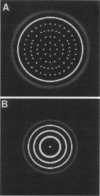
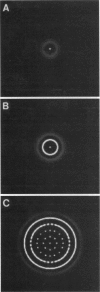
Abstract
We present experimental results on the bacterium Salmonella typhimurium which show that cells of chemotactic strains aggregate in response to gradients of amino acids, attractants that they themselves excrete. Depending on the conditions under which cells are cultured, they form periodic arrays of continuous or perforated rings, which arise sequentially within a spreading bacterial lawn. Based on these experiments, we develop a biologically realistic cell-chemotaxis model to describe the self-organization of bacteria. Numerical and analytical investigations of the model mechanism show how the two types of observed geometric patterns can be generated by the interaction of the cells with chemoattractant they produce.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adler J. Chemotaxis in bacteria. Science. 1966 Aug 12;153(3737):708–716. doi: 10.1126/science.153.3737.708. [DOI] [PubMed] [Google Scholar]
- Agladze K., Budriene L., Ivanitsky G., Krinsky V., Shakhbazyan V., Tsyganov M. Wave mechanisms of pattern formation in microbial populations. Proc Biol Sci. 1993 Aug 23;253(1337):131–135. doi: 10.1098/rspb.1993.0092. [DOI] [PubMed] [Google Scholar]
- Alt W., Lauffenburger D. A. Transient behavior of a chemotaxis system modelling certain types of tissue inflammation. J Math Biol. 1987;24(6):691–722. doi: 10.1007/BF00275511. [DOI] [PubMed] [Google Scholar]
- Berg H. C., Turner L. Chemotaxis of bacteria in glass capillary arrays. Escherichia coli, motility, microchannel plate, and light scattering. Biophys J. 1990 Oct;58(4):919–930. doi: 10.1016/S0006-3495(90)82436-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown D. A., Berg H. C. Temporal stimulation of chemotaxis in Escherichia coli. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1388–1392. doi: 10.1073/pnas.71.4.1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budrene E. O., Berg H. C. Complex patterns formed by motile cells of Escherichia coli. Nature. 1991 Feb 14;349(6310):630–633. doi: 10.1038/349630a0. [DOI] [PubMed] [Google Scholar]
- Keller E. F., Segel L. A. Initiation of slime mold aggregation viewed as an instability. J Theor Biol. 1970 Mar;26(3):399–415. doi: 10.1016/0022-5193(70)90092-5. [DOI] [PubMed] [Google Scholar]
- Keller E. F., Segel L. A. Traveling bands of chemotactic bacteria: a theoretical analysis. J Theor Biol. 1971 Feb;30(2):235–248. doi: 10.1016/0022-5193(71)90051-8. [DOI] [PubMed] [Google Scholar]
- Kennedy C. R., Aris R. Traveling waves in a simple population model involving growth and death. Bull Math Biol. 1980;42(3):397–429. doi: 10.1007/BF02460793. [DOI] [PubMed] [Google Scholar]
- Lapidus I. R., Schiller R. Model for the chemotactic response of a bacterial population. Biophys J. 1976 Jul;16(7):779–789. doi: 10.1016/S0006-3495(76)85728-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauffenburger D. A., Kennedy C. R. Localized bacterial infection in a distributed model for tissue inflammation. J Math Biol. 1983;16(2):141–163. doi: 10.1007/BF00276054. [DOI] [PubMed] [Google Scholar]
- Murray J. D., Deeming D. C., Ferguson M. W. Size-dependent pigmentation-pattern formation in embryos of Alligator mississippiensis: time of initiation of pattern generation mechanism. Proc R Soc Lond B Biol Sci. 1990 Apr 23;239(1296):279–293. doi: 10.1098/rspb.1990.0017. [DOI] [PubMed] [Google Scholar]
- Murray J. D., Myerscough M. R. Pigmentation pattern formation on snakes. J Theor Biol. 1991 Apr 7;149(3):339–360. doi: 10.1016/s0022-5193(05)80310-8. [DOI] [PubMed] [Google Scholar]
- Murray J. D. Parameter space for turing instability in reaction diffusion mechanisms: a comparison of models. J Theor Biol. 1982 Sep 7;98(1):143–163. doi: 10.1016/0022-5193(82)90063-7. [DOI] [PubMed] [Google Scholar]
- Nanjundiah V. Chemotaxis, signal relaying and aggregation morphology. J Theor Biol. 1973 Nov 5;42(1):63–105. doi: 10.1016/0022-5193(73)90149-5. [DOI] [PubMed] [Google Scholar]
- Nossal R. Growth and movement of rings of chemotactic bacteria. Exp Cell Res. 1972 Nov;75(1):138–142. doi: 10.1016/0014-4827(72)90529-0. [DOI] [PubMed] [Google Scholar]
- Oster G. F., Murray J. D. Pattern formation models and developmental constraints. J Exp Zool. 1989 Aug;251(2):186–202. doi: 10.1002/jez.1402510207. [DOI] [PubMed] [Google Scholar]
- Weis R. M., Koshland D. E., Jr Reversible receptor methylation is essential for normal chemotaxis of Escherichia coli in gradients of aspartic acid. Proc Natl Acad Sci U S A. 1988 Jan;85(1):83–87. doi: 10.1073/pnas.85.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfe A. J., Berg H. C. Migration of bacteria in semisolid agar. Proc Natl Acad Sci U S A. 1989 Sep;86(18):6973–6977. doi: 10.1073/pnas.86.18.6973. [DOI] [PMC free article] [PubMed] [Google Scholar]