Abstract
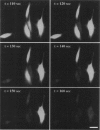
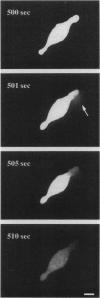
We have developed a video microscopy system designed for real-time measurement of single cell damage during photolysis under well defined physicochemical and photophysical conditions. Melanoma cells cultured in vitro were treated with the photosensitizer (PS), tin chlorin e6 (SnCe6) or immunoconjugate (SnCe6 conjugated to a anti-ICAM monoclonal antibody), and illuminated with a 10 mW He/Ne laser at a 630 nm wavelength. Cell membrane integrity was assessed using the vital dye calcein-AM. In experiments in which the laser power density and PS concentration were varied, it was determined that the time lag before cell rupture was inversely proportional to the estimated singlet oxygen flux to the cell surface. Microscopic examination of the lytic event indicated that photo-induced lysis was caused by a point rupture of the plasma membrane. The on-line nature of this microscopy system offers an opportunity to monitor the dynamics of the cell damage process and to gain insights into the mechanism governing photolytic cell injury processes.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Canaday D., Li P., Weichselbaum R., Astumian R. D., Lee R. C. Membrane permeability changes in gamma-irradiated muscle cells. Ann N Y Acad Sci. 1994 May 31;720:153–159. doi: 10.1111/j.1749-6632.1994.tb30443.x. [DOI] [PubMed] [Google Scholar]
- Comporti M. Three models of free radical-induced cell injury. Chem Biol Interact. 1989;72(1-2):1–56. doi: 10.1016/0009-2797(89)90016-1. [DOI] [PubMed] [Google Scholar]
- Deziel M. R., Girotti A. W. Lysis of resealed erythrocyte ghosts by photoactivated tetrapyrroles: estimation of photolesion dimensions. Int J Biochem. 1982;14(4):263–266. doi: 10.1016/0020-711x(82)90086-6. [DOI] [PubMed] [Google Scholar]
- Dougherty T. J. Photodynamic therapy. Photochem Photobiol. 1993 Dec;58(6):895–900. doi: 10.1111/j.1751-1097.1993.tb04990.x. [DOI] [PubMed] [Google Scholar]
- Fingar V. H., Wieman T. J., Wiehle S. A., Cerrito P. B. The role of microvascular damage in photodynamic therapy: the effect of treatment on vessel constriction, permeability, and leukocyte adhesion. Cancer Res. 1992 Sep 15;52(18):4914–4921. [PubMed] [Google Scholar]
- Girotti A. W., Deziel M. R. Photodynamic action of protoporphyrin on resealed erythrocyte membranes: mechanisms of release of trapped markers. Adv Exp Med Biol. 1983;160:213–225. doi: 10.1007/978-1-4684-4406-3_19. [DOI] [PubMed] [Google Scholar]
- Girotti A. W. Photodynamic lipid peroxidation in biological systems. Photochem Photobiol. 1990 Apr;51(4):497–509. doi: 10.1111/j.1751-1097.1990.tb01744.x. [DOI] [PubMed] [Google Scholar]
- Girotti A. W. Protoporphyrin-sensitized photodamage in isolated membranes of human erythrocytes. Biochemistry. 1979 Oct 2;18(20):4403–4411. doi: 10.1021/bi00587a021. [DOI] [PubMed] [Google Scholar]
- Gomer C. J. Preclinical examination of first and second generation photosensitizers used in photodynamic therapy. Photochem Photobiol. 1991 Dec;54(6):1093–1107. doi: 10.1111/j.1751-1097.1991.tb02133.x. [DOI] [PubMed] [Google Scholar]
- Hartman P. E., Dixon W. J., Dahl T. A., Daub M. E. Multiple modes of photodynamic action by cercosporin. Photochem Photobiol. 1988 May;47(5):699–703. doi: 10.1111/j.1751-1097.1988.tb02767.x. [DOI] [PubMed] [Google Scholar]
- Jiang F. N., Liu D. J., Neyndorff H., Chester M., Jiang S. Y., Levy J. G. Photodynamic killing of human squamous cell carcinoma cells using a monoclonal antibody-photosensitizer conjugate. J Natl Cancer Inst. 1991 Sep 4;83(17):1218–1225. doi: 10.1093/jnci/83.17.1218. [DOI] [PubMed] [Google Scholar]
- Lu X. M., Fischman A. J., Stevens E., Lee T. T., Strong L., Tompkins R. G., Yarmush M. L. Sn-chlorin e6 antibacterial immunoconjugates. An in vitro and in vivo analysis. J Immunol Methods. 1992 Nov 25;156(1):85–99. doi: 10.1016/0022-1759(92)90014-k. [DOI] [PubMed] [Google Scholar]
- Matthews W., Cook J., Mitchell J. B., Perry R. R., Evans S., Pass H. I. In vitro photodynamic therapy of human lung cancer: investigation of dose-rate effects. Cancer Res. 1989 Apr 1;49(7):1718–1721. [PubMed] [Google Scholar]
- Oseroff A. R., Ohuoha D., Hasan T., Bommer J. C., Yarmush M. L. Antibody-targeted photolysis: selective photodestruction of human T-cell leukemia cells using monoclonal antibody-chlorin e6 conjugates. Proc Natl Acad Sci U S A. 1986 Nov;83(22):8744–8748. doi: 10.1073/pnas.83.22.8744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter W. R., Mang T. S., Dougherty T. J. The theory of photodynamic therapy dosimetry: consequences of photo-destruction of sensitizer. Photochem Photobiol. 1987 Jul;46(1):97–101. doi: 10.1111/j.1751-1097.1987.tb04741.x. [DOI] [PubMed] [Google Scholar]
- Rakestraw S. L., Ford W. E., Tompkins R. G., Rodgers M. A., Thorpe W. P., Yarmush M. L. Antibody-targeted photolysis: in vitro immunological, photophysical, and cytotoxic properties of monoclonal antibody-dextran-Sn(IV) chlorin e6 immunoconjugates. Biotechnol Prog. 1992 Jan-Feb;8(1):30–39. doi: 10.1021/bp00013a006. [DOI] [PubMed] [Google Scholar]
- von Asmuth E. J., Smeets E. F., Ginsel L. A., Onderwater J. J., Leeuwenberg J. F., Buurman W. A. Evidence for endocytosis of E-selectin in human endothelial cells. Eur J Immunol. 1992 Oct;22(10):2519–2526. doi: 10.1002/eji.1830221009. [DOI] [PubMed] [Google Scholar]