Abstract
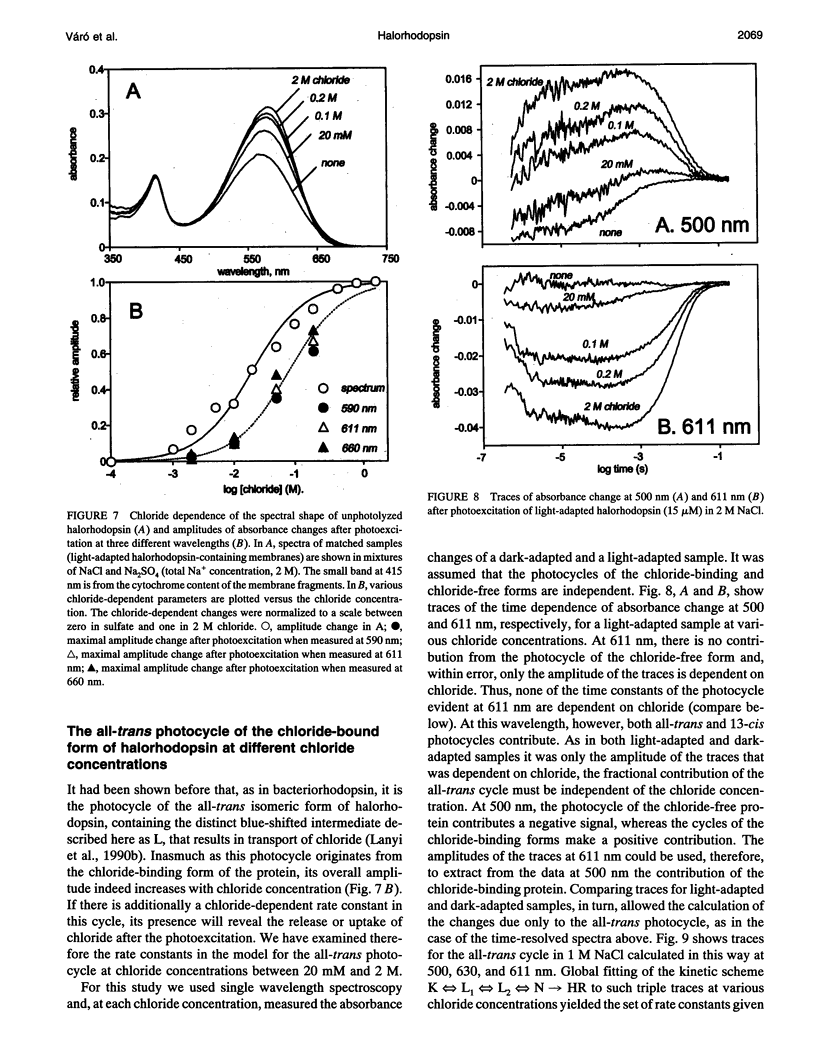
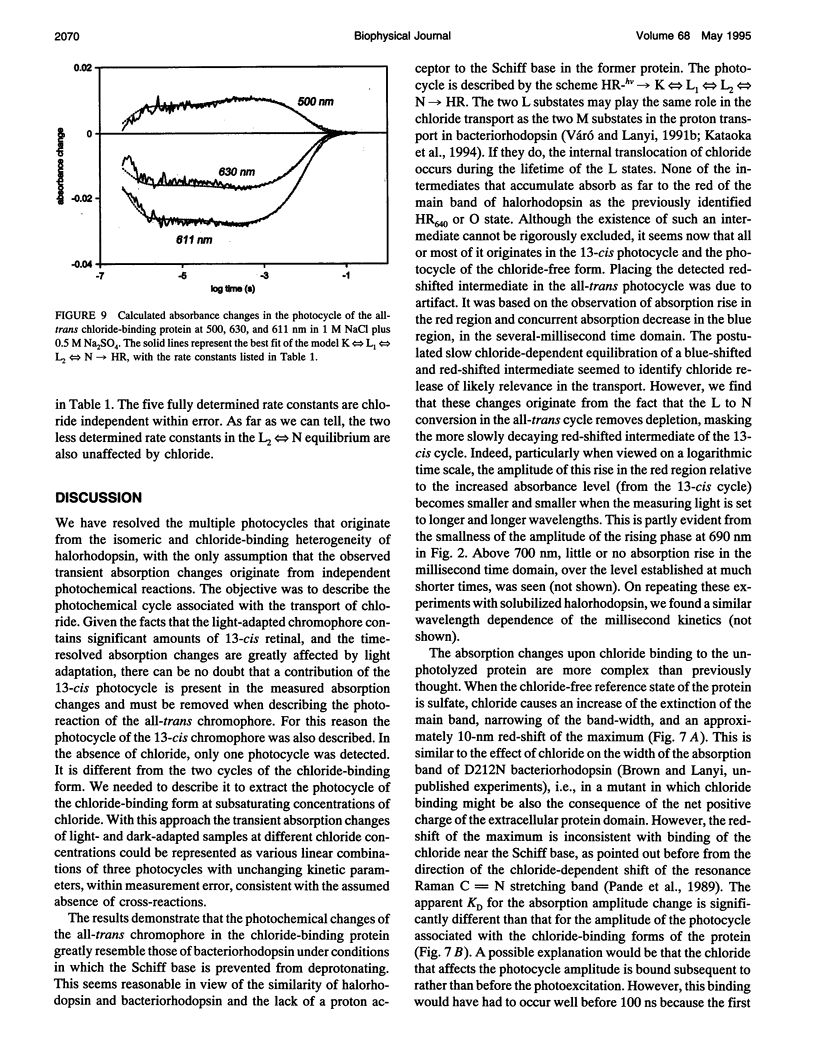
The light-driven chloride pump, halorhodopsin, is a mixture containing all-trans and 13-cis retinal chromophores under both light and dark-adapted conditions and can exist in chloride-free and chloride-binding forms. To describe the photochemical cycle of the all-trans, chloride-binding state that is associated with the transport, and thereby initiate study of the chloride translocation mechanism, one must first dissect the contributions of these species to the measured spectral changes. We resolved the multiple photochemical reactions by determining flash-induced difference spectra and photocycle kinetics in halorhodopsin-containing membranes prepared from Halobacterium salinarium, with light- and dark-adapted samples at various chloride concentrations. The high expression of cloned halorhodopsin made it possible to do these measurements with unfractionated cell envelope membranes in which the chromophore is photostable not only in the presence of NaCl but also in the Na2SO4 solution used for reference. Careful examination of the flash-induced changes at selected wavelengths allowed separating the spectral changes into components and assigning them to the individual photocycles. According to the results, a substantial revision of the photocycle model for H. salinarium halorhodopsin, and its dependence on chloride, is required. The cycle of the all-trans chloride-binding form is described by the scheme, HR-hv-->K<==>L1<==>L2<==>N-->HR, where HR, K, L, and N designate halorhodopsin and its photointermediates. Unlike the earlier models, this is very similar to the photoreaction of bacteriorhodopsin when deprotonation of the Schiff base is prevented (e.g., at low pH or in the D85N mutant). Also unlike in the earlier models, no step in this photocycle was noticeably affected when the chloride concentration was varied between 20 mM and 2 M in an attempt to identify a chloride-binding reaction.
Full text
PDF

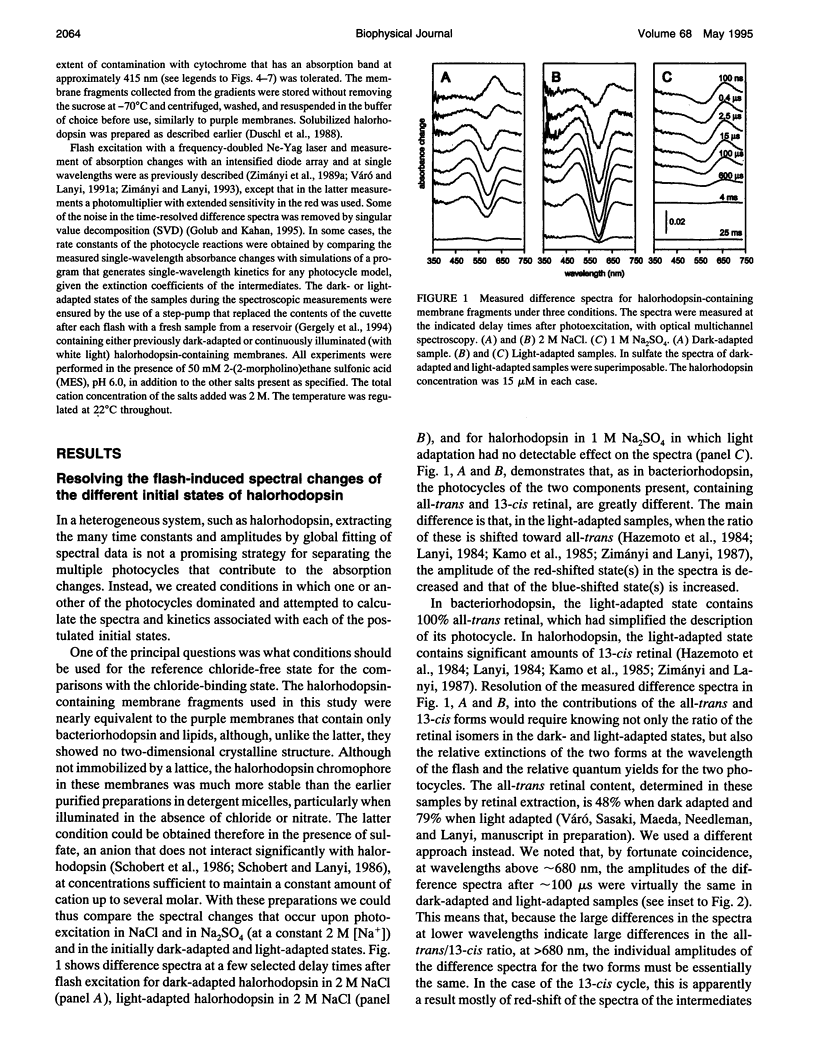
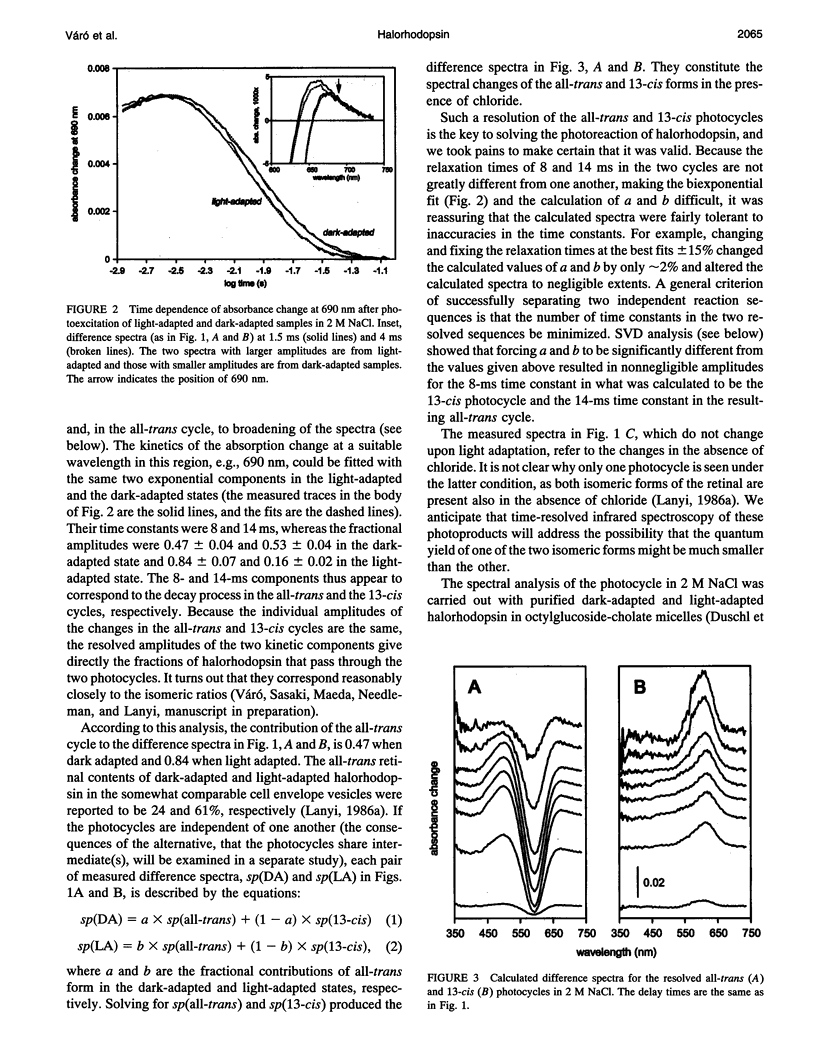
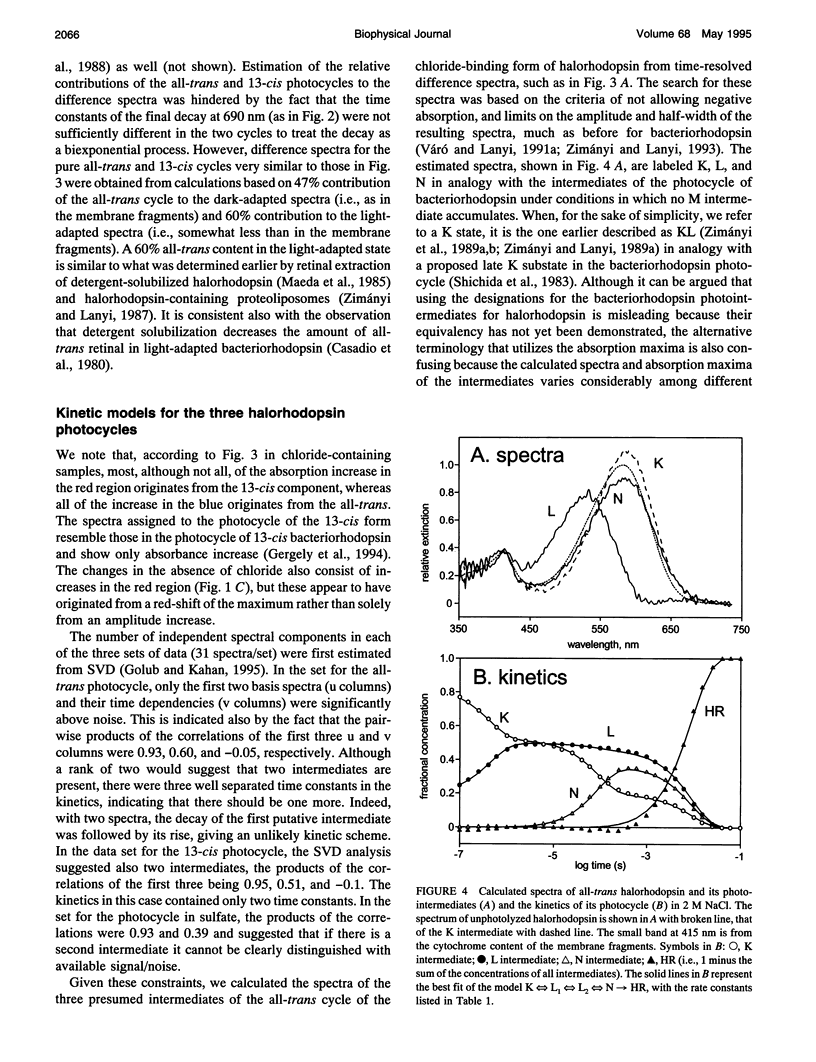

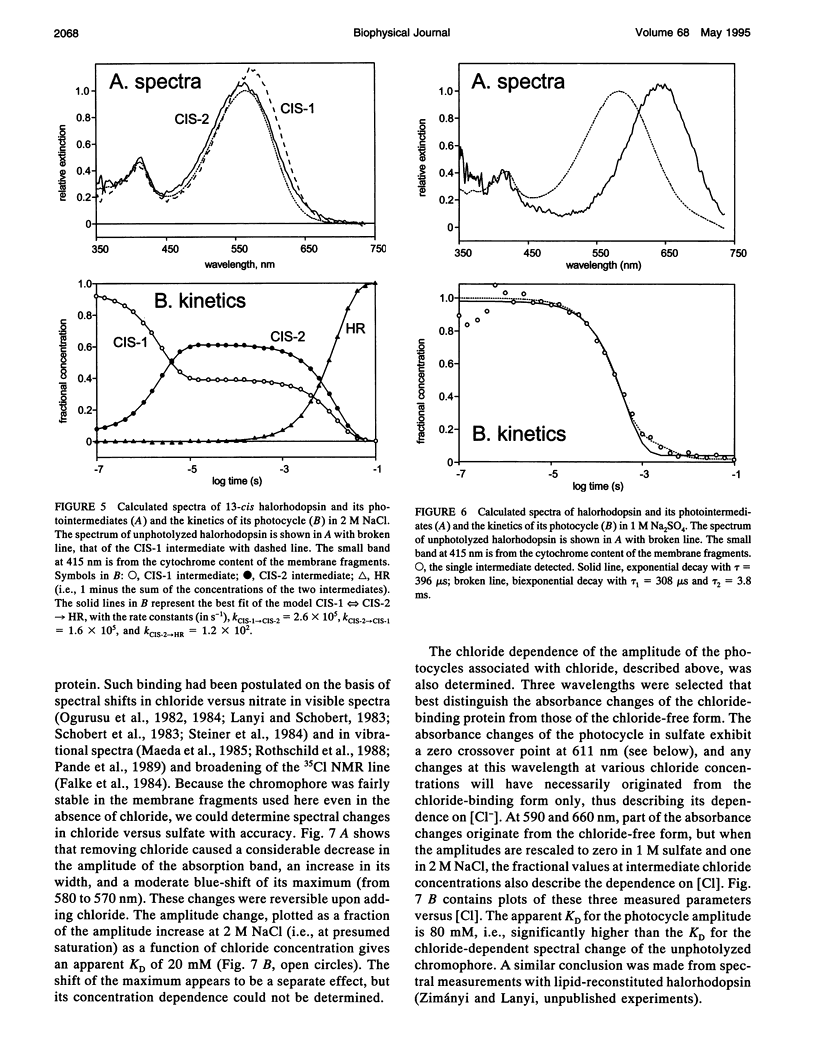


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ames J. B., Raap J., Lugtenburg J., Mathies R. A. Resonance Raman study of halorhodopsin photocycle kinetics, chromophore structure, and chloride-pumping mechanism. Biochemistry. 1992 Dec 22;31(50):12546–12554. doi: 10.1021/bi00165a002. [DOI] [PubMed] [Google Scholar]
- Blanck A., Oesterhelt D. The halo-opsin gene. II. Sequence, primary structure of halorhodopsin and comparison with bacteriorhodopsin. EMBO J. 1987 Jan;6(1):265–273. doi: 10.1002/j.1460-2075.1987.tb04749.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braiman M. S., Walter T. J., Briercheck D. M. Infrared spectroscopic detection of light-induced change in chloride-arginine interaction in halorhodopsin. Biochemistry. 1994 Feb 22;33(7):1629–1635. doi: 10.1021/bi00173a003. [DOI] [PubMed] [Google Scholar]
- Casadio R., Gutowitz H., Mowery P., Taylor M., Stoeckenius W. Light-dark adaptation of bacteriorhodopsin in triton-treated purple membrane. Biochim Biophys Acta. 1980 Mar 7;590(1):13–23. doi: 10.1016/0005-2728(80)90142-5. [DOI] [PubMed] [Google Scholar]
- Duschl A., Lanyi J. K., Zimányi L. Properties and photochemistry of a halorhodopsin from the haloalkalophile, Natronobacterium pharaonis. J Biol Chem. 1990 Jan 25;265(3):1261–1267. [PubMed] [Google Scholar]
- Duschl A., McCloskey M. A., Lanyi J. K. Functional reconstitution of halorhodopsin. Properties of halorhodopsin-containing proteoliposomes. J Biol Chem. 1988 Nov 15;263(32):17016–17022. [PubMed] [Google Scholar]
- Falke J. J., Chan S. I., Steiner M., Oesterhelt D., Towner P., Lanyi J. K. Halide binding by the purified halorhodopsin chromoprotein. II. New chloride-binding sites revealed by 35Cl NMR. J Biol Chem. 1984 Feb 25;259(4):2185–2189. [PubMed] [Google Scholar]
- Fodor S. P., Bogomolni R. A., Mathies R. A. Structure of the retinal chromophore in the hRL intermediate of halorhodopsin from resonance raman spectroscopy. Biochemistry. 1987 Oct 20;26(21):6775–6778. doi: 10.1021/bi00395a029. [DOI] [PubMed] [Google Scholar]
- Gergely C., Ganea C., Váró G. Combined optical and photoelectric study of the photocycle of 13-cis bacteriorhodopsin. Biophys J. 1994 Aug;67(2):855–861. doi: 10.1016/S0006-3495(94)80545-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harbison G. S., Smith S. O., Pardoen J. A., Winkel C., Lugtenburg J., Herzfeld J., Mathies R., Griffin R. G. Dark-adapted bacteriorhodopsin contains 13-cis, 15-syn and all-trans, 15-anti retinal Schiff bases. Proc Natl Acad Sci U S A. 1984 Mar;81(6):1706–1709. doi: 10.1073/pnas.81.6.1706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havelka W. A., Henderson R., Heymann J. A., Oesterhelt D. Projection structure of halorhodopsin from Halobacterium halobium at 6 A resolution obtained by electron cryo-microscopy. J Mol Biol. 1993 Dec 5;234(3):837–846. doi: 10.1006/jmbi.1993.1629. [DOI] [PubMed] [Google Scholar]
- Hazemoto N., Kamo N., Kobatake Y. Suggestion of existence of two forms of halorhodospin in alkaline solution. Biochem Biophys Res Commun. 1984 Jan 30;118(2):502–507. doi: 10.1016/0006-291x(84)91331-7. [DOI] [PubMed] [Google Scholar]
- Hazemoto N., Kamo N., Terayama Y., Kobatake Y., Tsuda M. Photochemistry of two rhodopsinlike pigments in bacteriorhodopsin-free mutant of Halobacterium halobium. Biophys J. 1983 Oct;44(1):59–64. doi: 10.1016/S0006-3495(83)84277-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegemann P., Oesterbelt D., Steiner M. The photocycle of the chloride pump halorhodopsin. I: Azide-catalyzed deprotonation of the chromophore is a side reaction of photocycle intermediates inactivating the pump. EMBO J. 1985 Sep;4(9):2347–2350. doi: 10.1002/j.1460-2075.1985.tb03937.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofrichter J., Henry E. R., Lozier R. H. Photocycles of bacteriorhodopsin in light- and dark-adapted purple membrane studied by time-resolved absorption spectroscopy. Biophys J. 1989 Oct;56(4):693–706. doi: 10.1016/S0006-3495(89)82716-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalisky O., Goldschmidt C. R., Ottolenghi M. On the photocycle and light adaptation of dark-adapted bacteriorhodopsin. Biophys J. 1977 Aug;19(2):185–189. doi: 10.1016/S0006-3495(77)85579-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamo N., Hazemoto N., Kobatake Y., Mukohata Y. Light and dark adaptation of halorhodopsin. Arch Biochem Biophys. 1985 Apr;238(1):90–96. doi: 10.1016/0003-9861(85)90144-4. [DOI] [PubMed] [Google Scholar]
- Kataoka M., Kamikubo H., Tokunaga F., Brown L. S., Yamazaki Y., Maeda A., Sheves M., Needleman R., Lanyi J. K. Energy coupling in an ion pump. The reprotonation switch of bacteriorhodopsin. J Mol Biol. 1994 Nov 4;243(4):621–638. doi: 10.1016/0022-2836(94)90037-x. [DOI] [PubMed] [Google Scholar]
- Lanyi J. K., Duschl A., Hatfield G. W., May K., Oesterhelt D. The primary structure of a halorhodopsin from Natronobacterium pharaonis. Structural, functional and evolutionary implications for bacterial rhodopsins and halorhodopsins. J Biol Chem. 1990 Jan 25;265(3):1253–1260. [PubMed] [Google Scholar]
- Lanyi J. K. Halorhodopsin, a light-driven electrogenic chloride-transport system. Physiol Rev. 1990 Apr;70(2):319–330. doi: 10.1152/physrev.1990.70.2.319. [DOI] [PubMed] [Google Scholar]
- Lanyi J. K. Halorhodopsin: a light-driven chloride ion pump. Annu Rev Biophys Biophys Chem. 1986;15:11–28. doi: 10.1146/annurev.bb.15.060186.000303. [DOI] [PubMed] [Google Scholar]
- Lanyi J. K. Light-dependent trans to cis isomerization of the retinal in halorhodopsin. FEBS Lett. 1984 Oct 1;175(2):337–342. doi: 10.1016/0014-5793(84)80764-4. [DOI] [PubMed] [Google Scholar]
- Lanyi J. K. Photochromism of halorhodopsin. cis/trans isomerization of the retinal around the 13-14 double bond. J Biol Chem. 1986 Oct 25;261(30):14025–14030. [PubMed] [Google Scholar]
- Lanyi J. K. Proton transfer and energy coupling in the bacteriorhodopsin photocycle. J Bioenerg Biomembr. 1992 Apr;24(2):169–179. doi: 10.1007/BF00762675. [DOI] [PubMed] [Google Scholar]
- Lanyi J. K. Proton translocation mechanism and energetics in the light-driven pump bacteriorhodopsin. Biochim Biophys Acta. 1993 Dec 7;1183(2):241–261. doi: 10.1016/0005-2728(93)90226-6. [DOI] [PubMed] [Google Scholar]
- Lanyi J. K., Vodyanoy V. Flash spectroscopic studies of the kinetics of the halorhodopsin photocycle. Biochemistry. 1986;25(6):1465–1470. doi: 10.1021/bi00354a042. [DOI] [PubMed] [Google Scholar]
- Mowery P. C., Lozier R. H., Chae Q., Tseng Y. W., Taylor M., Stoeckenius W. Effect of acid pH on the absorption spectra and photoreactions of bacteriorhodopsin. Biochemistry. 1979 Sep 18;18(19):4100–4107. doi: 10.1021/bi00586a007. [DOI] [PubMed] [Google Scholar]
- Needleman R., Chang M., Ni B., Váró G., Fornés J., White S. H., Lanyi J. K. Properties of Asp212----Asn bacteriorhodopsin suggest that Asp212 and Asp85 both participate in a counterion and proton acceptor complex near the Schiff base. J Biol Chem. 1991 Jun 25;266(18):11478–11484. [PubMed] [Google Scholar]
- Oesterhelt D., Hegemann P., Tittor J. The photocycle of the chloride pump halorhodopsin. II: Quantum yields and a kinetic model. EMBO J. 1985 Sep;4(9):2351–2356. doi: 10.1002/j.1460-2075.1985.tb03938.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oesterhelt D., Stoeckenius W. Isolation of the cell membrane of Halobacterium halobium and its fractionation into red and purple membrane. Methods Enzymol. 1974;31:667–678. doi: 10.1016/0076-6879(74)31072-5. [DOI] [PubMed] [Google Scholar]
- Oesterhelt D., Tittor J., Bamberg E. A unifying concept for ion translocation by retinal proteins. J Bioenerg Biomembr. 1992 Apr;24(2):181–191. doi: 10.1007/BF00762676. [DOI] [PubMed] [Google Scholar]
- Ogurusu T., Maeda A., Yoshizawa T. Absorption spectral properties of purified halorhodopsin. J Biochem. 1984 Apr;95(4):1073–1082. doi: 10.1093/oxfordjournals.jbchem.a134695. [DOI] [PubMed] [Google Scholar]
- Otomo J., Tomioka H., Sasabe H. Properties and the primary structure of a new halorhodopsin from halobacterial strain mex. Biochim Biophys Acta. 1992 Nov 23;1112(1):7–13. doi: 10.1016/0005-2736(92)90246-i. [DOI] [PubMed] [Google Scholar]
- Otto H., Marti T., Holz M., Mogi T., Stern L. J., Engel F., Khorana H. G., Heyn M. P. Substitution of amino acids Asp-85, Asp-212, and Arg-82 in bacteriorhodopsin affects the proton release phase of the pump and the pK of the Schiff base. Proc Natl Acad Sci U S A. 1990 Feb;87(3):1018–1022. doi: 10.1073/pnas.87.3.1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pande C., Lanyi J. K., Callender R. H. Effects of various anions on the Raman spectrum of halorhodopsin. Biophys J. 1989 Mar;55(3):425–431. doi: 10.1016/S0006-3495(89)82836-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothschild K. J., Bousché O., Braiman M. S., Hasselbacher C. A., Spudich J. L. Fourier transform infrared study of the halorhodopsin chloride pump. Biochemistry. 1988 Apr 5;27(7):2420–2424. doi: 10.1021/bi00407a026. [DOI] [PubMed] [Google Scholar]
- Scharf B., Engelhard M. Blue halorhodopsin from Natronobacterium pharaonis: wavelength regulation by anions. Biochemistry. 1994 May 31;33(21):6387–6393. doi: 10.1021/bi00187a002. [DOI] [PubMed] [Google Scholar]
- Schobert B., Lanyi J. K., Cragoe E. J., Jr Evidence for a halide-binding site in halorhodopsin. J Biol Chem. 1983 Dec 25;258(24):15158–15164. [PubMed] [Google Scholar]
- Schobert B., Lanyi J. K. Electrostatic interaction between anions bound to site I and the retinal Schiff base of halorhodopsin. Biochemistry. 1986 Jul 15;25(14):4163–4167. doi: 10.1021/bi00362a026. [DOI] [PubMed] [Google Scholar]
- Schobert B., Lanyi J. K. Halorhodopsin is a light-driven chloride pump. J Biol Chem. 1982 Sep 10;257(17):10306–10313. [PubMed] [Google Scholar]
- Schobert B., Lanyi J. K., Oesterhelt D. Effects of anion binding on the deprotonation reactions of halorhodopsin. J Biol Chem. 1986 Feb 25;261(6):2690–2696. [PubMed] [Google Scholar]
- Spencer D. B., Dewey T. G. Activation parameters for the halorhodopsin photocycle: a phase lifetime spectroscopic study of the 520- and 640-nanometer intermediates. Biochemistry. 1990 Mar 27;29(12):3140–3145. doi: 10.1021/bi00464a034. [DOI] [PubMed] [Google Scholar]
- Steiner M., Oesterhelt D., Ariki M., Lanyi J. K. Halide binding by the purified halorhodopsin chromoprotein. I. Effects on the chromophore. J Biol Chem. 1984 Feb 25;259(4):2179–2184. [PubMed] [Google Scholar]
- Stern L. J., Ahl P. L., Marti T., Mogi T., Duñach M., Berkowitz S., Rothschild K. J., Khorana H. G. Substitution of membrane-embedded aspartic acids in bacteriorhodopsin causes specific changes in different steps of the photochemical cycle. Biochemistry. 1989 Dec 26;28(26):10035–10042. doi: 10.1021/bi00452a023. [DOI] [PubMed] [Google Scholar]
- Thorgeirsson T. E., Milder S. J., Miercke L. J., Betlach M. C., Shand R. F., Stroud R. M., Kliger D. S. Effects of Asp-96----Asn, Asp-85----Asn, and Arg-82----Gln single-site substitutions on the photocycle of bacteriorhodopsin. Biochemistry. 1991 Sep 24;30(38):9133–9142. doi: 10.1021/bi00102a003. [DOI] [PubMed] [Google Scholar]
- Tittor J., Oesterhelt D., Maurer R., Desel H., Uhl R. The photochemical cycle of halorhodopsin: absolute spectra of intermediates obtained by flash photolysis and fast difference spectra measurements. Biophys J. 1987 Dec;52(6):999–1006. doi: 10.1016/S0006-3495(87)83292-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuda M., Hazemoto N., Kondo M., Kamo N., Kobatake Y., Terayama Y. Two photocycles in halobacterium halobium that lacks bacteriorhodopsin. Biochem Biophys Res Commun. 1982 Oct 15;108(3):970–976. doi: 10.1016/0006-291x(82)92094-0. [DOI] [PubMed] [Google Scholar]
- Váró G., Lanyi J. K. Kinetic and spectroscopic evidence for an irreversible step between deprotonation and reprotonation of the Schiff base in the bacteriorhodopsin photocycle. Biochemistry. 1991 May 21;30(20):5008–5015. doi: 10.1021/bi00234a024. [DOI] [PubMed] [Google Scholar]
- Váró G., Lanyi J. K. Photoreactions of bacteriorhodopsin at acid pH. Biophys J. 1989 Dec;56(6):1143–1151. doi: 10.1016/S0006-3495(89)82761-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Váró G., Lanyi J. K. Thermodynamics and energy coupling in the bacteriorhodopsin photocycle. Biochemistry. 1991 May 21;30(20):5016–5022. doi: 10.1021/bi00234a025. [DOI] [PubMed] [Google Scholar]
- Walter T. J., Braiman M. S. Anion-protein interactions during halorhodopsin pumping: halide binding at the protonated Schiff base. Biochemistry. 1994 Feb 22;33(7):1724–1733. doi: 10.1021/bi00173a015. [DOI] [PubMed] [Google Scholar]
- Zimányi L., Cao Y., Chang M., Ni B., Needleman R., Lanyi J. K. The two consecutive M substates in the photocycle of bacteriorhodopsin are affected specifically by the D85N and D96N residue replacements. Photochem Photobiol. 1992 Dec;56(6):1049–1055. doi: 10.1111/j.1751-1097.1992.tb09728.x. [DOI] [PubMed] [Google Scholar]
- Zimányi L., Keszthelyi L., Lanyi J. K. Transient spectroscopy of bacterial rhodopsins with an optical multichannel analyzer. 1. Comparison of the photocycles of bacteriorhodopsin and halorhodopsin. Biochemistry. 1989 Jun 13;28(12):5165–5172. doi: 10.1021/bi00438a038. [DOI] [PubMed] [Google Scholar]
- Zimányi L., Lanyi J. K. Deriving the intermediate spectra and photocycle kinetics from time-resolved difference spectra of bacteriorhodopsin. The simpler case of the recombinant D96N protein. Biophys J. 1993 Jan;64(1):240–251. doi: 10.1016/S0006-3495(93)81360-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimányi L., Lanyi J. K. Iso-halorhodopsin: a stable, 9-cis retinal containing photoproduct of halorhodopsin. Biophys J. 1987 Dec;52(6):1007–1013. doi: 10.1016/S0006-3495(87)83293-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimányi L., Lanyi J. K. Low-temperature photoreactions of halorhodopsin. 2. Description of the photocycle and its intermediates. Biochemistry. 1989 Feb 21;28(4):1662–1666. doi: 10.1021/bi00430a035. [DOI] [PubMed] [Google Scholar]
- Zimányi L., Lanyi J. K. Transient spectroscopy of bacterial rhodopsins with an optical multichannel analyzer. 2. Effects of anions on the halorhodopsin photocycle. Biochemistry. 1989 Jun 13;28(12):5172–5178. doi: 10.1021/bi00438a039. [DOI] [PubMed] [Google Scholar]
- Zimányi L., Ormos P., Lanyi J. K. Low-temperature photoreactions of halorhodopsin. 1. Detection of conformational substates of the chromoprotein. Biochemistry. 1989 Feb 21;28(4):1656–1661. doi: 10.1021/bi00430a034. [DOI] [PubMed] [Google Scholar]


