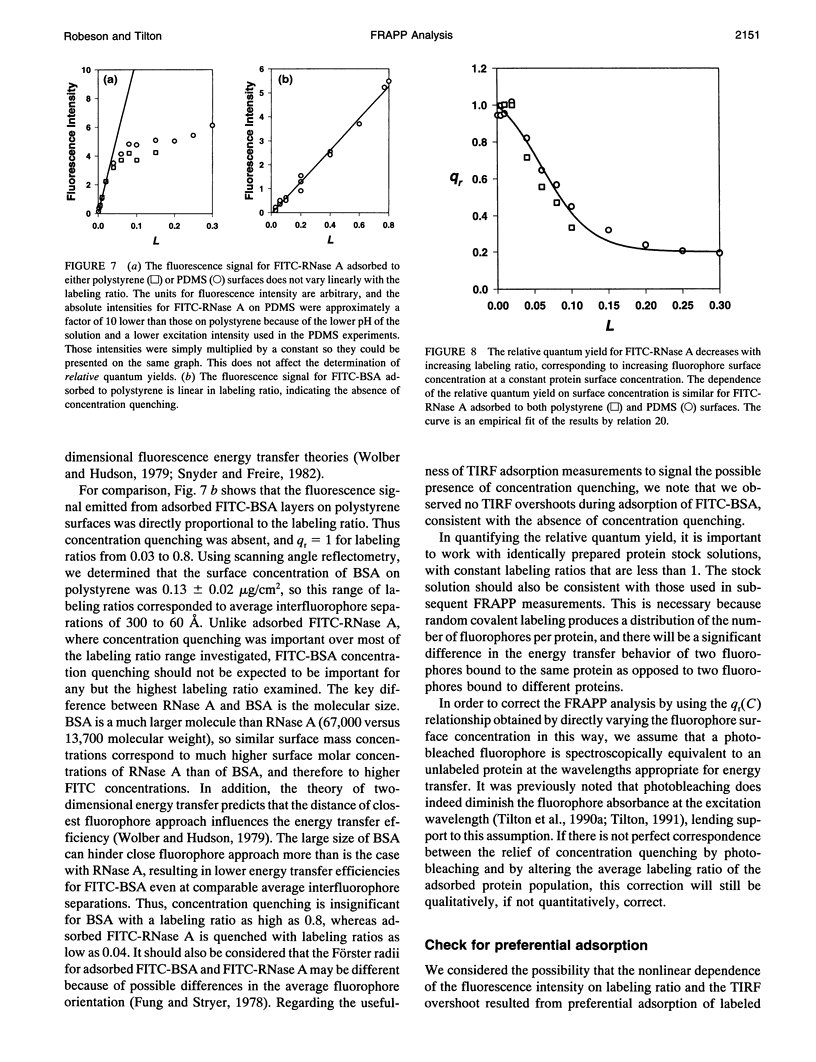
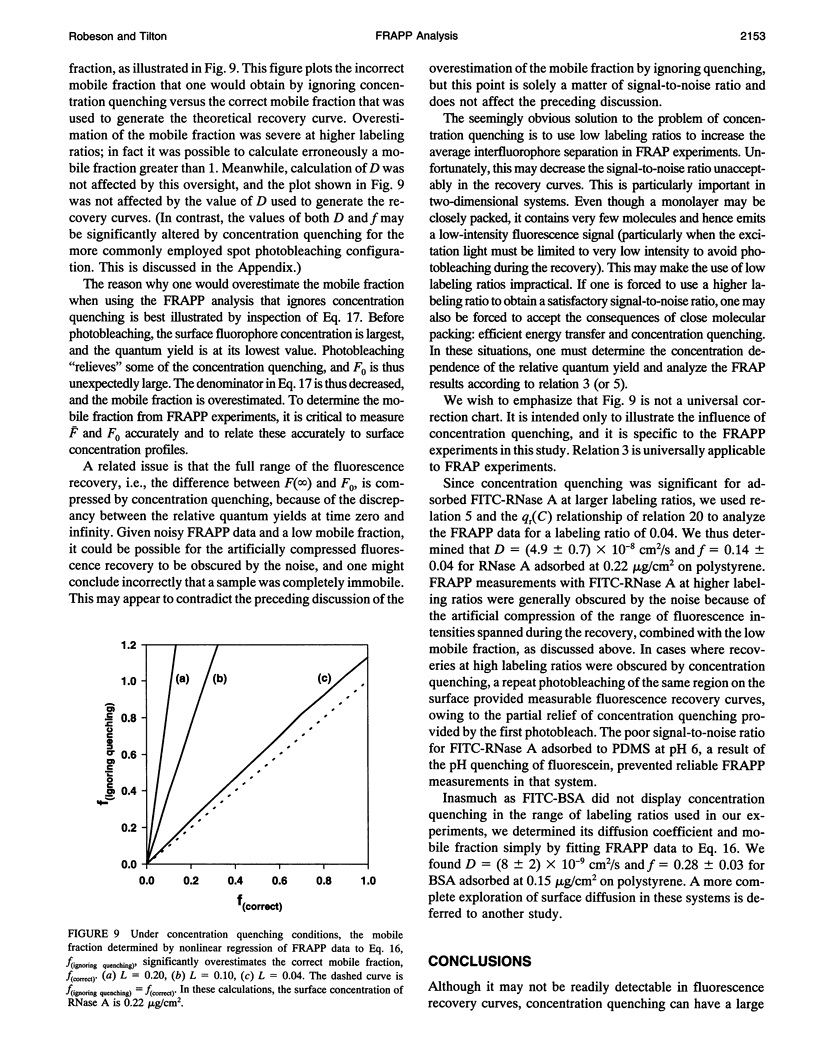
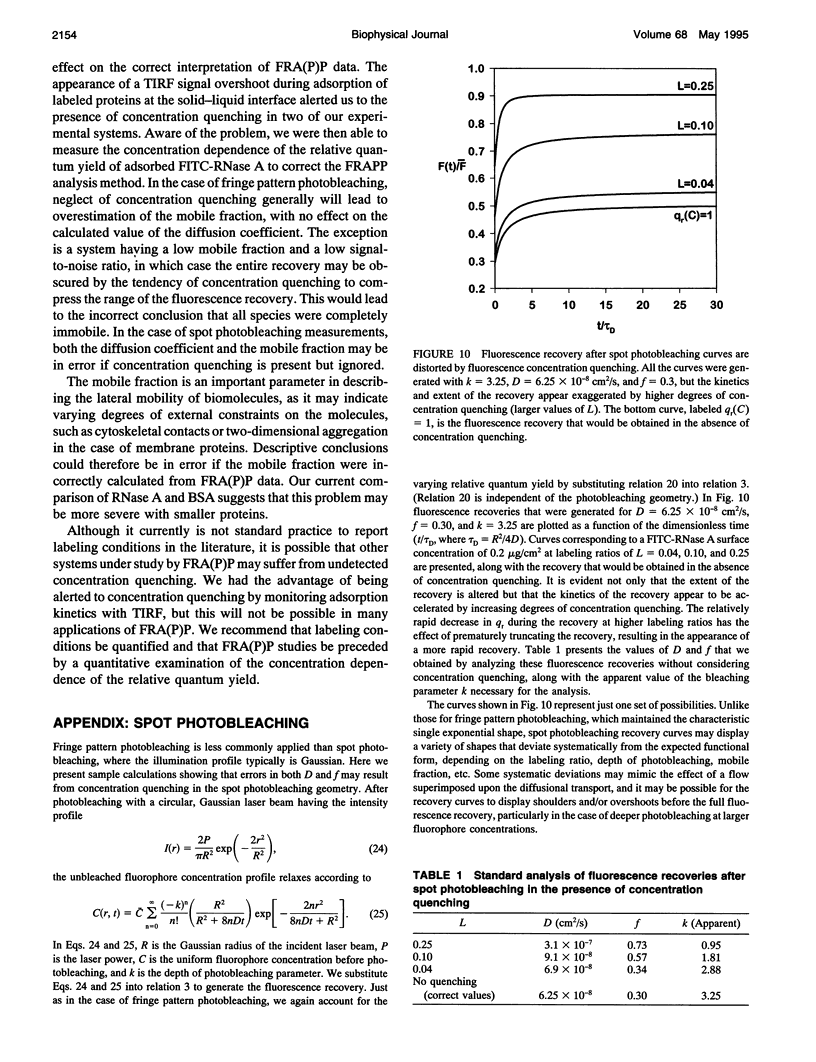
Abstract
Standard analysis of fluorescence recovery after photobleaching (FRAP) data is valid only if the quantum yield of unphotobleached fluorophores is independent of concentration, yet close molecular packing in two-dimensional systems may lead to significant fluorescence concentration quenching. Using total internal reflection fluorescence, we quantified the surface concentration dependence of the relative quantum yield of fluorescein isothiocyanate-labeled proteins adsorbed to polymeric surfaces before performing measurements of fluorescence recovery after pattern photobleaching. Adsorbed layers of FITC-labeled ribonuclease A displayed significant concentration quenching, and thus the standard FRAP analysis method was unacceptable. We present an extended FRAP analysis procedure that accounts for the changing quantum yield of diffusing fluorophores in systems that are influenced by concentration quenching. The extended analysis shows that if concentration quenching conditions prevail, there may be significant error in the transport parameters obtained from FRAP measurements by using the standard procedures.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abney J. R., Scalettar B. A., Thompson N. L. Evanescent interference patterns for fluorescence microscopy. Biophys J. 1992 Feb;61(2):542–552. doi: 10.1016/S0006-3495(92)81858-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axelrod D., Koppel D. E., Schlessinger J., Elson E., Webb W. W. Mobility measurement by analysis of fluorescence photobleaching recovery kinetics. Biophys J. 1976 Sep;16(9):1055–1069. doi: 10.1016/S0006-3495(76)85755-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burghardt T. P., Axelrod D. Total internal reflection/fluorescence photobleaching recovery study of serum albumin adsorption dynamics. Biophys J. 1981 Mar;33(3):455–467. doi: 10.1016/S0006-3495(81)84906-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elson E. L. Fluorescence photobleaching and correlation spectroscopy for translational diffusion in biological systems. Biochem Soc Trans. 1986 Oct;14(5):839–841. doi: 10.1042/bst0140839. [DOI] [PubMed] [Google Scholar]
- Fruchter R. G., Crestfield A. M. Preparation and properties of two active forms of ribonuclease dimer. J Biol Chem. 1965 Oct;240(10):3868–3874. [PubMed] [Google Scholar]
- Fung B. K., Stryer L. Surface density determination in membranes by fluorescence energy transfer. Biochemistry. 1978 Nov 28;17(24):5241–5248. doi: 10.1021/bi00617a025. [DOI] [PubMed] [Google Scholar]
- Golan D. E., Veatch W. Lateral mobility of band 3 in the human erythrocyte membrane studied by fluorescence photobleaching recovery: evidence for control by cytoskeletal interactions. Proc Natl Acad Sci U S A. 1980 May;77(5):2537–2541. doi: 10.1073/pnas.77.5.2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson K., Wojcieszyn J. The translational mobility of substances within the cytoplasmic matrix. Proc Natl Acad Sci U S A. 1984 Nov;81(21):6747–6751. doi: 10.1073/pnas.81.21.6747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koppel D. E. Fluorescence photobleaching recovery techniques for translational and slow rotational diffusion in solution and on cell surfaces. Biochem Soc Trans. 1986 Oct;14(5):842–845. doi: 10.1042/bst0140842. [DOI] [PubMed] [Google Scholar]
- Pachence J. M., Amador S., Maniara G., Vanderkooi J., Dutton P. L., Blasie J. K. Orientation and lateral mobility of cytochrome c on the surface of ultrathin lipid multilayer films. Biophys J. 1990 Aug;58(2):379–389. doi: 10.1016/S0006-3495(90)82384-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith L. M., Parce J. W., Smith B. A., McConnell H. M. Antibodies bound to lipid haptens in model membranes diffuse as rapidly as the lipids themselves. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4177–4179. doi: 10.1073/pnas.76.9.4177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder B., Freire E. Fluorescence energy transfer in two dimensions. A numeric solution for random and nonrandom distributions. Biophys J. 1982 Nov;40(2):137–148. doi: 10.1016/S0006-3495(82)84468-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stryer L. Fluorescence energy transfer as a spectroscopic ruler. Annu Rev Biochem. 1978;47:819–846. doi: 10.1146/annurev.bi.47.070178.004131. [DOI] [PubMed] [Google Scholar]
- Thompson N. L., Burghardt T. P., Axelrod D. Measuring surface dynamics of biomolecules by total internal reflection fluorescence with photobleaching recovery or correlation spectroscopy. Biophys J. 1981 Mar;33(3):435–454. doi: 10.1016/S0006-3495(81)84905-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilton R. D., Gast A. P., Robertson C. R. Surface diffusion of interacting proteins. Effect of concentration on the lateral mobility of adsorbed bovine serum albumin. Biophys J. 1990 Nov;58(5):1321–1326. doi: 10.1016/S0006-3495(90)82473-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weis R. M., Balakrishnan K., Smith B. A., McConnell H. M. Stimulation of fluorescence in a small contact region between rat basophil leukemia cells and planar lipid membrane targets by coherent evanescent radiation. J Biol Chem. 1982 Jun 10;257(11):6440–6445. [PubMed] [Google Scholar]
- Wolber P. K., Hudson B. S. An analytic solution to the Förster energy transfer problem in two dimensions. Biophys J. 1979 Nov;28(2):197–210. doi: 10.1016/S0006-3495(79)85171-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright L. L., Palmer A. G., 3rd, Thompson N. L. Inhomogeneous translational diffusion of monoclonal antibodies on phospholipid Langmuir-Blodgett films. Biophys J. 1988 Sep;54(3):463–470. doi: 10.1016/S0006-3495(88)82979-5. [DOI] [PMC free article] [PubMed] [Google Scholar]