Abstract
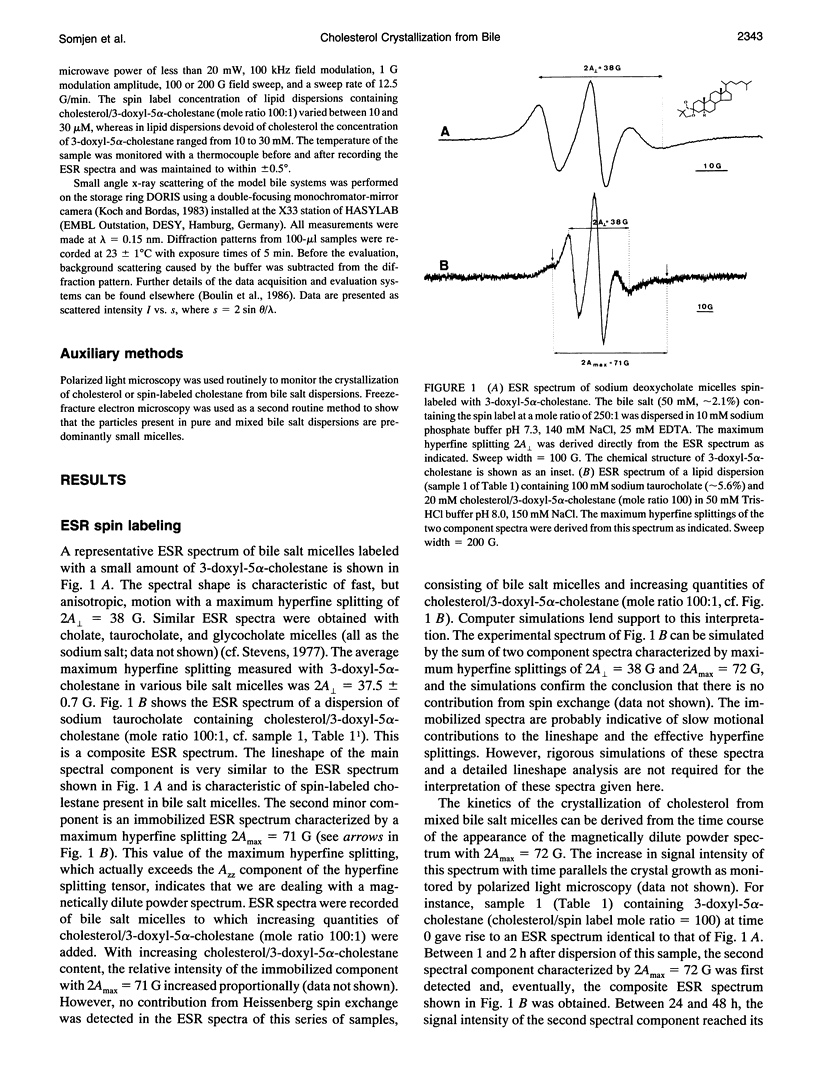
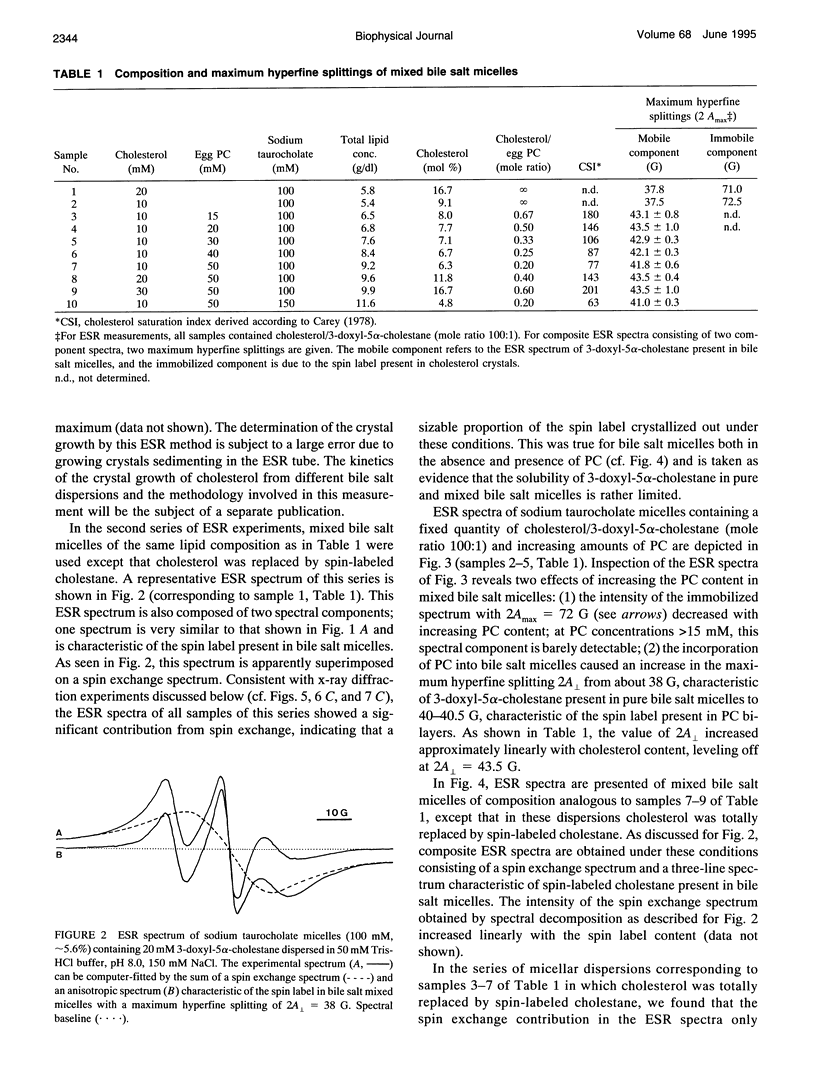
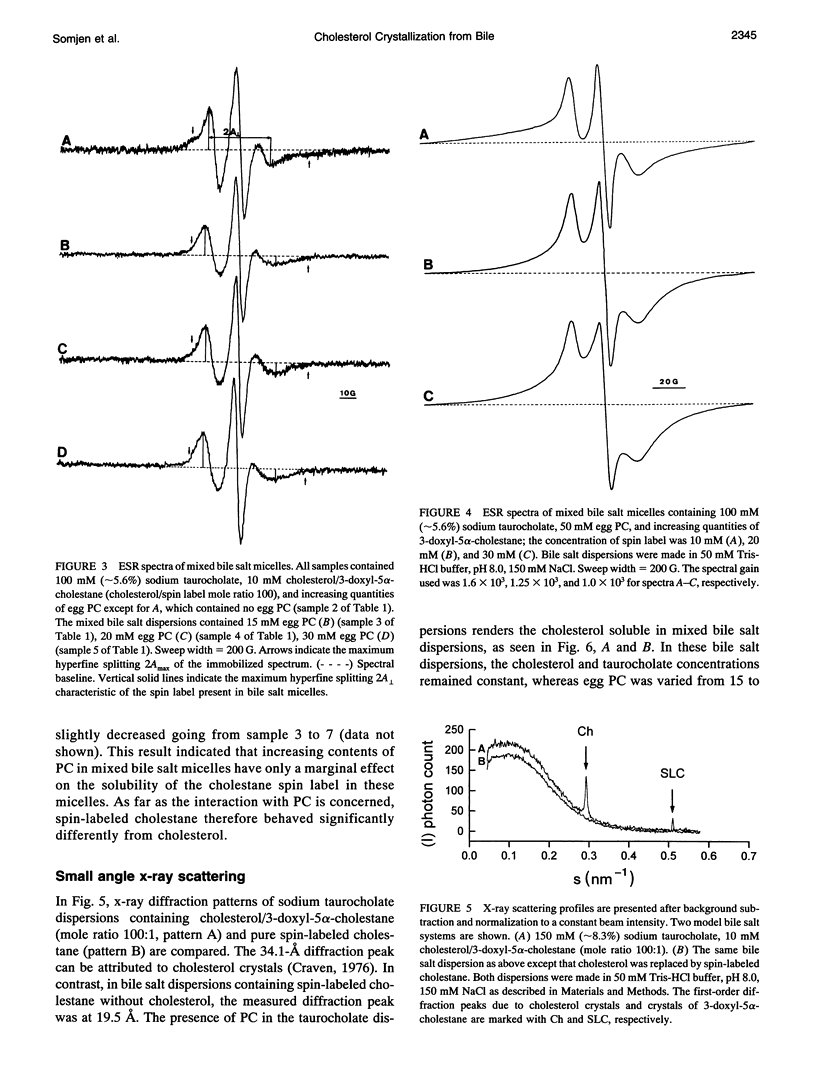
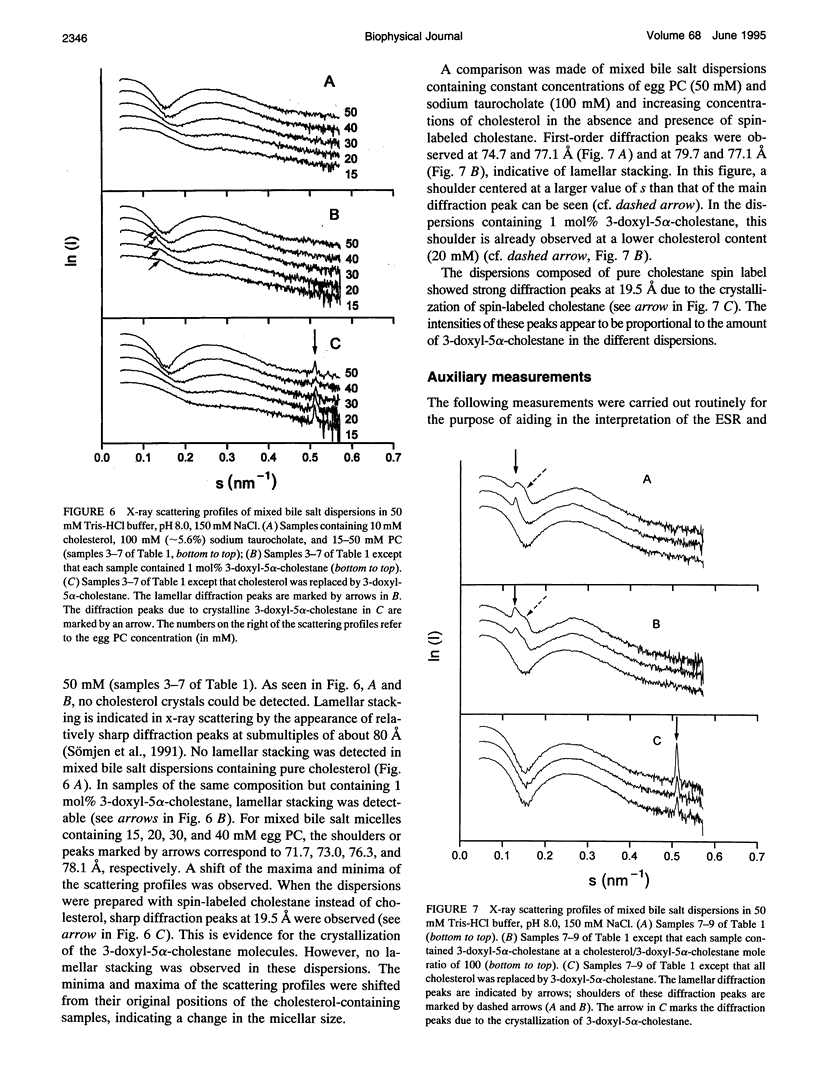
The behavior of mixed bile salt micelles consisting of sodium taurocholate, egg phosphatidylcholine, and cholesterol has been studied by ESR spin labeling and synchrotron x-ray scattering. Consistent with published phase diagrams, pure and mixed bile salt micelles have a limited capacity to incorporate and, hence, solubilize cholesterol. Excess cholesterol crystallizes out, a process that is readily detected both by ESR spin labeling using 3-doxyl-5 alpha-cholestane as a probe for cholesterol and synchrotron x-ray scattering. Both methods yield entirely consistent results. The crystallization of cholesterol from mixed bile salt micelles is indicated by the appearance of a magnetically dilute powder spectrum that is readily detected by visual inspection of the ESR spectra. Both the absence of Heissenberg spin exchange and the observation of a magnetically dilute powder spectrum provide evidence for the spin label co-crystallizing with cholesterol. In mixed bile salt micelles containing egg phosphatidylcholine, the solubility of cholesterol is increased as detected by both methods. With increasing content of phosphatidylcholine and increasing mole ratio cholesterol/phosphatidylcholine, the anisotropy of motion of the spin probe increases. The spin label 3-doxyl-5 alpha-cholestane is a useful substitute for cholesterol provided that it is used in dilute mixtures with excess cholesterol: the cholesterol/spin label mole ratio in these mixtures should be greater than 100. Despite the structural similarity between the two compounds, there are still significant differences in their physico-chemical properties. These differences come to the fore when cholesterol is totally replaced by the spin-label: 3-doxyl-5a-cholestane is significantly less soluble in bile salt and mixed bile salt micelles than cholesterol and, in contrast with cholesterol, it interacts only very weakly, if at all,with phosphatidylcholine. The potential of the ESR method for detecting cholesterol crystal growth in human bile is discussed.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bourgès M., Small D. M., Dervichian D. G. Biophysics of lipid associations. 3. The quaternary systems lecithin-bile salt-cholesterol-water. Biochim Biophys Acta. 1967 Oct 2;144(2):189–201. [PubMed] [Google Scholar]
- Cadenhead D. A., Demchak R. J., Muller-Landau F. Monolayer studies of 3-nitroxide cholestane. Ann N Y Acad Sci. 1972 Jun 20;195:218–223. [PubMed] [Google Scholar]
- Cadenhead D. A., Kellner B. M., Müller-Landau F. A comparison of a spin-label and a fluorescent cell membrane probe using pure and mixed monomolecular films. Biochim Biophys Acta. 1975 Mar 13;382(2):253–259. doi: 10.1016/0005-2736(75)90183-2. [DOI] [PubMed] [Google Scholar]
- Carey M. C. Critical tables for calculating the cholesterol saturation of native bile. J Lipid Res. 1978 Nov;19(8):945–955. [PubMed] [Google Scholar]
- Carey M. C., Small D. M. The physical chemistry of cholesterol solubility in bile. Relationship to gallstone formation and dissolution in man. J Clin Invest. 1978 Apr;61(4):998–1026. doi: 10.1172/JCI109025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craven B. M. Crystal structure of cholesterol monohydrate. Nature. 1976 Apr 22;260(5553):727–729. doi: 10.1038/260727a0. [DOI] [PubMed] [Google Scholar]
- Griffith O. H., Dehlinger P. J., Van S. P. Shape of the hydrophobic barrier of phospholipid bilayers (evidence for water penetration in biological membranes). J Membr Biol. 1974;15(2):159–192. doi: 10.1007/BF01870086. [DOI] [PubMed] [Google Scholar]
- Hsia J. C., Schneider H., Smith I. C. Spin label studies of oriented phospholipids: egg lecithin. Biochim Biophys Acta. 1970 Mar 10;202(2):399–402. doi: 10.1016/0005-2760(70)90207-9. [DOI] [PubMed] [Google Scholar]
- Johnson M. E. Apparent hydrogen bonding by strongly immobilized spin-labels. Biochemistry. 1981 Jun 9;20(12):3319–3328. doi: 10.1021/bi00515a001. [DOI] [PubMed] [Google Scholar]
- Kar L., Ney-Igner E., Freed J. H. Electron spin resonance and electron-spin-echo study of oriented multilayers of L alpha-dipalmitoylphosphatidylcholine water systems. Biophys J. 1985 Oct;48(4):569–595. doi: 10.1016/S0006-3495(85)83814-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh D. Electron spin resonance: spin labels. Mol Biol Biochem Biophys. 1981;31:51–142. doi: 10.1007/978-3-642-81537-9_2. [DOI] [PubMed] [Google Scholar]
- Mazer N. A., Benedek G. B., Carey M. C. Quasielastic light-scattering studies of aqueous biliary lipid systems. Mixed micelle formation in bile salt-lecithin solutions. Biochemistry. 1980 Feb 19;19(4):601–615. doi: 10.1021/bi00545a001. [DOI] [PubMed] [Google Scholar]
- Pope J. L. Crystallization of sodium taurocholate. J Lipid Res. 1967 Mar;8(2):146–147. [PubMed] [Google Scholar]
- Small D. M., Bourgès M. C., Dervichian D. G. The biophysics of lipidic associations. I. The ternary systems: lecithin-bile salt-water. Biochim Biophys Acta. 1966 Dec 7;125(3):563–580. [PubMed] [Google Scholar]
- Small D. M., Bourgès M., Dervichian D. G. Ternary and quaternary aqueous systems containing bile salt, lecithin, and cholesterol. Nature. 1966 Aug 20;211(5051):816–818. doi: 10.1038/211816a0. [DOI] [PubMed] [Google Scholar]
- Somjen G. J., Gilat T. A non-micellar mode of cholesterol transport in human bile. FEBS Lett. 1983 Jun 13;156(2):265–268. doi: 10.1016/0014-5793(83)80510-9. [DOI] [PubMed] [Google Scholar]
- Stevens R. D. An electron spin resonance study of cholestane spin label in aqueous mixtures of biliary lipids. J Lipid Res. 1977 Jul;18(4):417–422. [PubMed] [Google Scholar]
- Sömjen G. J., Coleman R., Koch M. H., Wachtel E., Billington D., Towns-Andrews E., Gilat T. The induction of lamellar stacking by cholesterol in lecithin-bile salt model systems and human bile studied by synchrotron X-radiation. FEBS Lett. 1991 Sep 9;289(2):163–166. doi: 10.1016/0014-5793(91)81060-l. [DOI] [PubMed] [Google Scholar]
- Sömjen G. J., Marikovsky Y., Lelkes P., Gilat T. Cholesterol-phospholipid vesicles in human bile: an ultrastructural study. Biochim Biophys Acta. 1986 Oct 24;879(1):14–21. doi: 10.1016/0005-2760(86)90260-2. [DOI] [PubMed] [Google Scholar]
- Walter A., Vinson P. K., Kaplun A., Talmon Y. Intermediate structures in the cholate-phosphatidylcholine vesicle-micelle transition. Biophys J. 1991 Dec;60(6):1315–1325. doi: 10.1016/S0006-3495(91)82169-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson M. D., Rudel L. L. Review of cholesterol absorption with emphasis on dietary and biliary cholesterol. J Lipid Res. 1994 Jun;35(6):943–955. [PubMed] [Google Scholar]


