Abstract
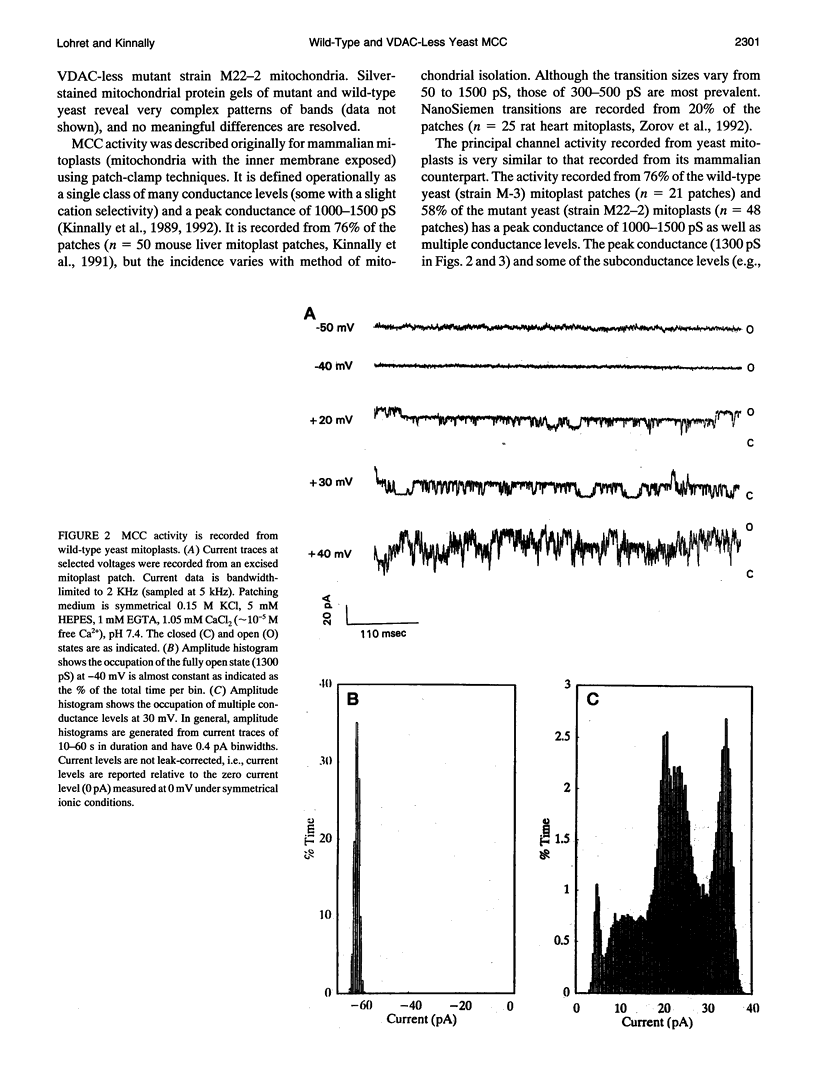
Yeast mitoplasts (mitochondria with the outer membrane stripped away) exhibit multiple conductance channel activity (MCC) in patch-clamp experiments that is very similar to the activity previously described in mammalian mitoplasts. The possible involvement of the voltage-dependent anion-selective channel (VDAC) of the outer membrane in MCC activity was explored by comparing the channel activity in wild-type yeast mitoplasts with that of a VDAC-deletion mutant. The channel activity recorded from the mutant is essentially the same as that of the wild-type in the voltage range of -40 to 30 mV. These observations indicate that VDAC is not required for MCC activity. Interestingly, the channel activity of the VDAC-less yeast mitoplasts exhibits altered gating properties at transmembrane potentials above and below this range. We conclude that the deletion of VDAC somehow results in a modification of MCC's voltage dependence. In fact, the voltage profile recorded from the VDAC-less mutant resembles that of VDAC.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Antonenko Y. N., Kinnally K. W., Tedeschi H. Identification of anion and cation pathways in the inner mitochondrial membrane by patch clamping of mouse liver mitoplasts. J Membr Biol. 1991 Nov;124(2):151–158. doi: 10.1007/BF01870459. [DOI] [PubMed] [Google Scholar]
- Antonenko Y. N., Smith D., Kinnally K. W., Tedeschi H. Single-channel activity induced in mitoplasts by alkaline pH. Biochim Biophys Acta. 1994 Sep 14;1194(2):247–254. doi: 10.1016/0005-2736(94)90306-9. [DOI] [PubMed] [Google Scholar]
- Blachly-Dyson E., Peng S., Colombini M., Forte M. Selectivity changes in site-directed mutants of the VDAC ion channel: structural implications. Science. 1990 Mar 9;247(4947):1233–1236. doi: 10.1126/science.1690454. [DOI] [PubMed] [Google Scholar]
- Campo M. L., Kinnally K. W., Tedeschi H. The effect of antimycin A on mouse liver inner mitochondrial membrane channel activity. J Biol Chem. 1992 Apr 25;267(12):8123–8127. [PubMed] [Google Scholar]
- Costa G., Kinnally K. W., Diwan J. J. Patch clamp analysis of a partially purified ion channel from rat liver mitochondria. Biochem Biophys Res Commun. 1991 Feb 28;175(1):305–310. doi: 10.1016/s0006-291x(05)81235-5. [DOI] [PubMed] [Google Scholar]
- Criado M., Keller B. U. A membrane fusion strategy for single-channel recordings of membranes usually non-accessible to patch-clamp pipette electrodes. FEBS Lett. 1987 Nov 16;224(1):172–176. doi: 10.1016/0014-5793(87)80442-8. [DOI] [PubMed] [Google Scholar]
- Daum G., Böhni P. C., Schatz G. Import of proteins into mitochondria. Cytochrome b2 and cytochrome c peroxidase are located in the intermembrane space of yeast mitochondria. J Biol Chem. 1982 Nov 10;257(21):13028–13033. [PubMed] [Google Scholar]
- De Pinto V., Ludwig O., Krause J., Benz R., Palmieri F. Porin pores of mitochondrial outer membranes from high and low eukaryotic cells: biochemical and biophysical characterization. Biochim Biophys Acta. 1987 Nov 19;894(2):109–119. doi: 10.1016/0005-2728(87)90180-0. [DOI] [PubMed] [Google Scholar]
- Decker G. L., Greenawalt J. W. Ultrastructural and biochemical studies of mitoplasts and outer membranes derived from French-pressed mitochondria. Advances in mitochondrial subfractionation. J Ultrastruct Res. 1977 Apr;59(1):44–56. doi: 10.1016/s0022-5320(77)80027-0. [DOI] [PubMed] [Google Scholar]
- Fèvre F., Chich J. F., Lauquin G. J., Henry J. P., Thieffry M. Comparison of mitochondrial cationic channels in wild-type and porin-deficient mutant yeast. FEBS Lett. 1990 Mar 26;262(2):201–204. doi: 10.1016/0014-5793(90)80189-p. [DOI] [PubMed] [Google Scholar]
- Gunter T. E., Pfeiffer D. R. Mechanisms by which mitochondria transport calcium. Am J Physiol. 1990 May;258(5 Pt 1):C755–C786. doi: 10.1152/ajpcell.1990.258.5.C755. [DOI] [PubMed] [Google Scholar]
- Kinnally K. W., Antonenko Y. N., Zorov D. B. Modulation of inner mitochondrial membrane channel activity. J Bioenerg Biomembr. 1992 Feb;24(1):99–110. doi: 10.1007/BF00769536. [DOI] [PubMed] [Google Scholar]
- Kinnally K. W., Campo M. L., Tedeschi H. Mitochondrial channel activity studied by patch-clamping mitoplasts. J Bioenerg Biomembr. 1989 Aug;21(4):497–506. doi: 10.1007/BF00762521. [DOI] [PubMed] [Google Scholar]
- Kinnally K. W., Zorov D. B., Antonenko Y. N., Snyder S. H., McEnery M. W., Tedeschi H. Mitochondrial benzodiazepine receptor linked to inner membrane ion channels by nanomolar actions of ligands. Proc Natl Acad Sci U S A. 1993 Feb 15;90(4):1374–1378. doi: 10.1073/pnas.90.4.1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinnally K. W., Zorov D., Antonenko Y., Perini S. Calcium modulation of mitochondrial inner membrane channel activity. Biochem Biophys Res Commun. 1991 May 15;176(3):1183–1188. doi: 10.1016/0006-291x(91)90410-9. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Mannella C. A. Structure of the outer mitochondrial membrane: ordered arrays of porelike subunits in outer-membrane fractions from Neurospora crassa mitochondria. J Cell Biol. 1982 Sep;94(3):680–687. doi: 10.1083/jcb.94.3.680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEnery M. W., Buhle E. L., Jr, Aebi U., Pedersen P. L. Proton ATPase of rat liver mitochondria. Preparation and visualization of a functional complex using the novel zwitterionic detergent 3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonate. J Biol Chem. 1984 Apr 10;259(7):4642–4651. [PubMed] [Google Scholar]
- McEnery M. W., Snowman A. M., Trifiletti R. R., Snyder S. H. Isolation of the mitochondrial benzodiazepine receptor: association with the voltage-dependent anion channel and the adenine nucleotide carrier. Proc Natl Acad Sci U S A. 1992 Apr 15;89(8):3170–3174. doi: 10.1073/pnas.89.8.3170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petronilli V., Szabò I., Zoratti M. The inner mitochondrial membrane contains ion-conducting channels similar to those found in bacteria. FEBS Lett. 1989 Dec 18;259(1):137–143. doi: 10.1016/0014-5793(89)81513-3. [DOI] [PubMed] [Google Scholar]
- Sorgato M. C., Keller B. U., Stühmer W. Patch-clamping of the inner mitochondrial membrane reveals a voltage-dependent ion channel. Nature. 1987 Dec 3;330(6147):498–500. doi: 10.1038/330498a0. [DOI] [PubMed] [Google Scholar]
- Szabó I., De Pinto V., Zoratti M. The mitochondrial permeability transition pore may comprise VDAC molecules. II. The electrophysiological properties of VDAC are compatible with those of the mitochondrial megachannel. FEBS Lett. 1993 Sep 13;330(2):206–210. doi: 10.1016/0014-5793(93)80274-x. [DOI] [PubMed] [Google Scholar]
- Szabó I., Zoratti M. The mitochondrial megachannel is the permeability transition pore. J Bioenerg Biomembr. 1992 Feb;24(1):111–117. doi: 10.1007/BF00769537. [DOI] [PubMed] [Google Scholar]
- Szabó I., Zoratti M. The mitochondrial permeability transition pore may comprise VDAC molecules. I. Binary structure and voltage dependence of the pore. FEBS Lett. 1993 Sep 13;330(2):201–205. doi: 10.1016/0014-5793(93)80273-w. [DOI] [PubMed] [Google Scholar]
- Thieffry M., Chich J. F., Goldschmidt D., Henry J. P. Incorporation in lipid bilayers of a large conductance cationic channel from mitochondrial membranes. EMBO J. 1988 May;7(5):1449–1454. doi: 10.1002/j.1460-2075.1988.tb02962.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorov D. B., Kinnally K. W., Perini S., Tedeschi H. Multiple conductance levels in rat heart inner mitochondrial membranes studied by patch clamping. Biochim Biophys Acta. 1992 Apr 13;1105(2):263–270. doi: 10.1016/0005-2736(92)90203-x. [DOI] [PubMed] [Google Scholar]