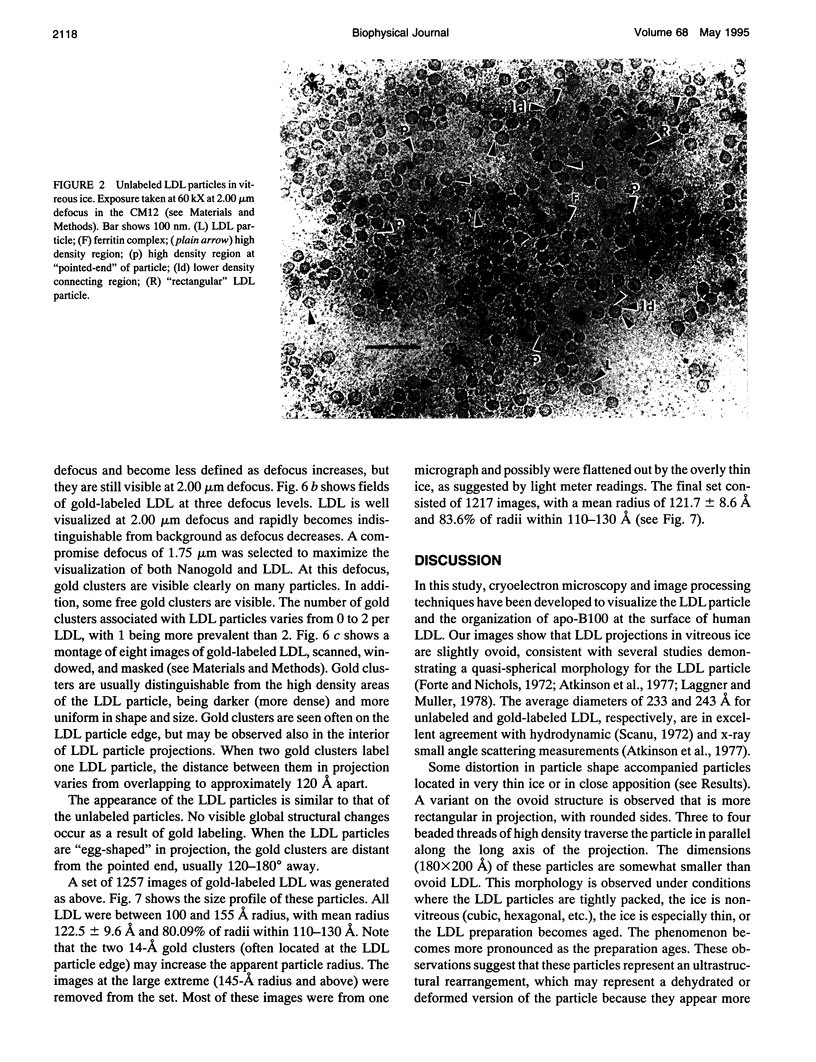
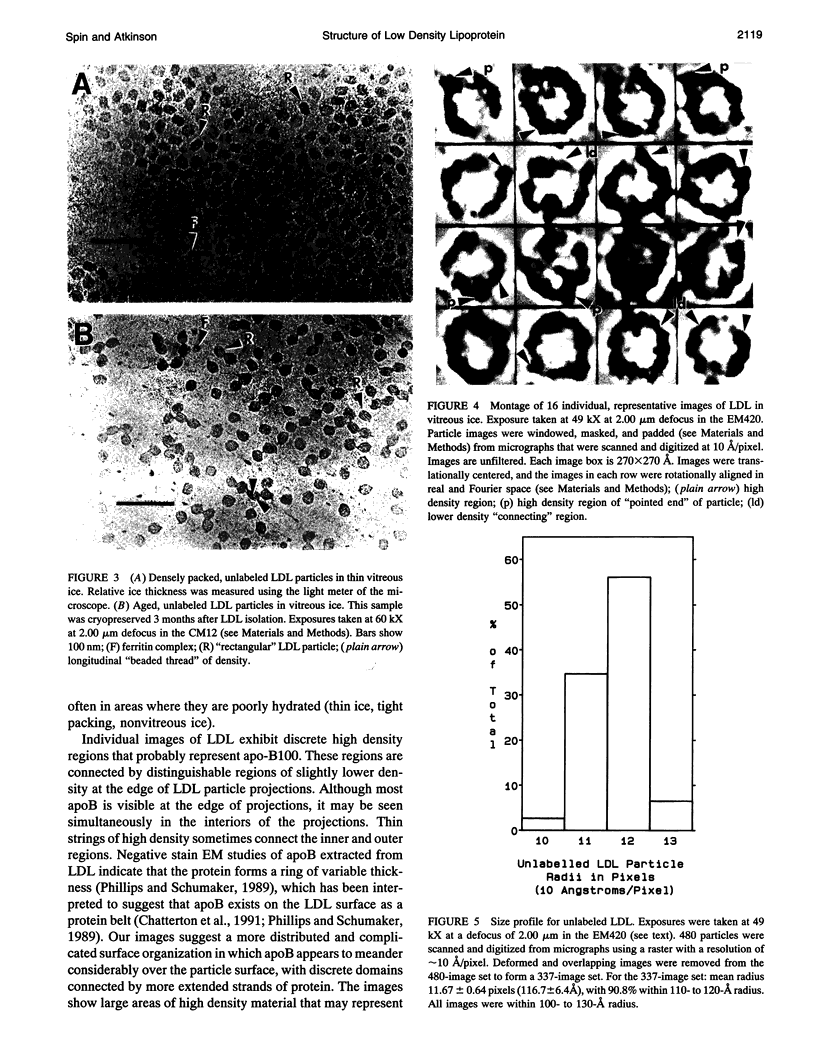
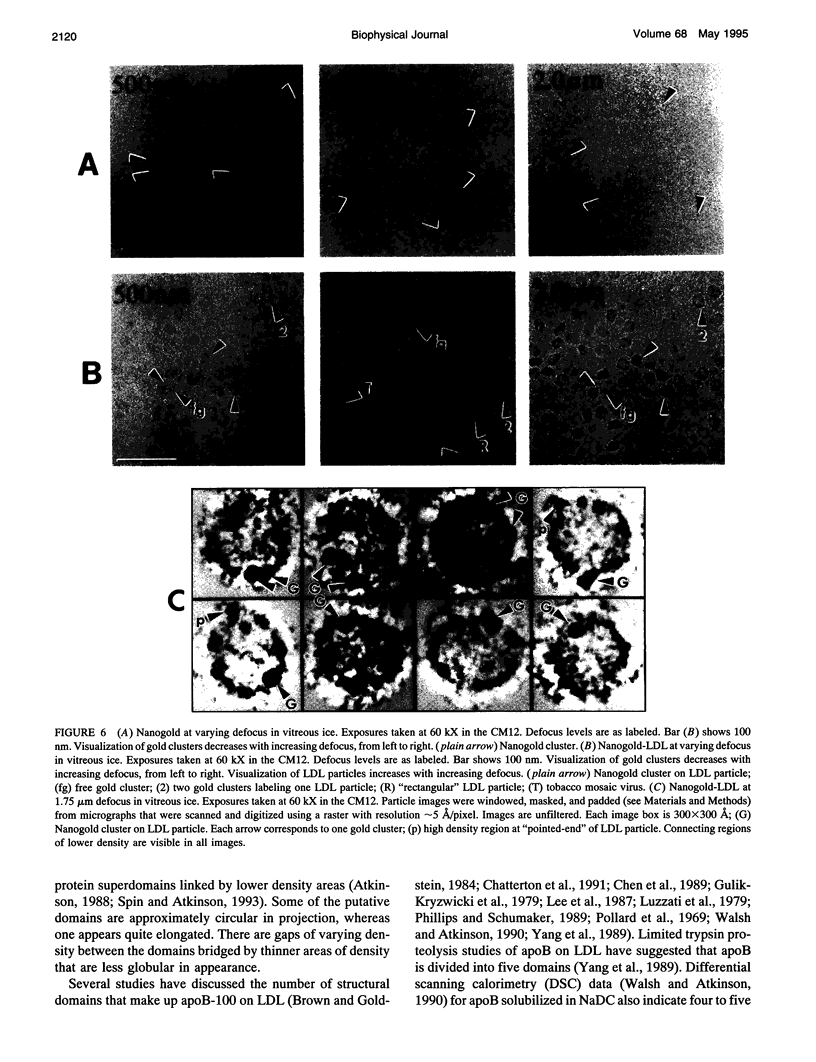
Abstract
In this report, images of low density lipoprotein (LDL) in vitreous ice at approximately 30 A resolution are presented. These images show that LDL is a quasi-spherical particle, approximately 220-240 A in diameter, with a region of low density (lipid) surrounded by a ring (in projection) of high density believed to represent apolipoprotein B-100. This ring is seen to be composed of four or five (depending on view) large regions of high density material that may represent protein superdomains. Analysis of LDL images obtained at slightly higher magnification reveals that areas of somewhat lower density connect these regions, in some cases crossing the projectional interiors of the LDL particles. Preliminary image analysis of LDL covalently labeled at Cys3734 and Cys4190 with 1.4-nm Nanogold clusters demonstrates that this methodology will provide an important site-specific marker in studies designed to map the organization of apoB at the surface of LDL.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adrian M., Dubochet J., Lepault J., McDowall A. W. Cryo-electron microscopy of viruses. Nature. 1984 Mar 1;308(5954):32–36. doi: 10.1038/308032a0. [DOI] [PubMed] [Google Scholar]
- Atkinson D., Deckelbaum R. J., Small D. M., Shipley G. G. Structure of human plasma low-density lipoproteins: molecular organization of the central core. Proc Natl Acad Sci U S A. 1977 Mar;74(3):1042–1046. doi: 10.1073/pnas.74.3.1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu S. K., Anderson R. G., Goldstein J. L., Brown M. S. Metabolism of cationized lipoproteins by human fibroblasts. Biochemical and morphologic correlations. J Cell Biol. 1977 Jul;74(1):119–135. doi: 10.1083/jcb.74.1.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown M. S., Goldstein J. L. How LDL receptors influence cholesterol and atherosclerosis. Sci Am. 1984 Nov;251(5):58–66. doi: 10.1038/scientificamerican1184-58. [DOI] [PubMed] [Google Scholar]
- Brown M. S., Goldstein J. L. Receptor-mediated control of cholesterol metabolism. Science. 1976 Jan 16;191(4223):150–154. doi: 10.1126/science.174194. [DOI] [PubMed] [Google Scholar]
- Cardin A. D., Witt K. R., Chao J., Margolius H. S., Donaldson V. H., Jackson R. L. Degradation of apolipoprotein B-100 of human plasma low density lipoproteins by tissue and plasma kallikreins. J Biol Chem. 1984 Jul 10;259(13):8522–8528. [PubMed] [Google Scholar]
- Chatterton J. E., Phillips M. L., Curtiss L. K., Milne R. W., Marcel Y. L., Schumaker V. N. Mapping apolipoprotein B on the low density lipoprotein surface by immunoelectron microscopy. J Biol Chem. 1991 Mar 25;266(9):5955–5962. [PubMed] [Google Scholar]
- Chen G. C., Zhu S., Hardman D. A., Schilling J. W., Lau K., Kane J. P. Structural domains of human apolipoprotein B-100. Differential accessibility to limited proteolysis of B-100 in low density and very low density lipoproteins. J Biol Chem. 1989 Aug 25;264(24):14369–14375. [PubMed] [Google Scholar]
- Cladaras C., Hadzopoulou-Cladaras M., Nolte R. T., Atkinson D., Zannis V. I. The complete sequence and structural analysis of human apolipoprotein B-100: relationship between apoB-100 and apoB-48 forms. EMBO J. 1986 Dec 20;5(13):3495–3507. doi: 10.1002/j.1460-2075.1986.tb04675.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman R. D., Kim T. W., Gotto A. M., Jr, Yang C. Y. Determination of cysteine on low-density lipoproteins using the fluorescent probe, 5-iodoacetamidofluoresceine. Biochim Biophys Acta. 1990 Jan 19;1037(1):129–132. doi: 10.1016/0167-4838(90)90111-r. [DOI] [PubMed] [Google Scholar]
- Deckelbaum R. J., Shipley G. G., Small D. M., Lees R. S., George P. K. Thermal transitions in human plasma low density lipoproteins. Science. 1975 Oct 24;190(4212):392–394. doi: 10.1126/science.170681. [DOI] [PubMed] [Google Scholar]
- Dubochet J., Adrian M., Chang J. J., Homo J. C., Lepault J., McDowall A. W., Schultz P. Cryo-electron microscopy of vitrified specimens. Q Rev Biophys. 1988 May;21(2):129–228. doi: 10.1017/s0033583500004297. [DOI] [PubMed] [Google Scholar]
- Forte T., Nichols A. V. Application of electron microscopy to the study of plasma lipoprotein structure. Adv Lipid Res. 1972;10:1–41. [PubMed] [Google Scholar]
- Frank J., Bretaudiere J. P., Carazo J. M., Verschoor A., Wagenknecht T. Classification of images of biomolecular assemblies: a study of ribosomes and ribosomal subunits of Escherichia coli. J Microsc. 1988 May;150(Pt 2):99–115. doi: 10.1111/j.1365-2818.1988.tb04602.x. [DOI] [PubMed] [Google Scholar]
- Frank J., Goldfarb W., Eisenberg D., Baker T. S. Reconstruction of glutamine synthetase using computer averaging. Ultramicroscopy. 1978;3(3):283–290. doi: 10.1016/s0304-3991(78)80038-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOFMAN J. W., RUBIN L., McGINLEY J. P., JONES H. B. Hyperlipoproteinemia. Am J Med. 1954 Oct;17(4):514–520. doi: 10.1016/0002-9343(54)90126-6. [DOI] [PubMed] [Google Scholar]
- Ginsburg G. S., Small D. M., Atkinson D. Microemulsions of phospholipids and cholesterol esters. Protein-free models of low density lipoprotein. J Biol Chem. 1982 Jul 25;257(14):8216–8227. [PubMed] [Google Scholar]
- Guevara J., Jr, Spurlino J., Jan A. Y., Yang C. Y., Tulinsky A., Prasad B. V., Gaubatz J. W., Morrisett J. D. Proposed mechanisms for binding of apo[a] kringle type 9 to apo B-100 in human lipoprotein[a]. Biophys J. 1993 Mar;64(3):686–700. doi: 10.1016/S0006-3495(93)81428-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulik-Krzywicki T., Yates M., Aggerbeck L. P. Structure of serum low-density lipoprotein. II. A freeze-etching electron microscopy study. J Mol Biol. 1979 Jul 5;131(3):475–484. doi: 10.1016/0022-2836(79)90003-2. [DOI] [PubMed] [Google Scholar]
- HAVEL R. J., EDER H. A., BRAGDON J. H. The distribution and chemical composition of ultracentrifugally separated lipoproteins in human serum. J Clin Invest. 1955 Sep;34(9):1345–1353. doi: 10.1172/JCI103182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hainfeld J. F., Furuya F. R. A 1.4-nm gold cluster covalently attached to antibodies improves immunolabeling. J Histochem Cytochem. 1992 Feb;40(2):177–184. doi: 10.1177/40.2.1552162. [DOI] [PubMed] [Google Scholar]
- Kannel W. B., Castelli W. P., Gordon T., McNamara P. M. Serum cholesterol, lipoproteins, and the risk of coronary heart disease. The Framingham study. Ann Intern Med. 1971 Jan;74(1):1–12. doi: 10.7326/0003-4819-74-1-1. [DOI] [PubMed] [Google Scholar]
- Knott T. J., Pease R. J., Powell L. M., Wallis S. C., Rall S. C., Jr, Innerarity T. L., Blackhart B., Taylor W. H., Marcel Y., Milne R. Complete protein sequence and identification of structural domains of human apolipoprotein B. Nature. 1986 Oct 23;323(6090):734–738. doi: 10.1038/323734a0. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laggner P., Müller K. W. The structure of serum lipoproteins as analysed by X-ray small-angle scattering. Q Rev Biophys. 1978 Aug;11(3):371–425. doi: 10.1017/s0033583500002304. [DOI] [PubMed] [Google Scholar]
- Lee D. M., Stiers D. L., Mok T. Apolipoprotein B is a globular protein--morphological studies by electron microscopy. Biochem Biophys Res Commun. 1987 Apr 14;144(1):210–216. doi: 10.1016/s0006-291x(87)80497-7. [DOI] [PubMed] [Google Scholar]
- Lepault J., Booy F. P., Dubochet J. Electron microscopy of frozen biological suspensions. J Microsc. 1983 Jan;129(Pt 1):89–102. doi: 10.1111/j.1365-2818.1983.tb04163.x. [DOI] [PubMed] [Google Scholar]
- Luzzati V., Tardieu A., Aggerbeck L. P. Structure of serum low-density lipoprotein. I. A solution X-ray scattering study of a hyperlipidemic monkey low-density lipoprotein. J Mol Biol. 1979 Jul 5;131(3):435–473. doi: 10.1016/0022-2836(79)90002-0. [DOI] [PubMed] [Google Scholar]
- Milligan R. A., Brisson A., Unwin P. N. Molecular structure determination of crystalline specimens in frozen aqueous solutions. Ultramicroscopy. 1984;13(1-2):1–9. doi: 10.1016/0304-3991(84)90051-2. [DOI] [PubMed] [Google Scholar]
- Noble R. P. Electrophoretic separation of plasma lipoproteins in agarose gel. J Lipid Res. 1968 Nov;9(6):693–700. [PubMed] [Google Scholar]
- Owen C., DeRosier D. A 13-A map of the actin-scruin filament from the limulus acrosomal process. J Cell Biol. 1993 Oct;123(2):337–344. doi: 10.1083/jcb.123.2.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson G. L. Review of the Folin phenol protein quantitation method of Lowry, Rosebrough, Farr and Randall. Anal Biochem. 1979 Dec;100(2):201–220. doi: 10.1016/0003-2697(79)90222-7. [DOI] [PubMed] [Google Scholar]
- Phillips M. L., Schumaker V. N. Conformation of apolipoprotein B after lipid extraction of low density lipoproteins attached to an electron microscope grid. J Lipid Res. 1989 Mar;30(3):415–422. [PubMed] [Google Scholar]
- Pollard H., Scanu A. M., Taylor E. W. On the geometrical arrangement of the protein subunits of human serum low-density lipoprotein: evidence for a dodecahedral model. Proc Natl Acad Sci U S A. 1969 Sep;64(1):304–310. doi: 10.1073/pnas.64.1.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redgrave T. G., Roberts D. C., West C. E. Separation of plasma lipoproteins by density-gradient ultracentrifugation. Anal Biochem. 1975 May 12;65(1-2):42–49. doi: 10.1016/0003-2697(75)90488-1. [DOI] [PubMed] [Google Scholar]
- Shen M. M., Krauss R. M., Lindgren F. T., Forte T. M. Heterogeneity of serum low density lipoproteins in normal human subjects. J Lipid Res. 1981 Feb;22(2):236–244. [PubMed] [Google Scholar]
- Sniderman A., Shapiro S., Marpole D., Skinner B., Teng B., Kwiterovich P. O., Jr Association of coronary atherosclerosis with hyperapobetalipoproteinemia [increased protein but normal cholesterol levels in human plasma low density (beta) lipoproteins]. Proc Natl Acad Sci U S A. 1980 Jan;77(1):604–608. doi: 10.1073/pnas.77.1.604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer A., Gorges R., Kostner G. M., Paltauf F., Hermetter A. Sulfhydryl-selective fluorescence labeling of lipoprotein(a) reveals evidence for one single disulfide linkage between apoproteins(a) and B-100. Biochemistry. 1991 Nov 26;30(47):11245–11249. doi: 10.1021/bi00111a008. [DOI] [PubMed] [Google Scholar]
- Wagenknecht T., Grassucci R., Frank J. Electron microscopy and computer image averaging of ice-embedded large ribosomal subunits from Escherichia coli. J Mol Biol. 1988 Jan 5;199(1):137–147. doi: 10.1016/0022-2836(88)90384-1. [DOI] [PubMed] [Google Scholar]
- Wagenknecht T., Grassucci R., Schaak D. Cryoelectron microscopy of frozen-hydrated alpha-ketoacid dehydrogenase complexes from Escherichia coli. J Biol Chem. 1990 Dec 25;265(36):22402–22408. [PubMed] [Google Scholar]
- Walsh M. T., Atkinson D. Calorimetric and spectroscopic investigation of the unfolding of human apolipoprotein B. J Lipid Res. 1990 Jun;31(6):1051–1062. [PubMed] [Google Scholar]
- Yang C. Y., Chen S. H., Gianturco S. H., Bradley W. A., Sparrow J. T., Tanimura M., Li W. H., Sparrow D. A., DeLoof H., Rosseneu M. Sequence, structure, receptor-binding domains and internal repeats of human apolipoprotein B-100. Nature. 1986 Oct 23;323(6090):738–742. doi: 10.1038/323738a0. [DOI] [PubMed] [Google Scholar]
- Yang C. Y., Gu Z. W., Weng S. A., Kim T. W., Chen S. H., Pownall H. J., Sharp P. M., Liu S. W., Li W. H., Gotto A. M., Jr Structure of apolipoprotein B-100 of human low density lipoproteins. Arteriosclerosis. 1989 Jan-Feb;9(1):96–108. doi: 10.1161/01.atv.9.1.96. [DOI] [PubMed] [Google Scholar]
- Yang C. Y., Kim T. W., Weng S. A., Lee B. R., Yang M. L., Gotto A. M., Jr Isolation and characterization of sulfhydryl and disulfide peptides of human apolipoprotein B-100. Proc Natl Acad Sci U S A. 1990 Jul;87(14):5523–5527. doi: 10.1073/pnas.87.14.5523. [DOI] [PMC free article] [PubMed] [Google Scholar]