268(4):2410–2415. [PubMed] [Google Scholar]
- Criddle A. H., Geeves M. A., Jeffries T. The use of actin labelled with N-(1-pyrenyl)iodoacetamide to study the interaction of actin with myosin subfragments and troponin/tropomyosin. Biochem J. 1985 Dec 1;232(2):343–349. doi: 10.1042/bj2320343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantzig J. A., Goldman Y. E., Millar N. C., Lacktis J., Homsher E. Reversal of the cross-bridge force-generating transition by photogeneration of phosphate in rabbit psoas muscle fibres. J Physiol. 1992;451:247–278. doi: 10.1113/jphysiol.1992.sp019163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drummond D. R., Hennessey E. S., Sparrow J. C. Characterisation of missense mutations in the Act88F gene of Drosophila melanogaster. Mol Gen Genet. 1991 Apr;226(1-2):70–80. doi: 10.1007/BF00273589. [DOI] [PubMed] [Google Scholar]
- Drummond D. R., Hennessey E. S., Sparrow J. C. Stability of mutant actins. Biochem J. 1991 Feb 15;274(Pt 1):301–303. doi: 10.1042/bj2740301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drummond D. R., Peckham M., Sparrow J. C., White D. C. Alteration in crossbridge kinetics caused by mutations in actin. Nature. 1990 Nov 29;348(6300):440–442. doi: 10.1038/348440a0. [DOI] [PubMed] [Google Scholar]
- Falkenthal S., Parker V. P., Mattox W. W., Davidson N. Drosophila melanogaster has only one myosin alkali light-chain gene which encodes a protein with considerable amino acid sequence homology to chicken myosin alkali light chains. Mol Cell Biol. 1984 May;4(5):956–965. doi: 10.1128/mcb.4.5.956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faulstich H., Zobeley S., Rinnerthaler G., Small J. V. Fluorescent phallotoxins as probes for filamentous actin. J Muscle Res Cell Motil. 1988 Oct;9(5):370–383. doi: 10.1007/BF01774064. [DOI] [PubMed] [Google Scholar]
- Fortune N. S., Geeves M. A., Ranatunga K. W. Tension responses to rapid pressure release in glycerinated rabbit muscle fibers. Proc Natl Acad Sci U S A. 1991 Aug 15;88(16):7323–7327. doi: 10.1073/pnas.88.16.7323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fyrberg E. A., Mahaffey J. W., Bond B. J., Davidson N. Transcripts of the six Drosophila actin genes accumulate in a stage- and tissue-specific manner. Cell. 1983 May;33(1):115–123. doi: 10.1016/0092-8674(83)90340-9. [DOI] [PubMed] [Google Scholar]
- Geeves M. A., Goldmann W. H. The influence of anions, ionic strength and organic solvents on the interaction between actin and myosin subfragment 1. Biochem Soc Trans. 1990 Aug;18(4):584–585. doi: 10.1042/bst0180584. [DOI] [PubMed] [Google Scholar]
- Geeves M. A. The dynamics of actin and myosin association and the crossbridge model of muscle contraction. Biochem J. 1991 Feb 15;274(Pt 1):1–14. doi: 10.1042/bj2740001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennessey E. S., Drummond D. R., Sparrow J. C. Molecular genetics of actin function. Biochem J. 1993 May 1;291(Pt 3):657–671. doi: 10.1042/bj2910657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higashi-Fujime S. Reconstitution of active movement in vitro based on the actin-myosin interaction. Int Rev Cytol. 1991;125:95–138. doi: 10.1016/s0074-7696(08)61217-6. [DOI] [PubMed] [Google Scholar]
- Hiratsuka T. New ribose-modified fluorescent analogs of adenine and guanine nucleotides available as substrates for various enzymes. Biochim Biophys Acta. 1983 Feb 15;742(3):496–508. doi: 10.1016/0167-4838(83)90267-4. [DOI] [PubMed] [Google Scholar]
- Hiromi Y., Hotta Y. Actin gene mutations in Drosophila; heat shock activation in the indirect flight muscles. EMBO J. 1985 Jul;4(7):1681–1687. doi: 10.1002/j.1460-2075.1985.tb03837.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiromi Y., Okamoto H., Gehring W. J., Hotta Y. Germline transformation with Drosophila mutant actin genes induces constitutive expression of heat shock genes. Cell. 1986 Jan 31;44(2):293–301. doi: 10.1016/0092-8674(86)90763-4. [DOI] [PubMed] [Google Scholar]
- Holmes K. C., Popp D., Gebhard W., Kabsch W. Atomic model of the actin filament. Nature. 1990 Sep 6;347(6288):44–49. doi: 10.1038/347044a0. [DOI] [PubMed] [Google Scholar]
- Homsher E., Millar N. C. Caged compounds and striated muscle contraction. Annu Rev Physiol. 1990;52:875–896. doi: 10.1146/annurev.ph.52.030190.004303. [DOI] [PubMed] [Google Scholar]
- Homsher E., Wang F., Sellers J. R. Factors affecting movement of F-actin filaments propelled by skeletal muscle heavy meromyosin. Am J Physiol. 1992 Mar;262(3 Pt 1):C714–C723. doi: 10.1152/ajpcell.1992.262.3.C714. [DOI] [PubMed] [Google Scholar]
- Horovitch S. J., Storti R. V., Rich A., Pardue M. L. Multiple actins in Drosophila melanogaster. J Cell Biol. 1979 Jul;82(1):86–92. doi: 10.1083/jcb.82.1.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johara M., Toyoshima Y. Y., Ishijima A., Kojima H., Yanagida T., Sutoh K. Charge-reversion mutagenesis of Dictyostelium actin to map the surface recognized by myosin during ATP-driven sliding motion. Proc Natl Acad Sci U S A. 1993 Mar 15;90(6):2127–2131. doi: 10.1073/pnas.90.6.2127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabsch W., Mannherz H. G., Suck D., Pai E. F., Holmes K. C. Atomic structure of the actin:DNase I complex. Nature. 1990 Sep 6;347(6288):37–44. doi: 10.1038/347037a0. [DOI] [PubMed] [Google Scholar]
- Kron S. J., Drubin D. G., Botstein D., Spudich J. A. Yeast actin filaments display ATP-dependent sliding movement over surfaces coated with rabbit muscle myosin. Proc Natl Acad Sci U S A. 1992 May 15;89(10):4466–4470. doi: 10.1073/pnas.89.10.4466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kron S. J., Spudich J. A. Fluorescent actin filaments move on myosin fixed to a glass surface. Proc Natl Acad Sci U S A. 1986 Sep;83(17):6272–6276. doi: 10.1073/pnas.83.17.6272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kron S. J., Toyoshima Y. Y., Uyeda T. Q., Spudich J. A. Assays for actin sliding movement over myosin-coated surfaces. Methods Enzymol. 1991;196:399–416. doi: 10.1016/0076-6879(91)96035-p. [DOI] [PubMed] [Google Scholar]
- Lehrer S. S., Kerwar G. Intrinsic fluorescence of actin. Biochemistry. 1972 Mar 28;11(7):1211–1217. doi: 10.1021/bi00757a015. [DOI] [PubMed] [Google Scholar]
- Mahaffey J. W., Coutu M. D., Fyrberg E. A., Inwood W. The flightless Drosophila mutant raised has two distinct genetic lesions affecting accumulation of myofibrillar proteins in flight muscles. Cell. 1985 Jan;40(1):101–110. doi: 10.1016/0092-8674(85)90313-7. [DOI] [PubMed] [Google Scholar]
- Margossian S. S., Lowey S. Preparation of myosin and its subfragments from rabbit skeletal muscle. Methods Enzymol. 1982;85(Pt B):55–71. doi: 10.1016/0076-6879(82)85009-x. [DOI] [PubMed] [Google Scholar]
- Milligan R. A., Whittaker M., Safer D. Molecular structure of F-actin and location of surface binding sites. Nature. 1990 Nov 15;348(6298):217–221. doi: 10.1038/348217a0. [DOI] [PubMed] [Google Scholar]
- Mogami K., Hotta Y. Isolation of Drosophila flightless mutants which affect myofibrillar proteins of indirect flight muscle. Mol Gen Genet. 1981;183(3):409–417. doi: 10.1007/BF00268758. [DOI] [PubMed] [Google Scholar]
- Pardee J. D., Spudich J. A. Purification of muscle actin. Methods Cell Biol. 1982;24:271–289. doi: 10.1016/s0091-679x(08)60661-5. [DOI] [PubMed] [Google Scholar]
- Rayment I., Holden H. M., Whittaker M., Yohn C. B., Lorenz M., Holmes K. C., Milligan R. A. Structure of the actin-myosin complex and its implications for muscle contraction. Science. 1993 Jul 2;261(5117):58–65. doi: 10.1126/science.8316858. [DOI] [PubMed] [Google Scholar]
- Rayment I., Rypniewski W. R., Schmidt-Bäse K., Smith R., Tomchick D. R., Benning M. M., Winkelmann D. A., Wesenberg G., Holden H. M. Three-dimensional structure of myosin subfragment-1: a molecular motor. Science. 1993 Jul 2;261(5117):50–58. doi: 10.1126/science.8316857. [DOI] [PubMed] [Google Scholar]
- Reedy M. C., Beall C., Fyrberg E. Formation of reverse rigor chevrons by myosin heads. Nature. 1989 Jun 8;339(6224):481–483. doi: 10.1038/339481a0. [DOI] [PubMed] [Google Scholar]
- Ritchie M. D., Geeves M. A., Woodward S. K., Manstein D. J. Kinetic characterization of a cytoplasmic myosin motor domain expressed in Dictyostelium discoideum. Proc Natl Acad Sci U S A. 1993 Sep 15;90(18):8619–8623. doi: 10.1073/pnas.90.18.8619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saide J. D., Chin-Bow S., Hogan-Sheldon J., Busquets-Turner L., Vigoreaux J. O., Valgeirsdottir K., Pardue M. L. Characterization of components of Z-bands in the fibrillar flight muscle of Drosophila melanogaster. J Cell Biol. 1989 Nov;109(5):2157–2167. doi: 10.1083/jcb.109.5.2157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai Y., Okamoto H., Mogami K., Matsuo H., Hotta Y. Heat shock gene activation by mutant actin is independent of myofibril degeneration in Drosophila muscle. J Biochem. 1991 May;109(5):670–673. doi: 10.1093/oxfordjournals.jbchem.a123438. [DOI] [PubMed] [Google Scholar]
- Sakai Y., Okamoto H., Mogami K., Yamada T., Hotta Y. Actin with tumor-related mutation is antimorphic in Drosophila muscle: two distinct modes of myofibrillar disruption by antimorphic actins. J Biochem. 1990 Mar;107(3):499–505. doi: 10.1093/oxfordjournals.jbchem.a123074. [DOI] [PubMed] [Google Scholar]
- Schröder R. R., Manstein D. J., Jahn W., Holden H., Rayment I., Holmes K. C., Spudich J. A. Three-dimensional atomic model of F-actin decorated with Dictyostelium myosin S1. Nature. 1993 Jul 8;364(6433):171–174. doi: 10.1038/364171a0. [DOI] [PubMed] [Google Scholar]
- Schutt C. E., Myslik J. C., Rozycki M. D., Goonesekere N. C., Lindberg U. The structure of crystalline profilin-beta-actin. Nature. 1993 Oct 28;365(6449):810–816. doi: 10.1038/365810a0. [DOI] [PubMed] [Google Scholar]
- Sellers J. R., Cuda G., Wang F., Homsher E. Myosin-specific adaptations of the motility assay. Methods Cell Biol. 1993;39:23–49. doi: 10.1016/s0091-679x(08)60159-4. [DOI] [PubMed] [Google Scholar]
- Siemankowski R. F., White H. D. Kinetics of the interaction between actin, ADP, and cardiac myosin-S1. J Biol Chem. 1984 Apr 25;259(8):5045–5053. [PubMed] [Google Scholar]
- Sparrow J., Reedy M., Ball E., Kyrtatas V., Molloy J., Durston J., Hennessey E., White D. Functional and ultrastructural effects of a missense mutation in the indirect flight muscle-specific actin gene of Drosophila melanogaster. J Mol Biol. 1991 Dec 20;222(4):963–982. doi: 10.1016/0022-2836(91)90588-w. [DOI] [PubMed] [Google Scholar]
- Sutoh K., Ando M., Sutoh K., Toyoshima Y. Y. Site-directed mutations of Dictyostelium actin: disruption of a negative charge cluster at the N terminus. Proc Natl Acad Sci U S A. 1991 Sep 1;88(17):7711–7714. doi: 10.1073/pnas.88.17.7711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutoh K. Identification of myosin-binding sites on the actin sequence. Biochemistry. 1982 Jul 20;21(15):3654–3661. doi: 10.1021/bi00258a020. [DOI] [PubMed] [Google Scholar]
- Szilagyi L., Lu R. C. Changes of lysine reactivities of actin in complex with myosin subfragment-1, tropomyosin and troponin. Biochim Biophys Acta. 1982 Dec 20;709(2):204–211. doi: 10.1016/0167-4838(82)90462-9. [DOI] [PubMed] [Google Scholar]
- Trayer I. P., Trayer H. R., Levine B. A. Evidence that the N-terminal region of A1-light chain of myosin interacts directly with the C-terminal region of actin. A proton magnetic resonance study. Eur J Biochem. 1987 Apr 1;164(1):259–266. doi: 10.1111/j.1432-1033.1987.tb11019.x. [DOI] [PubMed] [Google Scholar]
- Warrick H. M., Simmons R. M., Finer J. T., Uyeda T. Q., Chu S., Spudich J. A. In vitro methods for measuring force and velocity of the actin-myosin interaction using purified proteins. Methods Cell Biol. 1993;39:1–21. doi: 10.1016/s0091-679x(08)60158-2. [DOI] [PubMed] [Google Scholar]
- Weeds A. G., Taylor R. S. Separation of subfragment-1 isoenzymes from rabbit skeletal muscle myosin. Nature. 1975 Sep 4;257(5521):54–56. doi: 10.1038/257054a0. [DOI] [PubMed] [Google Scholar]
- White D. C. The elasticity of relaxed insect fibrillar flight muscle. J Physiol. 1983 Oct;343:31–57. doi: 10.1113/jphysiol.1983.sp014880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White H. D., Taylor E. W. Energetics and mechanism of actomyosin adenosine triphosphatase. Biochemistry. 1976 Dec 28;15(26):5818–5826. doi: 10.1021/bi00671a020. [DOI] [PubMed] [Google Scholar]
- Woodward S. K., Eccleston J. F., Geeves M. A. Kinetics of the interaction of 2'(3')-O-(N-methylanthraniloyl)-ATP with myosin subfragment 1 and actomyosin subfragment 1: characterization of two acto-S1-ADP complexes. Biochemistry. 1991 Jan 15;30(2):422–430. doi: 10.1021/bi00216a017. [DOI] [PubMed] [Google Scholar]




