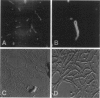
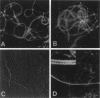
Abstract
Most polymers which comprise biological filaments assemble by two mechanisms: nucleation and elongation or a sequential, stepwise process involving a hierarchy of intermediate species. We report the application of atomic force microscopy (AFM) to the study of the early events in the sequential or stepwise mode of assembly of a macromolecular filament. Collagen monomers were assembled in vitro and the early structural intermediates of the assembly process were examined by AFM and correlated with turbidimetric alterations in the assembly mixture. The assembly of collagen involved a sequence of distinctive filamentous species which increased in both diameter and length over the time course of assembly. The first discrete population of collagen oligomers were 1-2 nm in diameter (300-500 nm in length); at later time points, filaments approximately 2-6 nm in diameter (> 10 microns in length) many with a conspicuous approximately 67-nm axial period were observed. Occasional mature collagen fibrils with a approximately 67-nm axial repeat were found late in the course of assembly. Our results are consistent with initial end-to-end axial association of monomers to form oligomers followed by lateral association into higher-order filaments. On this basis, there appears to be at least two distinctive types of structural interactions (axial and lateral) which are operative at different levels in the assembly hierarchy of collagen.
Full text
PDF

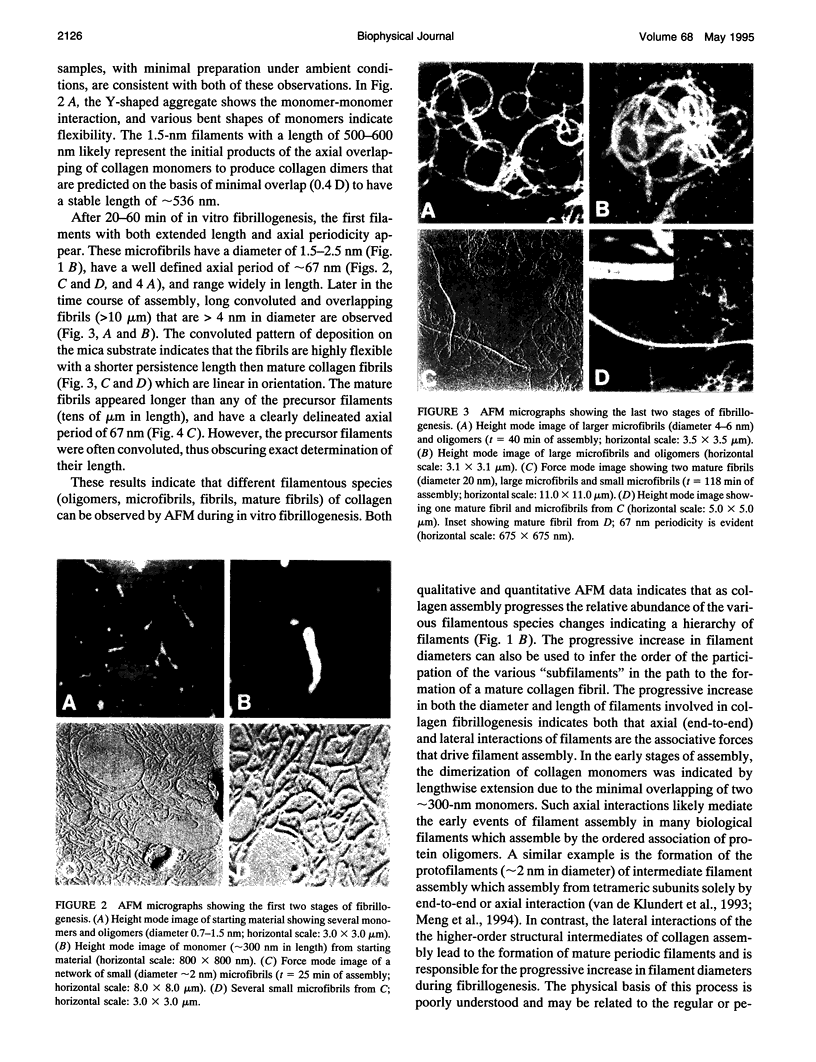
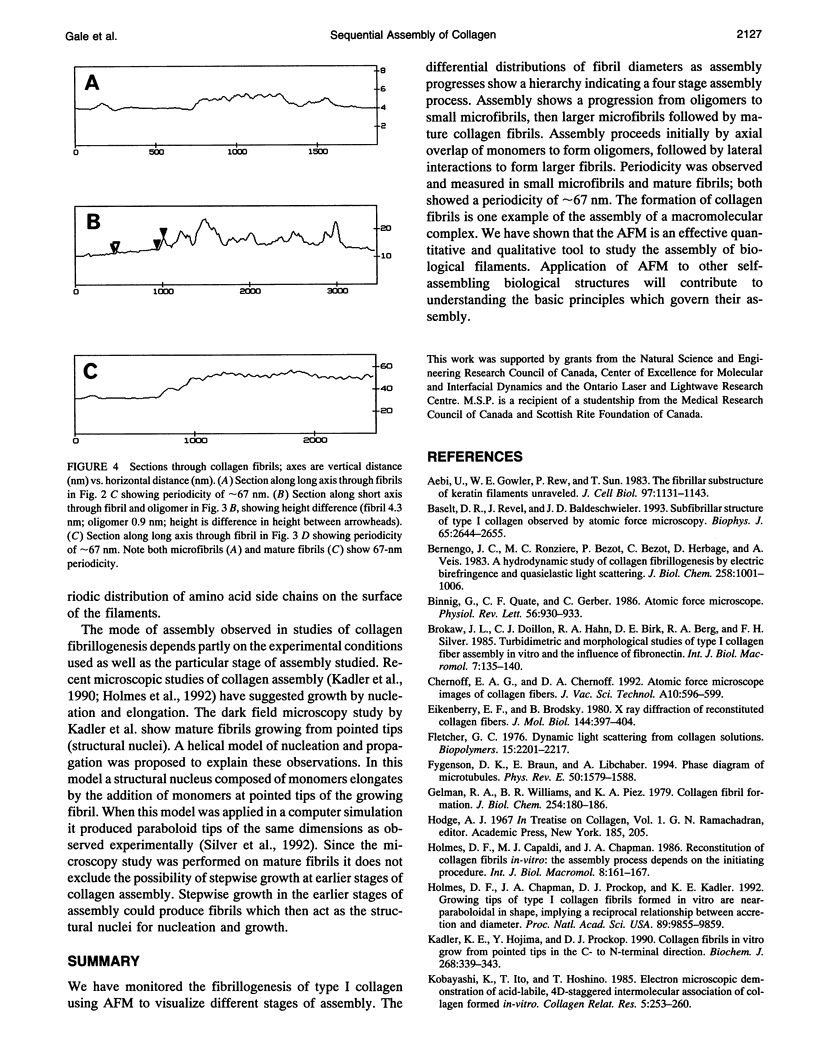

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aebi U., Fowler W. E., Rew P., Sun T. T. The fibrillar substructure of keratin filaments unraveled. J Cell Biol. 1983 Oct;97(4):1131–1143. doi: 10.1083/jcb.97.4.1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baselt D. R., Revel J. P., Baldeschwieler J. D. Subfibrillar structure of type I collagen observed by atomic force microscopy. Biophys J. 1993 Dec;65(6):2644–2655. doi: 10.1016/S0006-3495(93)81329-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernengo J. C., Ronziere M. C., Bezot P., Bezot C., Herbage D., Veis A. A hydrodynamic study of collagen fibrillogenesis by electric birefringence and quasielastic light scattering. J Biol Chem. 1983 Jan 25;258(2):1001–1006. [PubMed] [Google Scholar]
- Binnig G, Quate CF, Gerber C. Atomic force microscope. Phys Rev Lett. 1986 Mar 3;56(9):930–933. doi: 10.1103/PhysRevLett.56.930. [DOI] [PubMed] [Google Scholar]
- Eikenberry E. F., Brodsky B. X-ray diffraction of reconstituted collagen fibers. J Mol Biol. 1980 Dec 15;144(3):397–404. doi: 10.1016/0022-2836(80)90098-4. [DOI] [PubMed] [Google Scholar]
- Fletcher G. C. Dynamic light scattering from collagen solutions. I. Translational diffusion coefficient and aggregation effects. Biopolymers. 1976 Nov;15(11):2201–2217. doi: 10.1002/bip.1976.360151108. [DOI] [PubMed] [Google Scholar]
- Fygenson DK, Braun E, Libchaber A. Phase diagram of microtubules. Phys Rev E Stat Phys Plasmas Fluids Relat Interdiscip Topics. 1994 Aug;50(2):1579–1588. doi: 10.1103/physreve.50.1579. [DOI] [PubMed] [Google Scholar]
- Gelman R. A., Williams B. R., Piez K. A. Collagen fibril formation. Evidence for a multistep process. J Biol Chem. 1979 Jan 10;254(1):180–186. [PubMed] [Google Scholar]
- Holmes D. F., Chapman J. A., Prockop D. J., Kadler K. E. Growing tips of type I collagen fibrils formed in vitro are near-paraboloidal in shape, implying a reciprocal relationship between accretion and diameter. Proc Natl Acad Sci U S A. 1992 Oct 15;89(20):9855–9859. doi: 10.1073/pnas.89.20.9855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadler K. E., Hojima Y., Prockop D. J. Collagen fibrils in vitro grow from pointed tips in the C- to N-terminal direction. Biochem J. 1990 Jun 1;268(2):339–343. doi: 10.1042/bj2680339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi K., Ito T., Hoshino T. Electron microscopic demonstration of acid-labile, 4D-staggered intermolecular association of collagen formed in vitro. Coll Relat Res. 1985 Jun;5(3):253–260. doi: 10.1016/s0174-173x(85)80015-7. [DOI] [PubMed] [Google Scholar]
- Lal R., John S. A. Biological applications of atomic force microscopy. Am J Physiol. 1994 Jan;266(1 Pt 1):C1–21. doi: 10.1152/ajpcell.1994.266.1.C1. [DOI] [PubMed] [Google Scholar]
- Leikin S., Rau D. C., Parsegian V. A. Direct measurement of forces between self-assembled proteins: temperature-dependent exponential forces between collagen triple helices. Proc Natl Acad Sci U S A. 1994 Jan 4;91(1):276–280. doi: 10.1073/pnas.91.1.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacBeath J. R., Shackleton D. R., Hulmes D. J. Tyrosine-rich acidic matrix protein (TRAMP) accelerates collagen fibril formation in vitro. J Biol Chem. 1993 Sep 15;268(26):19826–19832. [PubMed] [Google Scholar]
- Meng J. J., Khan S., Ip W. Charge interactions in the rod domain drive formation of tetramers during intermediate filament assembly. J Biol Chem. 1994 Jul 15;269(28):18679–18685. [PubMed] [Google Scholar]
- Notbohm H., Mosler S., Müller P. K., Brinckmann J. In vitro formation and aggregation of heterotypic collagen I and III fibrils. Int J Biol Macromol. 1993 Oct;15(5):299–304. doi: 10.1016/0141-8130(93)90030-p. [DOI] [PubMed] [Google Scholar]
- Pollanen M. S., Markiewicz P., Bergeron C., Goh M. C. Twisted ribbon structure of paired helical filaments revealed by atomic force microscopy. Am J Pathol. 1994 May;144(5):869–873. [PMC free article] [PubMed] [Google Scholar]
- Revenko I., Sommer F., Minh D. T., Garrone R., Franc J. M. Atomic force microscopy study of the collagen fibre structure. Biol Cell. 1994;80(1):67–69. doi: 10.1016/0248-4900(94)90019-1. [DOI] [PubMed] [Google Scholar]
- Silver D., Miller J., Harrison R., Prockop D. J. Helical model of nucleation and propagation to account for the growth of type I collagen fibrils from symmetrical pointed tips: a special example of self-assembly of rod-like monomers. Proc Natl Acad Sci U S A. 1992 Oct 15;89(20):9860–9864. doi: 10.1073/pnas.89.20.9860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver F. H., Langley K. H., Trelstad R. L. Type I collagen fibrillogenesis: initiation via reversible linear and lateral growth steps. Biopolymers. 1979 Oct;18(10):2523–2535. doi: 10.1002/bip.1979.360181011. [DOI] [PubMed] [Google Scholar]
- Smyrk T. C. Colon cancer connections. Cancer syndrome meets molecular biology meets histopathology. Am J Pathol. 1994 Jul;145(1):1–6. [PMC free article] [PubMed] [Google Scholar]
- Wallace D. G. Early assembly pathways of type I collagen. Biopolymers. 1992 May;32(5):497–515. doi: 10.1002/bip.360320506. [DOI] [PubMed] [Google Scholar]
- Ward N. P., Hulmes D. J., Chapman J. A. Collagen self-assembly in vitro: electron microscopy of initial aggregates formed during the lag phase. J Mol Biol. 1986 Jul 5;190(1):107–112. doi: 10.1016/0022-2836(86)90079-3. [DOI] [PubMed] [Google Scholar]
- van de Klundert F. A., Raats J. M., Bloemendal H. Intermediate filaments: regulation of gene expression and assembly. Eur J Biochem. 1993 Jun 1;214(2):351–366. doi: 10.1111/j.1432-1033.1993.tb17931.x. [DOI] [PubMed] [Google Scholar]