Abstract
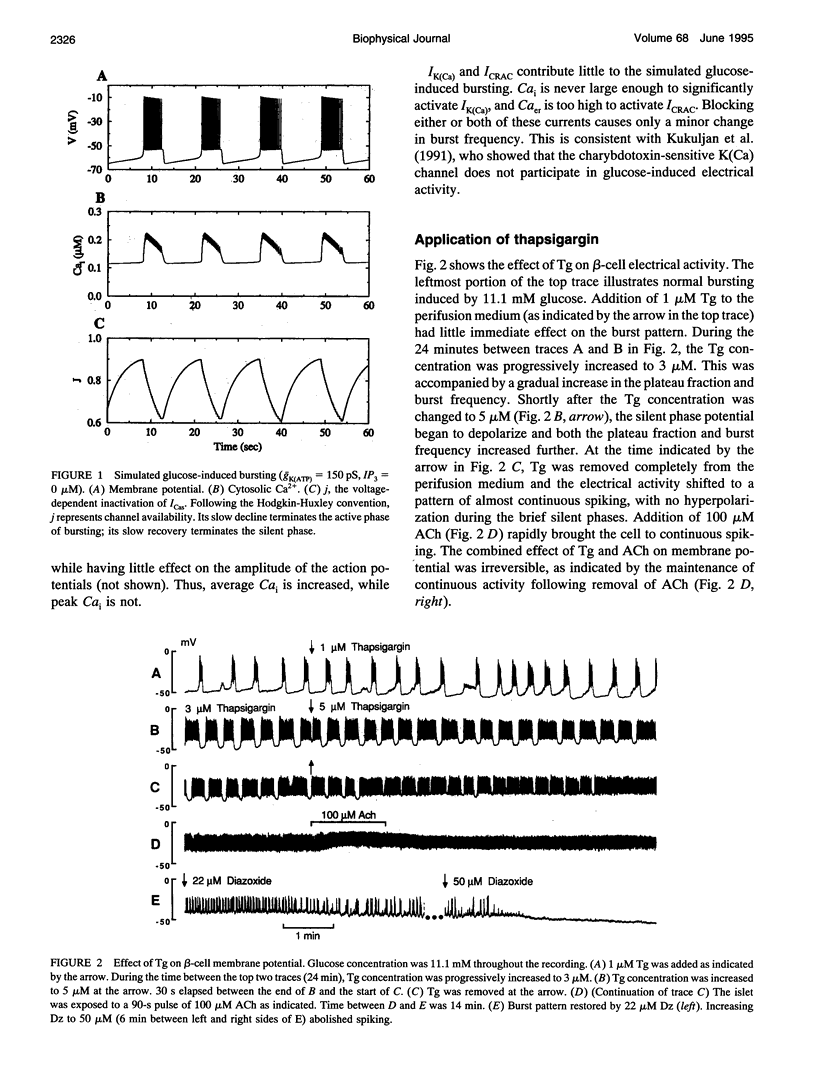
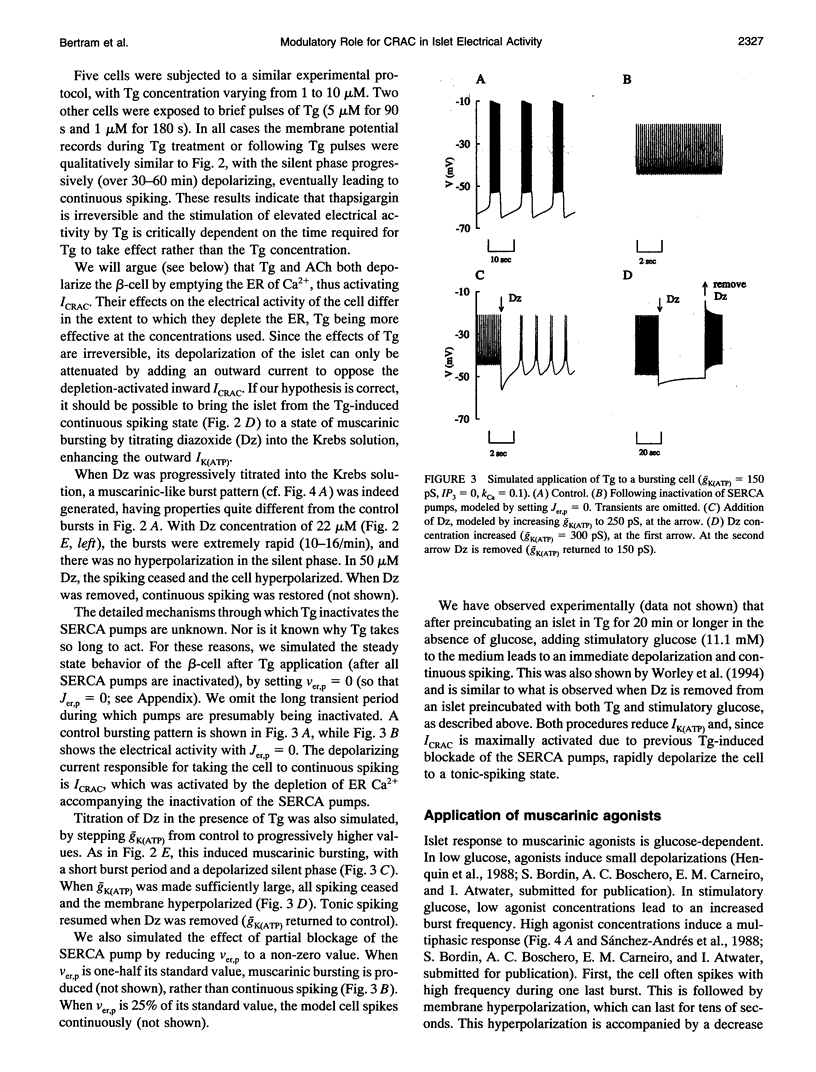
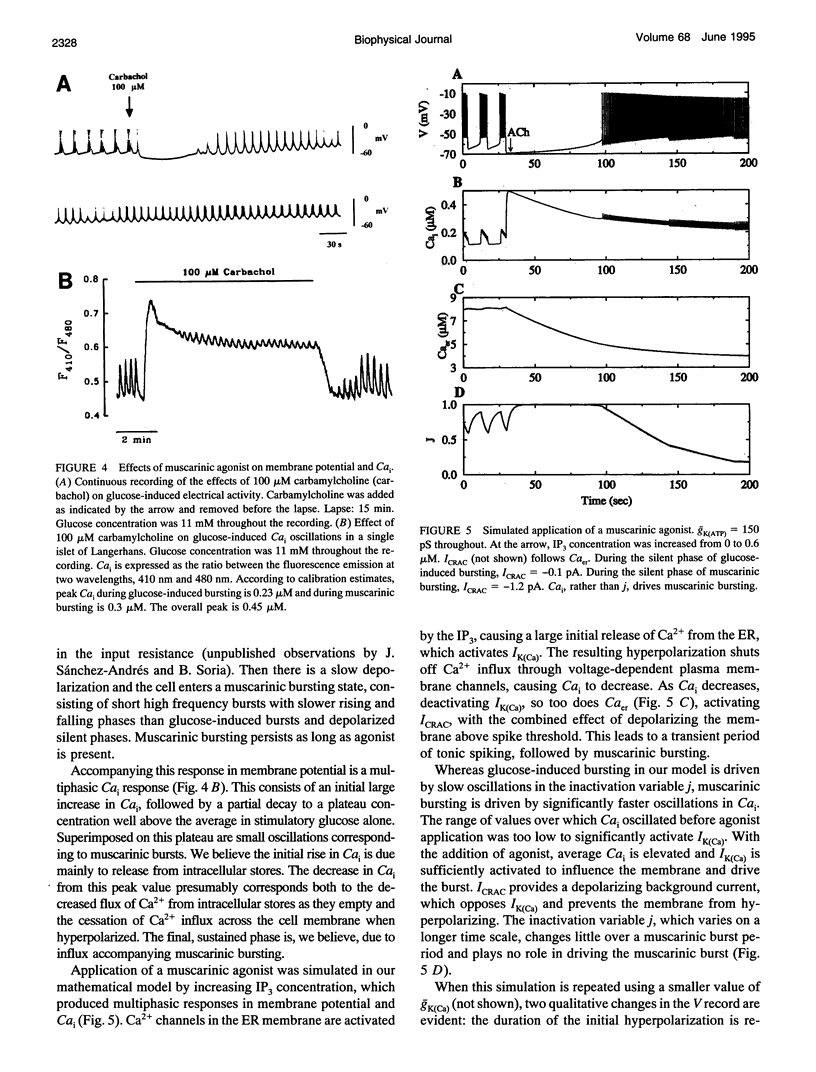
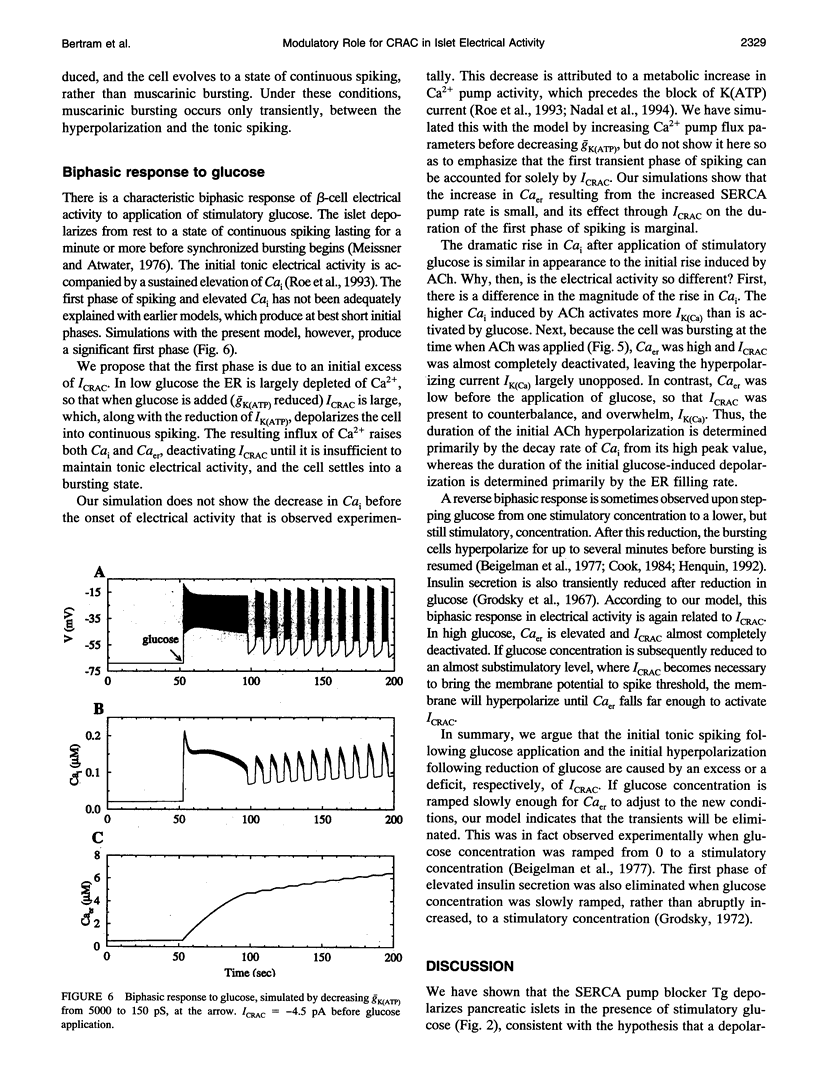
S. Bordin and colleagues have proposed that the depolarizing effects of acetylcholine and other muscarinic agonists on pancreatic beta-cells are mediated by a calcium release-activated current (CRAC). We support this hypothesis with additional data, and present a theoretical model which accounts for most known data on muscarinic effects. Additional phenomena, such as the biphasic responses of beta-cells to changes in glucose concentration and the depolarizing effects of the sarco-endoplasmic reticulum calcium ATPase pump poison thapsigargin, are also accounted for by our model. The ability of this single hypothesis, that CRAC is present in beta-cells, to explain so many phenomena motivates a more complete characterization of this current.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ammälä C., Bokvist K., Larsson O., Berggren P. O., Rorsman P. Demonstration of a novel apamin-insensitive calcium-activated K+ channel in mouse pancreatic B cells. Pflugers Arch. 1993 Feb;422(5):443–448. doi: 10.1007/BF00375069. [DOI] [PubMed] [Google Scholar]
- Ammälä C., Larsson O., Berggren P. O., Bokvist K., Juntti-Berggren L., Kindmark H., Rorsman P. Inositol trisphosphate-dependent periodic activation of a Ca(2+)-activated K+ conductance in glucose-stimulated pancreatic beta-cells. Nature. 1991 Oct 31;353(6347):849–852. doi: 10.1038/353849a0. [DOI] [PubMed] [Google Scholar]
- Ashcroft F. M., Rorsman P. Electrophysiology of the pancreatic beta-cell. Prog Biophys Mol Biol. 1989;54(2):87–143. doi: 10.1016/0079-6107(89)90013-8. [DOI] [PubMed] [Google Scholar]
- Atwater I., Ribalet B., Rojas E. Cyclic changes in potential and resistance of the beta-cell membrane induced by glucose in islets of Langerhans from mouse. J Physiol. 1978 May;278:117–139. doi: 10.1113/jphysiol.1978.sp012296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahnson T. D., Pandol S. J., Dionne V. E. Cyclic GMP modulates depletion-activated Ca2+ entry in pancreatic acinar cells. J Biol Chem. 1993 May 25;268(15):10808–10812. [PubMed] [Google Scholar]
- Beigelman P. M., Ribalet B., Atwater I. Electric activity of mouse pancreatic beta-cells. II. Effects of glucose and arginine. J Physiol (Paris) 1977 Jul;73(2):201–217. [PubMed] [Google Scholar]
- Berridge M. J., Irvine R. F. Inositol phosphates and cell signalling. Nature. 1989 Sep 21;341(6239):197–205. doi: 10.1038/341197a0. [DOI] [PubMed] [Google Scholar]
- Bozem M., Nenquin M., Henquin J. C. The ionic, electrical, and secretory effects of protein kinase C activation in mouse pancreatic B-cells: studies with a phorbol ester. Endocrinology. 1987 Sep;121(3):1025–1033. doi: 10.1210/endo-121-3-1025. [DOI] [PubMed] [Google Scholar]
- Cook D. L., Crill W. E., Porte D., Jr Glucose and acetylcholine have different effects on the plateau pacemaker of pancreatic islet cells. Diabetes. 1981 Jul;30(7):558–561. doi: 10.2337/diab.30.7.558. [DOI] [PubMed] [Google Scholar]
- Cook D. L. Electrical pacemaker mechanisms of pancreatic islet cells. Fed Proc. 1984 Jun;43(9):2368–2372. [PubMed] [Google Scholar]
- Cook D. L., Ikeuchi M., Fujimoto W. Y. Lowering of pHi inhibits Ca2+-activated K+ channels in pancreatic B-cells. Nature. 1984 Sep 20;311(5983):269–271. doi: 10.1038/311269a0. [DOI] [PubMed] [Google Scholar]
- Gilon P., Shepherd R. M., Henquin J. C. Oscillations of secretion driven by oscillations of cytoplasmic Ca2+ as evidences in single pancreatic islets. J Biol Chem. 1993 Oct 25;268(30):22265–22268. [PubMed] [Google Scholar]
- Grodsky G. M. A threshold distribution hypothesis for packet storage of insulin and its mathematical modeling. J Clin Invest. 1972 Aug;51(8):2047–2059. doi: 10.1172/JCI107011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Henquin J. C. Adenosine triphosphate-sensitive K+ channels may not be the sole regulators of glucose-induced electrical activity in pancreatic B-cells. Endocrinology. 1992 Jul;131(1):127–131. doi: 10.1210/endo.131.1.1611991. [DOI] [PubMed] [Google Scholar]
- Henquin J. C., Garcia M. C., Bozem M., Hermans M. P., Nenquin M. Muscarinic control of pancreatic B cell function involves sodium-dependent depolarization and calcium influx. Endocrinology. 1988 May;122(5):2134–2142. doi: 10.1210/endo-122-5-2134. [DOI] [PubMed] [Google Scholar]
- Hoth M., Penner R. Depletion of intracellular calcium stores activates a calcium current in mast cells. Nature. 1992 Jan 23;355(6358):353–356. doi: 10.1038/355353a0. [DOI] [PubMed] [Google Scholar]
- Jones P. M., Stutchfield J., Howell S. L. Effects of Ca2+ and a phorbol ester on insulin secretion from islets of Langerhans permeabilised by high-voltage discharge. FEBS Lett. 1985 Oct 21;191(1):102–106. doi: 10.1016/0014-5793(85)81002-4. [DOI] [PubMed] [Google Scholar]
- Keizer J., De Young G. Effect of voltage-gated plasma membrane Ca2+ fluxes on IP3-linked Ca2+ oscillations. Cell Calcium. 1993 May;14(5):397–410. doi: 10.1016/0143-4160(93)90044-7. [DOI] [PubMed] [Google Scholar]
- Kukuljan M., Goncalves A. A., Atwater I. Charybdotoxin-sensitive K(Ca) channel is not involved in glucose-induced electrical activity in pancreatic beta-cells. J Membr Biol. 1991 Jan;119(2):187–195. doi: 10.1007/BF01871418. [DOI] [PubMed] [Google Scholar]
- Leech C. A., Holz G. G., 4th, Habener J. F. Voltage-independent calcium channels mediate slow oscillations of cytosolic calcium that are glucose dependent in pancreatic beta-cells. Endocrinology. 1994 Jul;135(1):365–372. doi: 10.1210/endo.135.1.8013370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lernmark A. The preparation of, and studies on, free cell suspensions from mouse pancreatic islets. Diabetologia. 1974 Oct;10(5):431–438. doi: 10.1007/BF01221634. [DOI] [PubMed] [Google Scholar]
- Li Y. X., Rinzel J. Equations for InsP3 receptor-mediated [Ca2+]i oscillations derived from a detailed kinetic model: a Hodgkin-Huxley like formalism. J Theor Biol. 1994 Feb 21;166(4):461–473. doi: 10.1006/jtbi.1994.1041. [DOI] [PubMed] [Google Scholar]
- Meissner H. P., Atwater I. J. The kinetics of electrical activity of beta cells in response to a "square wave" stimulation with glucose or glibenclamide. Horm Metab Res. 1976 Jan;8(1):11–16. doi: 10.1055/s-0028-1093685. [DOI] [PubMed] [Google Scholar]
- Mertz L. M., Baum B. J., Ambudkar I. S. Refill status of the agonist-sensitive Ca2+ pool regulates Mn2+ influx into parotid acini. J Biol Chem. 1990 Sep 5;265(25):15010–15014. [PubMed] [Google Scholar]
- Nadal A., Valdeolmillos M., Soria B. Metabolic regulation of intracellular calcium concentration in mouse pancreatic islets of Langerhans. Am J Physiol. 1994 Nov;267(5 Pt 1):E769–E774. doi: 10.1152/ajpendo.1994.267.5.E769. [DOI] [PubMed] [Google Scholar]
- Parekh A. B., Terlau H., Stühmer W. Depletion of InsP3 stores activates a Ca2+ and K+ current by means of a phosphatase and a diffusible messenger. Nature. 1993 Aug 26;364(6440):814–818. doi: 10.1038/364814a0. [DOI] [PubMed] [Google Scholar]
- Putney J. W., Jr Capacitative calcium entry revisited. Cell Calcium. 1990 Nov-Dec;11(10):611–624. doi: 10.1016/0143-4160(90)90016-n. [DOI] [PubMed] [Google Scholar]
- Randriamampita C., Tsien R. Y. Emptying of intracellular Ca2+ stores releases a novel small messenger that stimulates Ca2+ influx. Nature. 1993 Aug 26;364(6440):809–814. doi: 10.1038/364809a0. [DOI] [PubMed] [Google Scholar]
- Roe M. W., Lancaster M. E., Mertz R. J., Worley J. F., 3rd, Dukes I. D. Voltage-dependent intracellular calcium release from mouse islets stimulated by glucose. J Biol Chem. 1993 May 15;268(14):9953–9956. [PubMed] [Google Scholar]
- Rojas E., Carroll P. B., Ricordi C., Boschero A. C., Stojilkovic S. S., Atwater I. Control of cytosolic free calcium in cultured human pancreatic beta-cells occurs by external calcium-dependent and independent mechanisms. Endocrinology. 1994 Apr;134(4):1771–1781. doi: 10.1210/endo.134.4.8137742. [DOI] [PubMed] [Google Scholar]
- Santos R. M., Rojas E. Muscarinic receptor modulation of glucose-induced electrical activity in mouse pancreatic B-cells. FEBS Lett. 1989 Jun 5;249(2):411–417. doi: 10.1016/0014-5793(89)80669-6. [DOI] [PubMed] [Google Scholar]
- Santos R. M., Rosario L. M., Nadal A., Garcia-Sancho J., Soria B., Valdeolmillos M. Widespread synchronous [Ca2+]i oscillations due to bursting electrical activity in single pancreatic islets. Pflugers Arch. 1991 May;418(4):417–422. doi: 10.1007/BF00550880. [DOI] [PubMed] [Google Scholar]
- Satin L. S., Cook D. L. Evidence for two calcium currents in insulin-secreting cells. Pflugers Arch. 1988 Apr;411(4):401–409. doi: 10.1007/BF00587719. [DOI] [PubMed] [Google Scholar]
- Silva A. M., Rosário L. M., Santos R. M. Background Ca2+ influx mediated by a dihydropyridine- and voltage-insensitive channel in pancreatic beta-cells. Modulation by Ni2+, diphenylamine-2-carboxylate, and glucose metabolism. J Biol Chem. 1994 Jun 24;269(25):17095–17103. [PubMed] [Google Scholar]
- Smolen P., Keizer J. Slow voltage inactivation of Ca2+ currents and bursting mechanisms for the mouse pancreatic beta-cell. J Membr Biol. 1992 Apr;127(1):9–19. doi: 10.1007/BF00232754. [DOI] [PubMed] [Google Scholar]
- Sánchez-Andrés J. V., Ripoll C., Soria B. Evidence that muscarinic potentiation of insulin release is initiated by an early transient calcium entry. FEBS Lett. 1988 Apr 11;231(1):143–147. doi: 10.1016/0014-5793(88)80719-1. [DOI] [PubMed] [Google Scholar]
- Takemura H., Hughes A. R., Thastrup O., Putney J. W., Jr Activation of calcium entry by the tumor promoter thapsigargin in parotid acinar cells. Evidence that an intracellular calcium pool and not an inositol phosphate regulates calcium fluxes at the plasma membrane. J Biol Chem. 1989 Jul 25;264(21):12266–12271. [PubMed] [Google Scholar]
- Valdeolmillos M., Santos R. M., Contreras D., Soria B., Rosario L. M. Glucose-induced oscillations of intracellular Ca2+ concentration resembling bursting electrical activity in single mouse islets of Langerhans. FEBS Lett. 1989 Dec 18;259(1):19–23. doi: 10.1016/0014-5793(89)81484-x. [DOI] [PubMed] [Google Scholar]
- Woods S. C., Porte D., Jr Neural control of the endocrine pancreas. Physiol Rev. 1974 Jul;54(3):596–619. doi: 10.1152/physrev.1974.54.3.596. [DOI] [PubMed] [Google Scholar]
- Worley J. F., 3rd, McIntyre M. S., Spencer B., Mertz R. J., Roe M. W., Dukes I. D. Endoplasmic reticulum calcium store regulates membrane potential in mouse islet beta-cells. J Biol Chem. 1994 May 20;269(20):14359–14362. [PubMed] [Google Scholar]
- Zweifach A., Lewis R. S. Mitogen-regulated Ca2+ current of T lymphocytes is activated by depletion of intracellular Ca2+ stores. Proc Natl Acad Sci U S A. 1993 Jul 1;90(13):6295–6299. doi: 10.1073/pnas.90.13.6295. [DOI] [PMC free article] [PubMed] [Google Scholar]



