Abstract
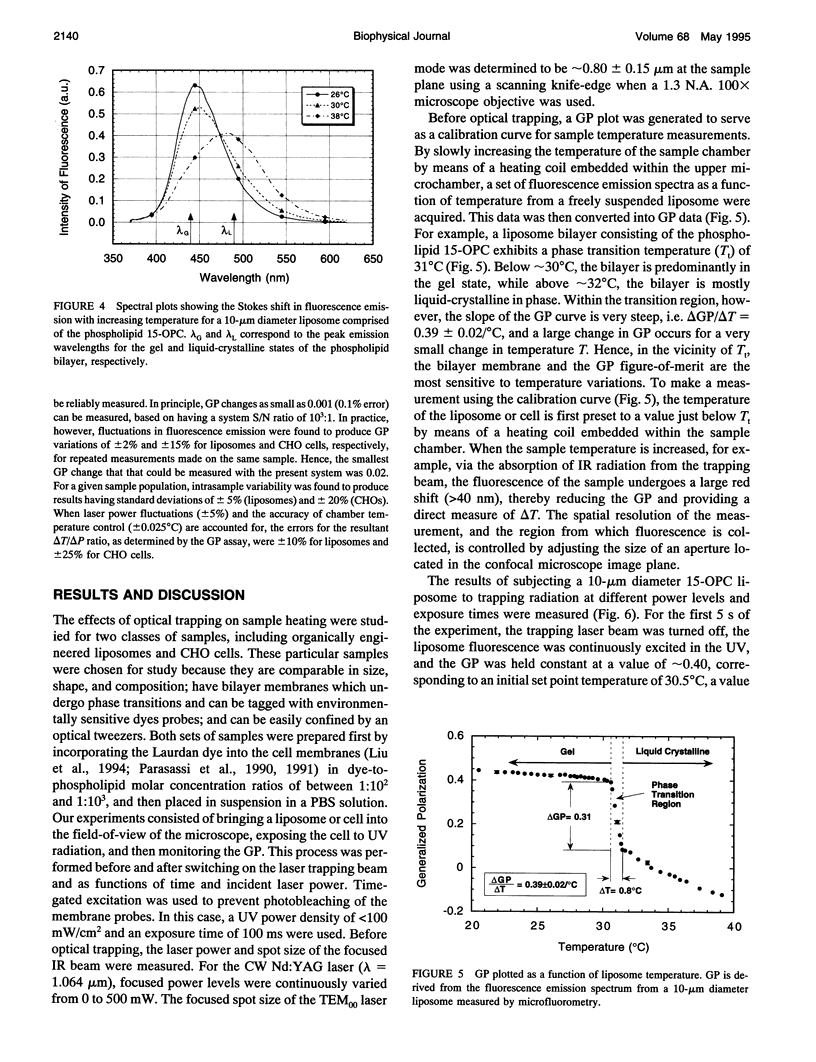
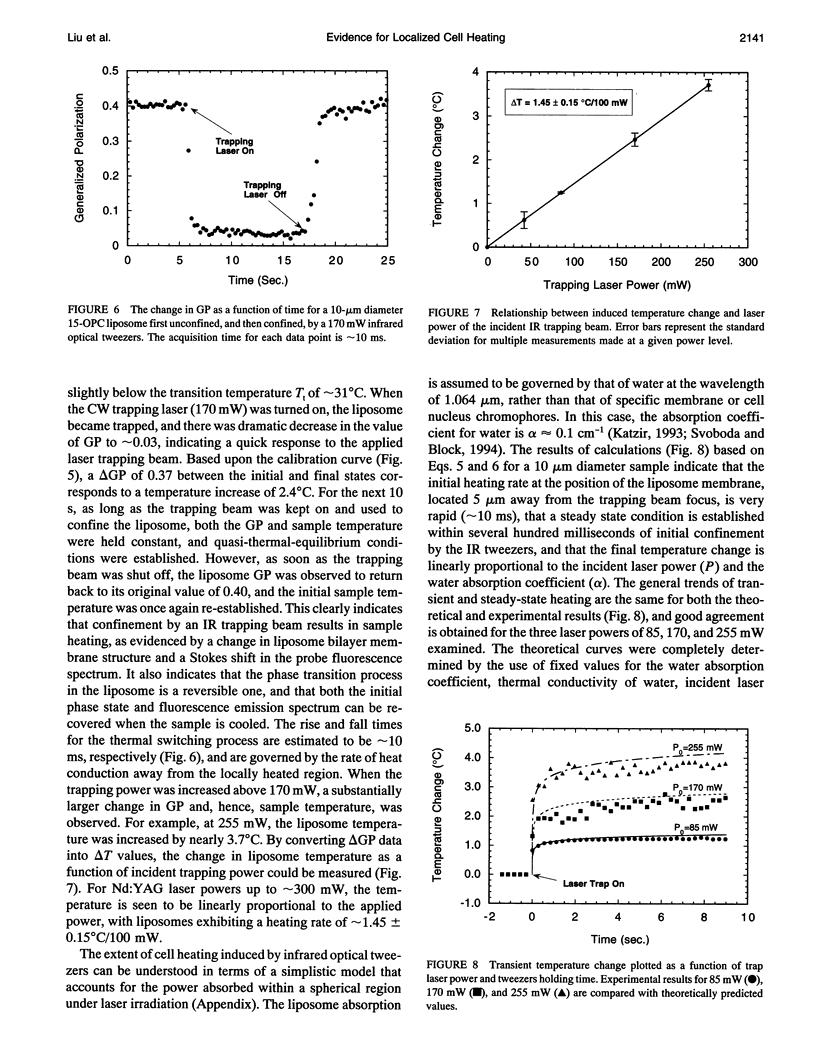
The confinement of liposomes and Chinese hamster ovary (CHO) cells by infrared (IR) optical tweezers is shown to result in sample heating and temperature increases by several degrees centigrade, as measured by a noninvasive, spatially resolved fluorescence detection technique. For micron-sized spherical liposome vesicles having bilayer membranes composed of the phospholipid 1,2-diacyl-pentadecanoyl-glycero-phosphocholine (15-OPC), a temperature rise of approximately 1.45 +/- 0.15 degrees C/100 mW is observed when the vesicles are held stationary with a 1.064 microns optical tweezers having a power density of approximately 10(7) W/cm2 and a focused spot size of approximately 0.8 micron. The increase in sample temperature is found to scale linearly with applied optical power in the 40 to 250 mW range. Under the same trapping conditions, CHO cells exhibit an average temperature rise of nearly 1.15 +/- 0.25 degrees C/100 mW. The extent of cell heating induced by infrared tweezers confinement can be described by a heat conduction model that accounts for the absorption of infrared (IR) laser radiation in the aqueous cell core and membrane regions, respectively. The observed results are relevant to the assessment of the noninvasive nature of infrared trapping beams in micromanipulation applications and cell physiological studies.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashkin A., Dziedzic J. M. Internal cell manipulation using infrared laser traps. Proc Natl Acad Sci U S A. 1989 Oct;86(20):7914–7918. doi: 10.1073/pnas.86.20.7914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashkin A., Dziedzic J. M. Optical trapping and manipulation of viruses and bacteria. Science. 1987 Mar 20;235(4795):1517–1520. doi: 10.1126/science.3547653. [DOI] [PubMed] [Google Scholar]
- Ashkin A., Dziedzic J. M., Yamane T. Optical trapping and manipulation of single cells using infrared laser beams. Nature. 1987 Dec 24;330(6150):769–771. doi: 10.1038/330769a0. [DOI] [PubMed] [Google Scholar]
- Berns M. W., Wright W. H., Tromberg B. J., Profeta G. A., Andrews J. J., Walter R. J. Use of a laser-induced optical force trap to study chromosome movement on the mitotic spindle. Proc Natl Acad Sci U S A. 1989 Jun;86(12):4539–4543. doi: 10.1073/pnas.86.12.4539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Block S. M., Goldstein L. S., Schnapp B. J. Bead movement by single kinesin molecules studied with optical tweezers. Nature. 1990 Nov 22;348(6299):348–352. doi: 10.1038/348348a0. [DOI] [PubMed] [Google Scholar]
- Colon J. M., Sarosi P., McGovern P. G., Askin A., Dziedzic J. M., Skurnick J., Weiss G., Bonder E. M. Controlled micromanipulation of human sperm in three dimensions with an infrared laser optical trap: effect on sperm velocity. Fertil Steril. 1992 Mar;57(3):695–698. doi: 10.1016/s0015-0282(16)54926-7. [DOI] [PubMed] [Google Scholar]
- Dynlacht J. R., Fox M. H. Heat-induced changes in the membrane fluidity of Chinese hamster ovary cells measured by flow cytometry. Radiat Res. 1992 Apr;130(1):48–54. [PubMed] [Google Scholar]
- Finer J. T., Simmons R. M., Spudich J. A. Single myosin molecule mechanics: piconewton forces and nanometre steps. Nature. 1994 Mar 10;368(6467):113–119. doi: 10.1038/368113a0. [DOI] [PubMed] [Google Scholar]
- Kuo S. C., Sheetz M. P. Force of single kinesin molecules measured with optical tweezers. Science. 1993 Apr 9;260(5105):232–234. doi: 10.1126/science.8469975. [DOI] [PubMed] [Google Scholar]
- Kuo S. C., Sheetz M. P. Optical tweezers in cell biology. Trends Cell Biol. 1992 Apr;2(4):116–118. doi: 10.1016/0962-8924(92)90016-g. [DOI] [PubMed] [Google Scholar]
- Liang H., Wright W. H., Rieder C. L., Salmon E. D., Profeta G., Andrews J., Liu Y., Sonek G. J., Berns M. W. Directed movement of chromosome arms and fragments in mitotic newt lung cells using optical scissors and optical tweezers. Exp Cell Res. 1994 Jul;213(1):308–312. doi: 10.1006/excr.1994.1203. [DOI] [PubMed] [Google Scholar]
- Oku N., Scheerer J. F., MacDonald R. C. Preparation of giant liposomes. Biochim Biophys Acta. 1982 Nov 22;692(3):384–388. doi: 10.1016/0005-2736(82)90388-1. [DOI] [PubMed] [Google Scholar]
- Parasassi T., De Stasio G., Ravagnan G., Rusch R. M., Gratton E. Quantitation of lipid phases in phospholipid vesicles by the generalized polarization of Laurdan fluorescence. Biophys J. 1991 Jul;60(1):179–189. doi: 10.1016/S0006-3495(91)82041-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parasassi T., De Stasio G., d'Ubaldo A., Gratton E. Phase fluctuation in phospholipid membranes revealed by Laurdan fluorescence. Biophys J. 1990 Jun;57(6):1179–1186. doi: 10.1016/S0006-3495(90)82637-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins T. T., Quake S. R., Smith D. E., Chu S. Relaxation of a single DNA molecule observed by optical microscopy. Science. 1994 May 6;264(5160):822–826. doi: 10.1126/science.8171336. [DOI] [PubMed] [Google Scholar]
- Perkins T. T., Smith D. E., Chu S. Direct observation of tube-like motion of a single polymer chain. Science. 1994 May 6;264(5160):819–822. doi: 10.1126/science.8171335. [DOI] [PubMed] [Google Scholar]
- Schütze K., Clement-Sengewald A., Ashkin A. Zona drilling and sperm insertion with combined laser microbeam and optical tweezers. Fertil Steril. 1994 Apr;61(4):783–786. [PubMed] [Google Scholar]
- Schütze K., Clement-Sengewald A. Catch and move--cut or fuse. Nature. 1994 Apr 14;368(6472):667–669. doi: 10.1038/368667a0. [DOI] [PubMed] [Google Scholar]
- Svoboda K., Block S. M. Biological applications of optical forces. Annu Rev Biophys Biomol Struct. 1994;23:247–285. doi: 10.1146/annurev.bb.23.060194.001335. [DOI] [PubMed] [Google Scholar]
- Svoboda K., Schmidt C. F., Schnapp B. J., Block S. M. Direct observation of kinesin stepping by optical trapping interferometry. Nature. 1993 Oct 21;365(6448):721–727. doi: 10.1038/365721a0. [DOI] [PubMed] [Google Scholar]
- Tadir Y., Wright W. H., Vafa O., Ord T., Asch R. H., Berns M. W. Micromanipulation of sperm by a laser generated optical trap. Fertil Steril. 1989 Nov;52(5):870–873. [PubMed] [Google Scholar]
- Vorobjev I. A., Liang H., Wright W. H., Berns M. W. Optical trapping for chromosome manipulation: a wavelength dependence of induced chromosome bridges. Biophys J. 1993 Feb;64(2):533–538. doi: 10.1016/S0006-3495(93)81398-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yatvin M. B., Tegmo-Larsson I. M., Dennis W. H. Temperature- and pH-sensitive liposomes for drug targeting. Methods Enzymol. 1987;149:77–87. doi: 10.1016/0076-6879(87)49045-9. [DOI] [PubMed] [Google Scholar]




