Abstract
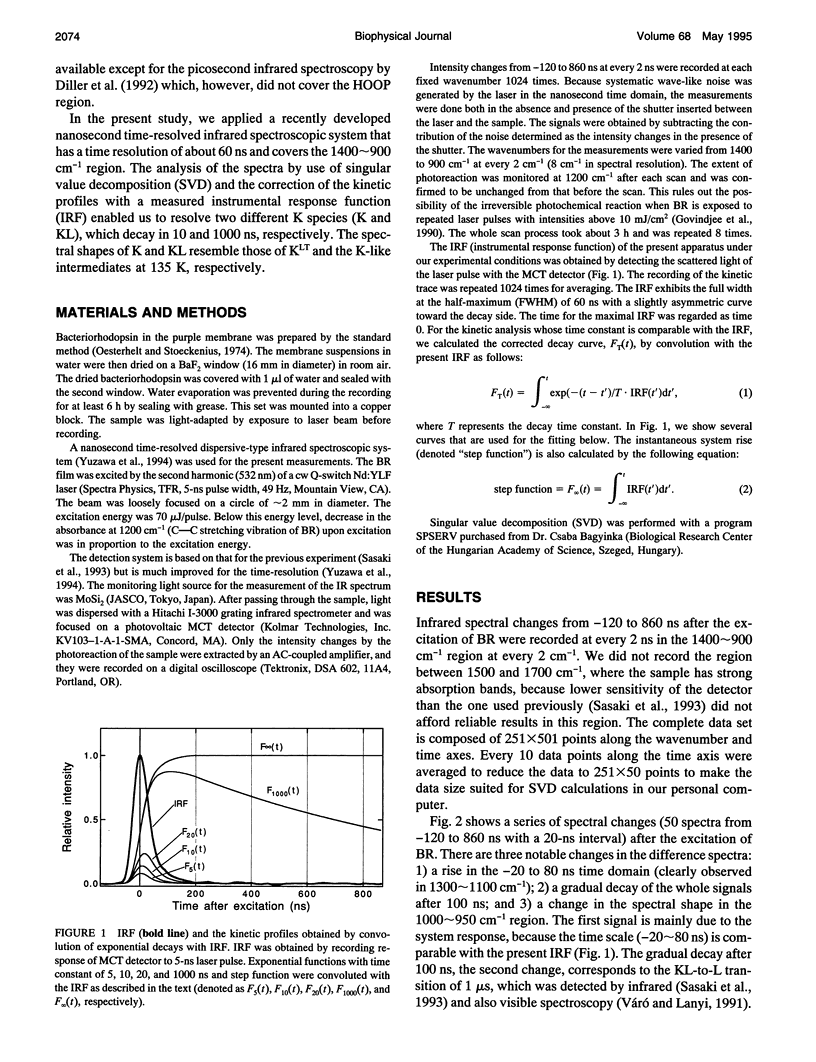
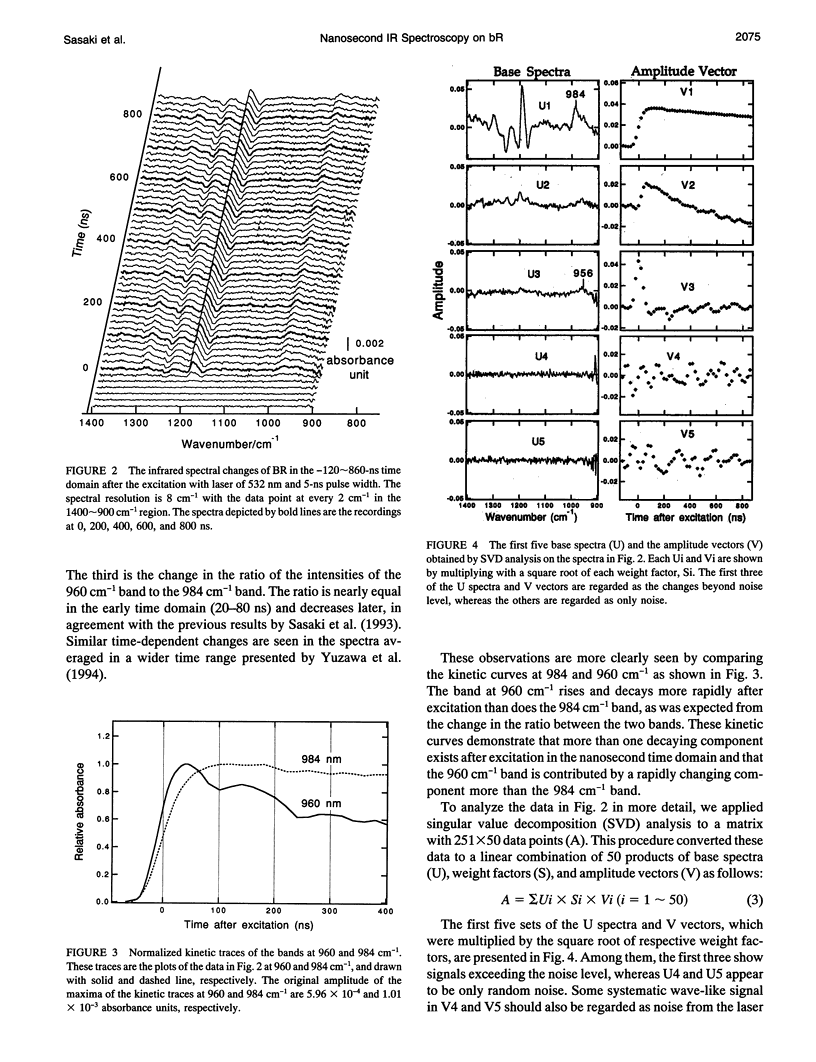
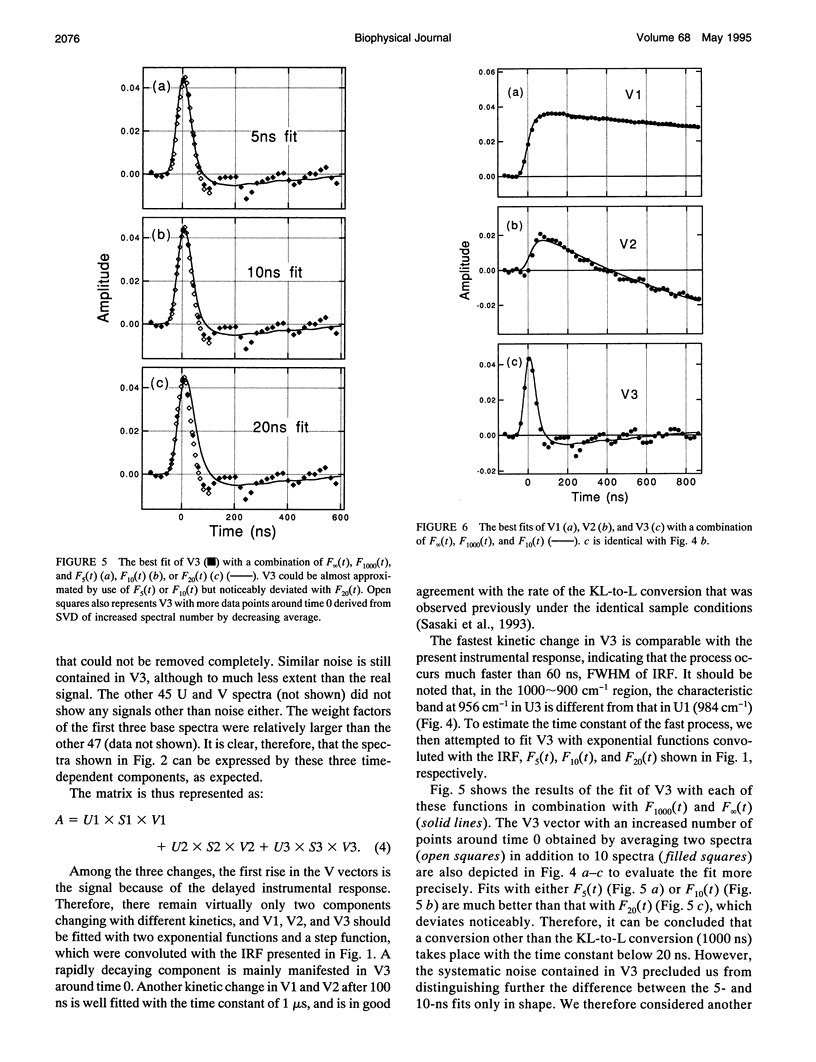
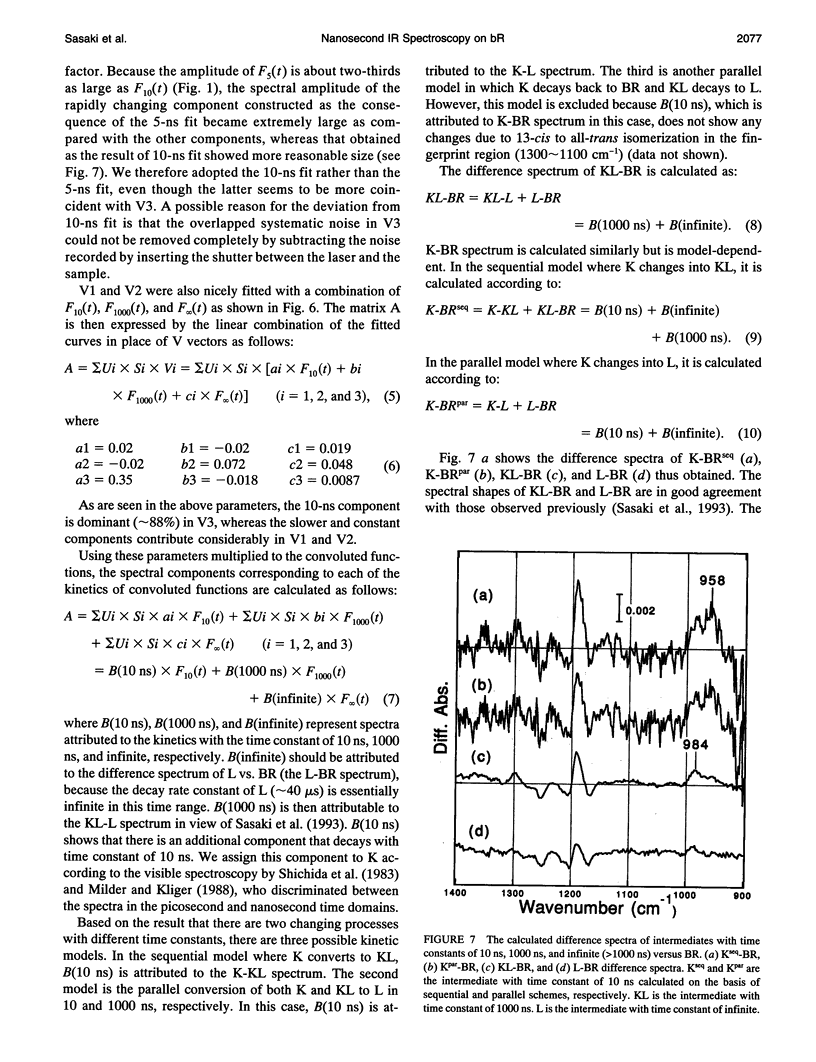
The photochemical reaction process of bacteriorhodopsin in the nanosecond time range (-120-860 ns) was measured in the 1400-900 cm-1 region with an improved time resolved dispersive-type infrared spectrometer. The system is equipped with a newly developed detection unit whose instrumental response to a 5-ns laser pulse has a full width of the half-maximum of 60 ns. It provides highly accurate data that enabled us to extract a kinetic process one order of magnitude faster than the instrumental response. The spectral changes in the 1400-900 cm-1 region were analyzed by singular value decomposition and resolved into three components. These components were separated by fitting with 10- and 1000-ns exponential functions and a step function, which were convoluted with the instrumental response function. The components with decay time constants of 10 and 1000 ns are named K and KL, respectively, on the basis of previous visible spectroscopy. The spectral shapes of K and KL are distinguishable by their hydrogen-out-of-plane (HOOP) modes, at 958 and 984 cm-1, respectively. The former corresponds to the K intermediate recorded at 77 K and the latter to a K-like photoproduct at 135 K. On the basis of published data, these bands are assigned to the 15-HOOP mode, indicating that the K and KL differ in a twist around the C14-C15 bond.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Braiman M. S., Mogi T., Marti T., Stern L. J., Khorana H. G., Rothschild K. J. Vibrational spectroscopy of bacteriorhodopsin mutants: light-driven proton transport involves protonation changes of aspartic acid residues 85, 96, and 212. Biochemistry. 1988 Nov 15;27(23):8516–8520. doi: 10.1021/bi00423a002. [DOI] [PubMed] [Google Scholar]
- Braiman M. S., Mogi T., Stern L. J., Hackett N. R., Chao B. H., Khorana H. G., Rothschild K. J. Vibrational spectroscopy of bacteriorhodopsin mutants: I. Tyrosine-185 protonates and deprotonates during the photocycle. Proteins. 1988;3(4):219–229. doi: 10.1002/prot.340030403. [DOI] [PubMed] [Google Scholar]
- Diller R., Iannone M., Cowen B. R., Maiti S., Bogomolni R. A., Hochstrasser R. M. Picosecond dynamics of bacteriorhodopsin, probed by time-resolved infrared spectroscopy. Biochemistry. 1992 Jun 23;31(24):5567–5572. doi: 10.1021/bi00139a020. [DOI] [PubMed] [Google Scholar]
- Fodor S. P., Pollard W. T., Gebhard R., van den Berg E. M., Lugtenburg J., Mathies R. A. Bacteriorhodopsin's L550 intermediate contains a C14-C15 s-trans-retinal chromophore. Proc Natl Acad Sci U S A. 1988 Apr;85(7):2156–2160. doi: 10.1073/pnas.85.7.2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govindjee R., Balashov S. P., Ebrey T. G. Quantum efficiency of the photochemical cycle of bacteriorhodopsin. Biophys J. 1990 Sep;58(3):597–608. doi: 10.1016/S0006-3495(90)82403-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanyi J. K. Proton translocation mechanism and energetics in the light-driven pump bacteriorhodopsin. Biochim Biophys Acta. 1993 Dec 7;1183(2):241–261. doi: 10.1016/0005-2728(93)90226-6. [DOI] [PubMed] [Google Scholar]
- Lozier R. H., Bogomolni R. A., Stoeckenius W. Bacteriorhodopsin: a light-driven proton pump in Halobacterium Halobium. Biophys J. 1975 Sep;15(9):955–962. doi: 10.1016/S0006-3495(75)85875-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda A., Sasaki J., Shichida Y., Yoshizawa T., Chang M., Ni B., Needleman R., Lanyi J. K. Structures of aspartic acid-96 in the L and N intermediates of bacteriorhodopsin: analysis by Fourier transform infrared spectroscopy. Biochemistry. 1992 May 19;31(19):4684–4690. doi: 10.1021/bi00134a022. [DOI] [PubMed] [Google Scholar]
- Maeda A., Sasaki J., Shichida Y., Yoshizawa T. Water structural changes in the bacteriorhodopsin photocycle: analysis by Fourier transform infrared spectroscopy. Biochemistry. 1992 Jan 21;31(2):462–467. doi: 10.1021/bi00117a023. [DOI] [PubMed] [Google Scholar]
- Maeda A., Sasaki J., Yamazaki Y., Needleman R., Lanyi J. K. Interaction of aspartate-85 with a water molecule and the protonated Schiff base in the L intermediate of bacteriorhodopsin: a Fourier-transform infrared spectroscopic study. Biochemistry. 1994 Feb 22;33(7):1713–1717. doi: 10.1021/bi00173a013. [DOI] [PubMed] [Google Scholar]
- Mathies R. A., Brito Cruz C. H., Pollard W. T., Shank C. V. Direct observation of the femtosecond excited-state cis-trans isomerization in bacteriorhodopsin. Science. 1988 May 6;240(4853):777–779. doi: 10.1126/science.3363359. [DOI] [PubMed] [Google Scholar]
- Mathies R. A., Lin S. W., Ames J. B., Pollard W. T. From femtoseconds to biology: mechanism of bacteriorhodopsin's light-driven proton pump. Annu Rev Biophys Biophys Chem. 1991;20:491–518. doi: 10.1146/annurev.bb.20.060191.002423. [DOI] [PubMed] [Google Scholar]
- Milder S. J., Kliger D. S. A time-resolved spectral study of the K and KL intermediates of bacteriorhodopsin. Biophys J. 1988 Mar;53(3):465–468. doi: 10.1016/S0006-3495(88)83124-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oesterhelt D., Stoeckenius W. Isolation of the cell membrane of Halobacterium halobium and its fractionation into red and purple membrane. Methods Enzymol. 1974;31:667–678. doi: 10.1016/0076-6879(74)31072-5. [DOI] [PubMed] [Google Scholar]
- Rothschild K. J., He Y. W., Sonar S., Marti T., Khorana H. G. Vibrational spectroscopy of bacteriorhodopsin mutants. Evidence that Thr-46 and Thr-89 form part of a transient network of hydrogen bonds. J Biol Chem. 1992 Jan 25;267(3):1615–1622. [PubMed] [Google Scholar]
- Rothschild K. J., Marrero H. Infrared evidence that the Schiff base of bacteriorhodopsin is protonated: bR570 and K intermediates. Proc Natl Acad Sci U S A. 1982 Jul;79(13):4045–4049. doi: 10.1073/pnas.79.13.4045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothschild K. J., Roepe P., Gillespie J. Fourier transform infrared spectroscopic evidence for the existence of two conformations of the bacteriorhodopsin primary photoproduct at low temperature. Biochim Biophys Acta. 1985 Jun 26;808(1):140–148. doi: 10.1016/0005-2728(85)90036-2. [DOI] [PubMed] [Google Scholar]
- Sasaki J., Maeda A., Kato C., Hamaguchi H. Time-resolved infrared spectral analysis of the KL-to-L conversion in the photocycle of bacteriorhodopsin. Biochemistry. 1993 Jan 26;32(3):867–871. doi: 10.1021/bi00054a018. [DOI] [PubMed] [Google Scholar]
- Siebert F., Mäntele W. Investigation of the primary photochemistry of bacteriorhodopsin by low-temperature Fourier-transform infrared spectroscopy. Eur J Biochem. 1983 Feb 15;130(3):565–573. doi: 10.1111/j.1432-1033.1983.tb07187.x. [DOI] [PubMed] [Google Scholar]
- Stoeckenius W., Lozier R. H. Light energy conversion in Halobacterium halobium. J Supramol Struct. 1974;2(5-6):769–774. doi: 10.1002/jss.400020519. [DOI] [PubMed] [Google Scholar]
- Tokunaga F., Iwasa T., Yoshizawa T. Photochemical reaction of bacteriorhodopsin. FEBS Lett. 1976 Dec 15;72(1):33–38. doi: 10.1016/0014-5793(76)80807-1. [DOI] [PubMed] [Google Scholar]
- Váró G., Lanyi J. K. Thermodynamics and energy coupling in the bacteriorhodopsin photocycle. Biochemistry. 1991 May 21;30(20):5016–5022. doi: 10.1021/bi00234a025. [DOI] [PubMed] [Google Scholar]
- Yamazaki Y., Sasaki J., Hatanaka M., Kandori H., Maeda A., Needleman R., Shinada T., Yoshihara K., Brown L. S., Lanyi J. K. Interaction of tryptophan-182 with the retinal 9-methyl group in the L intermediate of bacteriorhodopsin. Biochemistry. 1995 Jan 17;34(2):577–582. doi: 10.1021/bi00002a024. [DOI] [PubMed] [Google Scholar]
- Zhou F., Windemuth A., Schulten K. Molecular dynamics study of the proton pump cycle of bacteriorhodopsin. Biochemistry. 1993 Mar 9;32(9):2291–2306. doi: 10.1021/bi00060a022. [DOI] [PubMed] [Google Scholar]


