Abstract
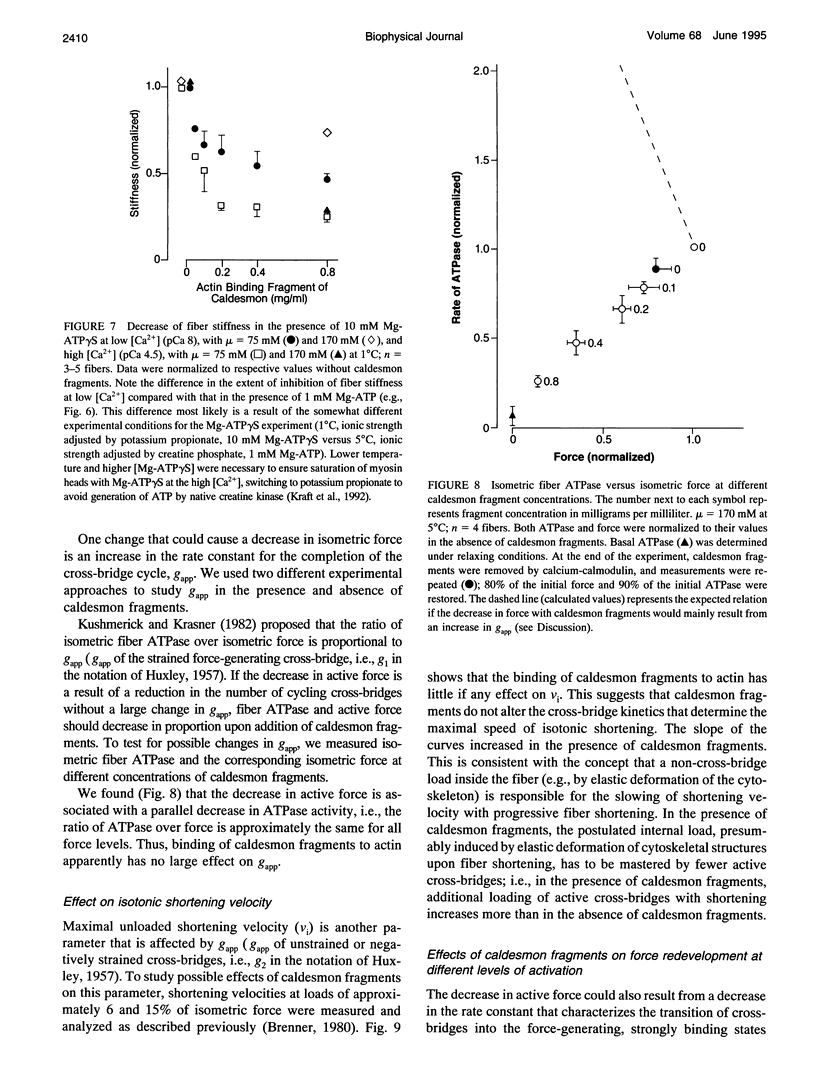
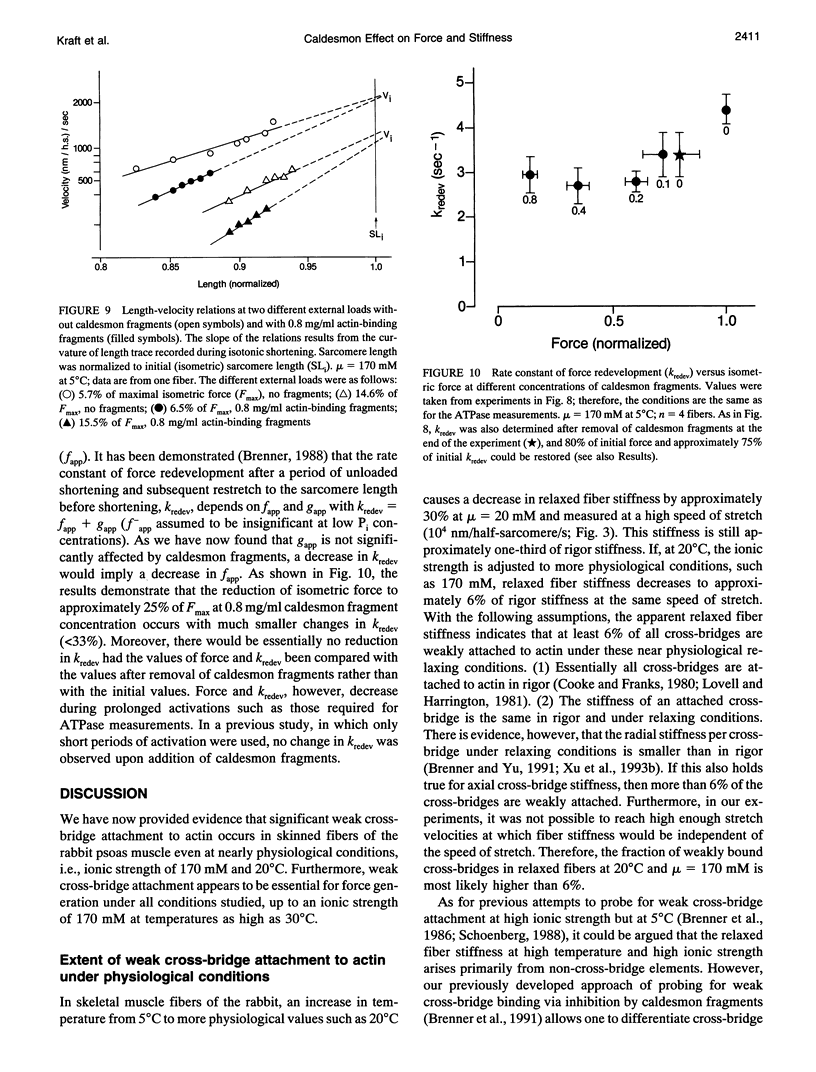
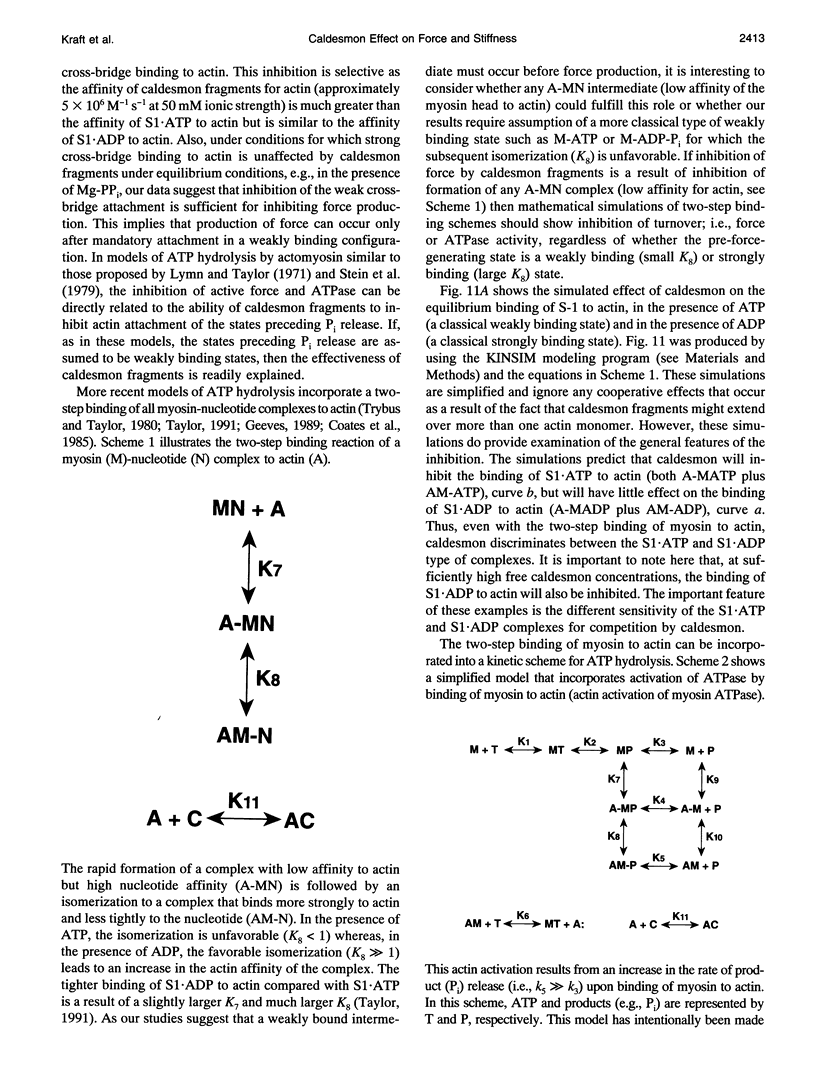
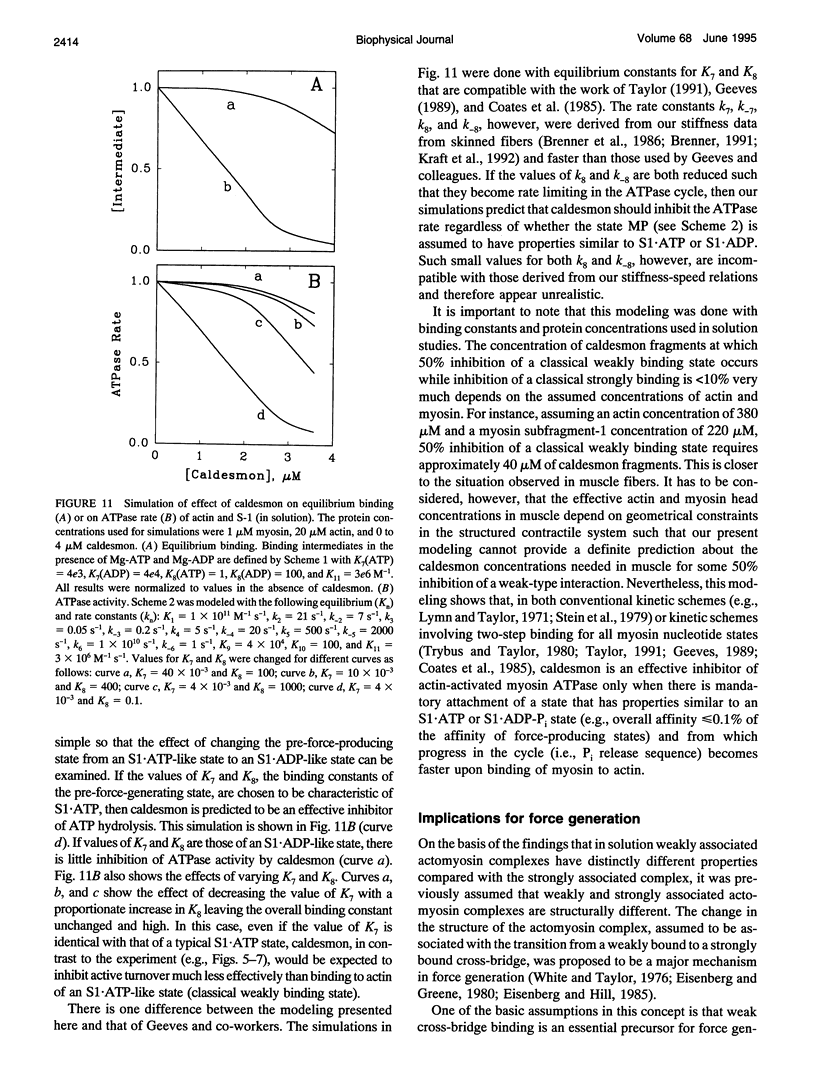
Previously we showed that stiffness of relaxed fibers and active force generated in single skinned fibers of rabbit psoas muscle are inhibited in parallel by actin-binding fragments of caldesmon, an actin-associated protein of smooth muscle, under conditions in which a large fraction of cross-bridges is weakly attached to actin (ionic strength of 50 mM and temperature of 5 degrees C). These results suggested that weak cross-bridge attachment to actin is essential for force generation. The present study provides evidence that this is also true for physiological ionic strength (170 mM) at temperatures up to 30 degrees C, suggesting that weak cross-bridge binding to actin is generally required for force generation. In addition, we show that the inhibition of active force is not a result of changes in cross-bridge cycling kinetics but apparently results from selective inhibition of weak cross-bridge binding to actin. Together with our previous biochemical, mechanical, and structural studies, these findings support the proposal that weak cross-bridge attachment to actin is an essential intermediate on the path to force generation and are consistent with the concept that isometric force mainly results from an increase in strain of the attached cross-bridge as a result of a structural change associated with the transition from a weakly bound to a strongly bound actomyosin complex. This mechanism is different from the processes responsible for quick tension recovery that were proposed by Huxley and Simmons (Proposed mechanism of force generation in striated muscle. Nature. 233:533-538.) to represent the elementary mechanism of force generation.
Full text
PDF


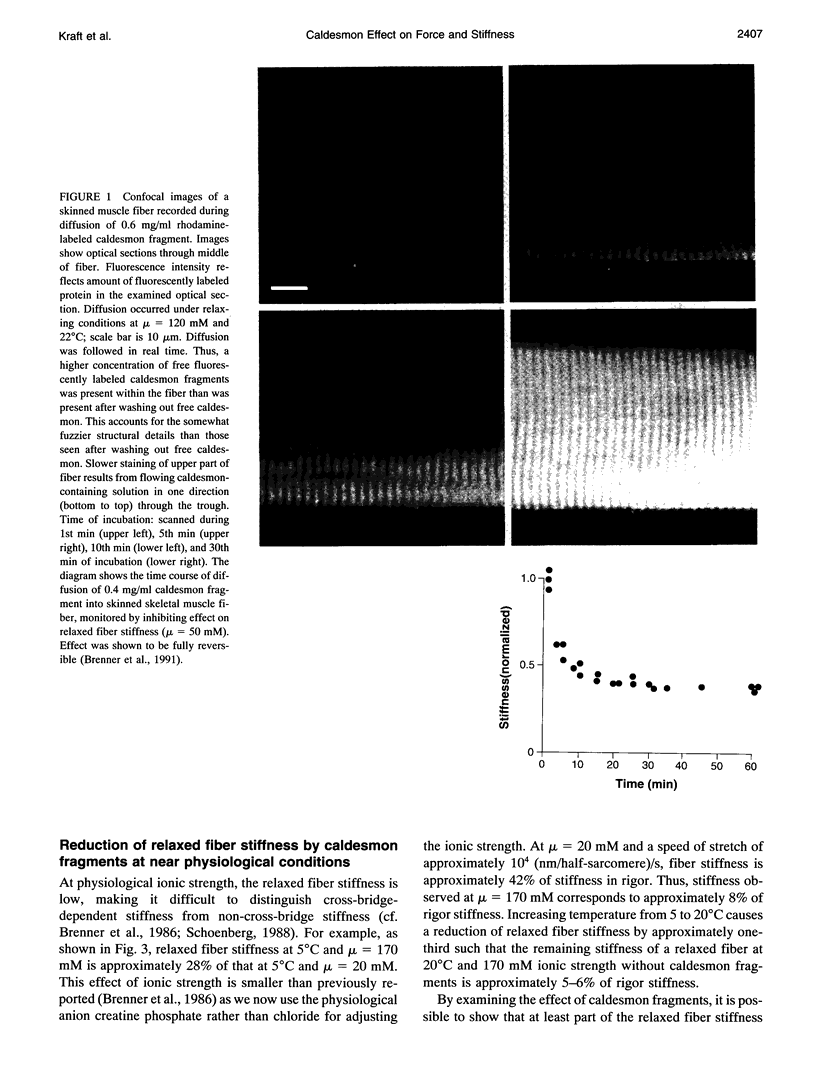
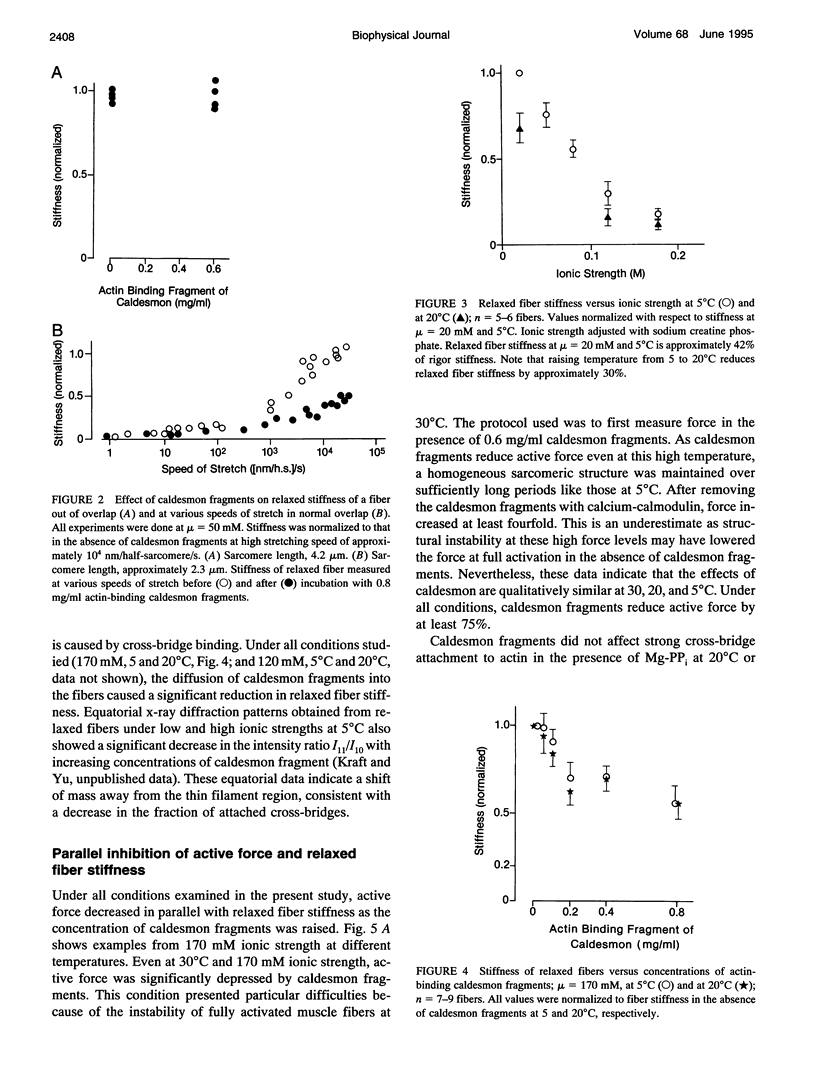
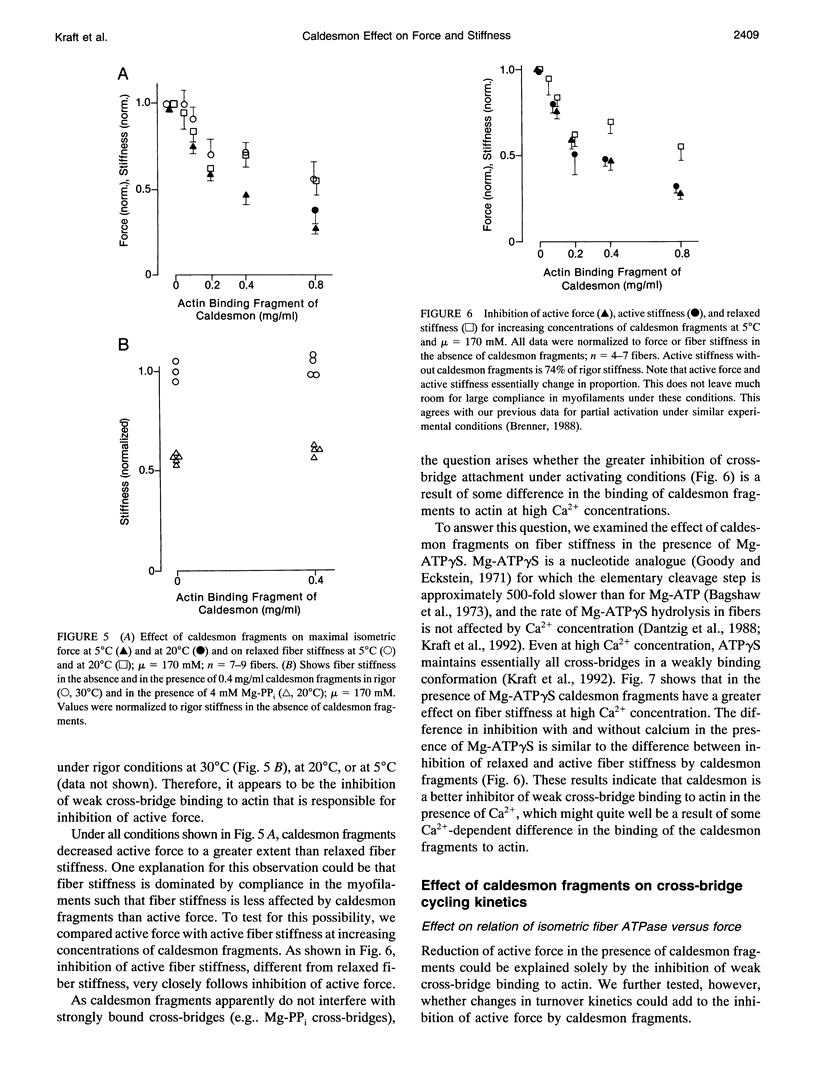





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barshop B. A., Wrenn R. F., Frieden C. Analysis of numerical methods for computer simulation of kinetic processes: development of KINSIM--a flexible, portable system. Anal Biochem. 1983 Apr 1;130(1):134–145. doi: 10.1016/0003-2697(83)90660-7. [DOI] [PubMed] [Google Scholar]
- Berger C. L., Svensson E. C., Thomas D. D. Photolysis of a photolabile precursor of ATP (caged ATP) induces microsecond rotational motions of myosin heads bound to actin. Proc Natl Acad Sci U S A. 1989 Nov;86(22):8753–8757. doi: 10.1073/pnas.86.22.8753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremel R. D., Weber A. Cooperation within actin filament in vertebrate skeletal muscle. Nat New Biol. 1972 Jul 26;238(82):97–101. doi: 10.1038/newbio238097a0. [DOI] [PubMed] [Google Scholar]
- Brenner B., Chalovich J. M., Greene L. E., Eisenberg E., Schoenberg M. Stiffness of skinned rabbit psoas fibers in MgATP and MgPPi solution. Biophys J. 1986 Oct;50(4):685–691. doi: 10.1016/S0006-3495(86)83509-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner B. Effect of Ca2+ on cross-bridge turnover kinetics in skinned single rabbit psoas fibers: implications for regulation of muscle contraction. Proc Natl Acad Sci U S A. 1988 May;85(9):3265–3269. doi: 10.1073/pnas.85.9.3265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner B., Eisenberg E. Rate of force generation in muscle: correlation with actomyosin ATPase activity in solution. Proc Natl Acad Sci U S A. 1986 May;83(10):3542–3546. doi: 10.1073/pnas.83.10.3542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner B. Rapid dissociation and reassociation of actomyosin cross-bridges during force generation: a newly observed facet of cross-bridge action in muscle. Proc Natl Acad Sci U S A. 1991 Dec 1;88(23):10490–10494. doi: 10.1073/pnas.88.23.10490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner B. Technique for stabilizing the striation pattern in maximally calcium-activated skinned rabbit psoas fibers. Biophys J. 1983 Jan;41(1):99–102. doi: 10.1016/S0006-3495(83)84411-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner B. The cross-bridge cycle in muscle. Mechanical, biochemical, and structural studies on single skinned rabbit psoas fibers to characterize cross-bridge kinetics in muscle for correlation with the actomyosin-ATPase in solution. Basic Res Cardiol. 1986;81 (Suppl 1):1–15. doi: 10.1007/978-3-662-11374-5_1. [DOI] [PubMed] [Google Scholar]
- Brenner B., Yu L. C., Chalovich J. M. Parallel inhibition of active force and relaxed fiber stiffness in skeletal muscle by caldesmon: implications for the pathway to force generation. Proc Natl Acad Sci U S A. 1991 Jul 1;88(13):5739–5743. doi: 10.1073/pnas.88.13.5739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner B., Yu L. C. Characterization of radial force and radial stiffness in Ca(2+)-activated skinned fibres of the rabbit psoas muscle. J Physiol. 1991 Sep;441:703–718. doi: 10.1113/jphysiol.1991.sp018774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner B., Yu L. C. Structural changes in the actomyosin cross-bridges associated with force generation. Proc Natl Acad Sci U S A. 1993 Jun 1;90(11):5252–5256. doi: 10.1073/pnas.90.11.5252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bretscher A. Smooth muscle caldesmon. Rapid purification and F-actin cross-linking properties. J Biol Chem. 1984 Oct 25;259(20):12873–12880. [PubMed] [Google Scholar]
- Chalovich J. M. Actin mediated regulation of muscle contraction. Pharmacol Ther. 1992;55(2):95–148. doi: 10.1016/0163-7258(92)90013-p. [DOI] [PubMed] [Google Scholar]
- Chalovich J. M., Bryan J., Benson C. E., Velaz L. Localization and characterization of a 7.3-kDa region of caldesmon which reversibly inhibits actomyosin ATPase activity. J Biol Chem. 1992 Aug 15;267(23):16644–16650. [PMC free article] [PubMed] [Google Scholar]
- Chalovich J. M., Chock P. B., Eisenberg E. Mechanism of action of troponin . tropomyosin. Inhibition of actomyosin ATPase activity without inhibition of myosin binding to actin. J Biol Chem. 1981 Jan 25;256(2):575–578. [PMC free article] [PubMed] [Google Scholar]
- Chalovich J. M., Greene L. E., Eisenberg E. Crosslinked myosin subfragment 1: a stable analogue of the subfragment-1.ATP complex. Proc Natl Acad Sci U S A. 1983 Aug;80(16):4909–4913. doi: 10.1073/pnas.80.16.4909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chase P. B., Kushmerick M. J. Effects of pH on contraction of rabbit fast and slow skeletal muscle fibers. Biophys J. 1988 Jun;53(6):935–946. doi: 10.1016/S0006-3495(88)83174-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coates J. H., Criddle A. H., Geeves M. A. Pressure-relaxation studies of pyrene-labelled actin and myosin subfragment 1 from rabbit skeletal muscle. Evidence for two states of acto-subfragment 1. Biochem J. 1985 Dec 1;232(2):351–356. doi: 10.1042/bj2320351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke R., Franks K. All myosin heads form bonds with actin in rigor rabbit skeletal muscle. Biochemistry. 1980 May 13;19(10):2265–2269. doi: 10.1021/bi00551a042. [DOI] [PubMed] [Google Scholar]
- Craig R., Greene L. E., Eisenberg E. Structure of the actin-myosin complex in the presence of ATP. Proc Natl Acad Sci U S A. 1985 May;82(10):3247–3251. doi: 10.1073/pnas.82.10.3247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantzig J. A., Walker J. W., Trentham D. R., Goldman Y. E. Relaxation of muscle fibers with adenosine 5'-[gamma-thio]triphosphate (ATP[gamma S]) and by laser photolysis of caged ATP[gamma S]: evidence for Ca2+-dependent affinity of rapidly detaching zero-force cross-bridges. Proc Natl Acad Sci U S A. 1988 Sep;85(18):6716–6720. doi: 10.1073/pnas.85.18.6716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg E., Greene L. E. The relation of muscle biochemistry to muscle physiology. Annu Rev Physiol. 1980;42:293–309. doi: 10.1146/annurev.ph.42.030180.001453. [DOI] [PubMed] [Google Scholar]
- Eisenberg E., Hill T. L. Muscle contraction and free energy transduction in biological systems. Science. 1985 Mar 1;227(4690):999–1006. doi: 10.1126/science.3156404. [DOI] [PubMed] [Google Scholar]
- Fajer P. G., Fajer E. A., Schoenberg M., Thomas D. D. Orientational disorder and motion of weakly attached cross-bridges. Biophys J. 1991 Sep;60(3):642–649. doi: 10.1016/S0006-3495(91)82093-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford L. E., Huxley A. F., Simmons R. M. Tension responses to sudden length change in stimulated frog muscle fibres near slack length. J Physiol. 1977 Jul;269(2):441–515. doi: 10.1113/jphysiol.1977.sp011911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geeves M. A. Dynamic interaction between actin and myosin subfragment 1 in the presence of ADP. Biochemistry. 1989 Jul 11;28(14):5864–5871. doi: 10.1021/bi00440a024. [DOI] [PubMed] [Google Scholar]
- Granzier H. L., Wang K. Passive tension and stiffness of vertebrate skeletal and insect flight muscles: the contribution of weak cross-bridges and elastic filaments. Biophys J. 1993 Nov;65(5):2141–2159. doi: 10.1016/S0006-3495(93)81262-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene L. E., Eisenberg E. Cooperative binding of myosin subfragment-1 to the actin-troponin-tropomyosin complex. Proc Natl Acad Sci U S A. 1980 May;77(5):2616–2620. doi: 10.1073/pnas.77.5.2616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene L. E., Eisenberg E. Dissociation of the actin.subfragment 1 complex by adenyl-5'-yl imidodiphosphate, ADP, and PPi. J Biol Chem. 1980 Jan 25;255(2):543–548. [PubMed] [Google Scholar]
- Greene L. E., Eisenberg E. Relationship between regulated actomyosin ATPase activity and cooperative binding of myosin to regulated actin. Cell Biophys. 1988 Jan-Jun;12:59–71. doi: 10.1007/BF02918350. [DOI] [PubMed] [Google Scholar]
- Greene L. E., Sellers J. R., Eisenberg E., Adelstein R. S. Binding of gizzard smooth muscle myosin subfragment 1 to actin in the presence and absence of adenosine 5'-triphosphate. Biochemistry. 1983 Feb 1;22(3):530–535. doi: 10.1021/bi00272a002. [DOI] [PubMed] [Google Scholar]
- HUXLEY A. F. Muscle structure and theories of contraction. Prog Biophys Biophys Chem. 1957;7:255–318. [PubMed] [Google Scholar]
- Hemric M. E., Chalovich J. M. Effect of caldesmon on the ATPase activity and the binding of smooth and skeletal myosin subfragments to actin. J Biol Chem. 1988 Feb 5;263(4):1878–1885. [PubMed] [Google Scholar]
- Highsmith S. The effects of temperature and salts on myosin subfragment-1 and F-actin association. Arch Biochem Biophys. 1977 Apr 30;180(2):404–408. doi: 10.1016/0003-9861(77)90054-6. [DOI] [PubMed] [Google Scholar]
- Hill T. L. Theoretical formalism for the sliding filament model of contraction of striated muscle. Part I. Prog Biophys Mol Biol. 1974;28:267–340. doi: 10.1016/0079-6107(74)90020-0. [DOI] [PubMed] [Google Scholar]
- Holmes K. C., Popp D., Gebhard W., Kabsch W. Atomic model of the actin filament. Nature. 1990 Sep 6;347(6288):44–49. doi: 10.1038/347044a0. [DOI] [PubMed] [Google Scholar]
- Huxley A. F., Simmons R. M. Proposed mechanism of force generation in striated muscle. Nature. 1971 Oct 22;233(5321):533–538. doi: 10.1038/233533a0. [DOI] [PubMed] [Google Scholar]
- Huxley H. E., Simmons R. M., Faruqi A. R., Kress M., Bordas J., Koch M. H. Changes in the X-ray reflections from contracting muscle during rapid mechanical transients and their structural implications. J Mol Biol. 1983 Sep 15;169(2):469–506. doi: 10.1016/s0022-2836(83)80062-x. [DOI] [PubMed] [Google Scholar]
- Irving M., Lombardi V., Piazzesi G., Ferenczi M. A. Myosin head movements are synchronous with the elementary force-generating process in muscle. Nature. 1992 May 14;357(6374):156–158. doi: 10.1038/357156a0. [DOI] [PubMed] [Google Scholar]
- Konrad M., Goody R. S. Kinetic and thermodynamic properties of the ternary complex between F-actin, myosin subfragment 1 and adenosine 5'-[beta, gamma-imido]triphosphate. Eur J Biochem. 1982 Nov 15;128(2-3):547–555. doi: 10.1111/j.1432-1033.1982.tb07000.x. [DOI] [PubMed] [Google Scholar]
- Kraft T., Yu L. C., Kuhn H. J., Brenner B. Effect of Ca2+ on weak cross-bridge interaction with actin in the presence of adenosine 5'-[gamma-thio]triphosphate). Proc Natl Acad Sci U S A. 1992 Dec 1;89(23):11362–11366. doi: 10.1073/pnas.89.23.11362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kushmerick M. J., Krasner B. Force and ATPase rate in skinned skeletal muscle fibers. Fed Proc. 1982 May;41(7):2232–2237. [PubMed] [Google Scholar]
- Lovell S. J., Harrington W. F. Measurement of the fraction of myosin heads bound to actin in rabbit skeletal myofibrils in rigor. J Mol Biol. 1981 Jul 15;149(4):659–674. doi: 10.1016/0022-2836(81)90352-1. [DOI] [PubMed] [Google Scholar]
- Lymn R. W., Taylor E. W. Mechanism of adenosine triphosphate hydrolysis by actomyosin. Biochemistry. 1971 Dec 7;10(25):4617–4624. doi: 10.1021/bi00801a004. [DOI] [PubMed] [Google Scholar]
- Margossian S. S., Lowey S. Interaction of myosin subfragments with F-actin. Biochemistry. 1978 Dec 12;17(25):5431–5439. doi: 10.1021/bi00618a017. [DOI] [PubMed] [Google Scholar]
- Marston S. B. The rates of formation and dissociation of actin-myosin complexes. Effects of solvent, temperature, nucleotide binding and head-head interactions. Biochem J. 1982 May 1;203(2):453–460. doi: 10.1042/bj2030453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marston S., Weber A. The dissociation constant of the actin-heavy meromyosin subfragment-1 complex. Biochemistry. 1975 Aug 26;14(17):3868–3873. doi: 10.1021/bi00688a021. [DOI] [PubMed] [Google Scholar]
- Matsuda T., Podolsky R. J. X-ray evidence for two structural states of the actomyosin cross-bridge in muscle fibers. Proc Natl Acad Sci U S A. 1984 Apr;81(8):2364–2368. doi: 10.1073/pnas.81.8.2364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milligan R. A., Whittaker M., Safer D. Molecular structure of F-actin and location of surface binding sites. Nature. 1990 Nov 15;348(6298):217–221. doi: 10.1038/348217a0. [DOI] [PubMed] [Google Scholar]
- Pollard T. D., Bhandari D., Maupin P., Wachsstock D., Weeds A. G., Zot H. G. Direct visualization by electron microscopy of the weakly bound intermediates in the actomyosin adenosine triphosphatase cycle. Biophys J. 1993 Feb;64(2):454–471. doi: 10.1016/S0006-3495(93)81387-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rayment I., Holden H. M., Whittaker M., Yohn C. B., Lorenz M., Holmes K. C., Milligan R. A. Structure of the actin-myosin complex and its implications for muscle contraction. Science. 1993 Jul 2;261(5117):58–65. doi: 10.1126/science.8316858. [DOI] [PubMed] [Google Scholar]
- Rayment I., Rypniewski W. R., Schmidt-Bäse K., Smith R., Tomchick D. R., Benning M. M., Winkelmann D. A., Wesenberg G., Holden H. M. Three-dimensional structure of myosin subfragment-1: a molecular motor. Science. 1993 Jul 2;261(5117):50–58. doi: 10.1126/science.8316857. [DOI] [PubMed] [Google Scholar]
- Schoenberg M. Characterization of the myosin adenosine triphosphate (M.ATP) crossbridge in rabbit and frog skeletal muscle fibers. Biophys J. 1988 Jul;54(1):135–148. doi: 10.1016/S0006-3495(88)82938-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schröder R. R., Manstein D. J., Jahn W., Holden H., Rayment I., Holmes K. C., Spudich J. A. Three-dimensional atomic model of F-actin decorated with Dictyostelium myosin S1. Nature. 1993 Jul 8;364(6433):171–174. doi: 10.1038/364171a0. [DOI] [PubMed] [Google Scholar]
- Stein L. A., Schwarz R. P., Jr, Chock P. B., Eisenberg E. Mechanism of actomyosin adenosine triphosphatase. Evidence that adenosine 5'-triphosphate hydrolysis can occur without dissociation of the actomyosin complex. Biochemistry. 1979 Sep 4;18(18):3895–3909. doi: 10.1021/bi00585a009. [DOI] [PubMed] [Google Scholar]
- Taylor E. W. Kinetic studies on the association and dissociation of myosin subfragment 1 and actin. J Biol Chem. 1991 Jan 5;266(1):294–302. [PubMed] [Google Scholar]
- Trybus K. M., Taylor E. W. Kinetic studies of the cooperative binding of subfragment 1 to regulated actin. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7209–7213. doi: 10.1073/pnas.77.12.7209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velaz L., Chen Y. D., Chalovich J. M. Characterization of a caldesmon fragment that competes with myosin-ATP binding to actin. Biophys J. 1993 Aug;65(2):892–898. doi: 10.1016/S0006-3495(93)81113-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner P. D., Giniger E. Calcium-sensitive binding of heavy meromyosin to regulated actin in the presence of ATP. J Biol Chem. 1981 Dec 25;256(24):12647–12650. [PubMed] [Google Scholar]
- Walker M., White H., Belknap B., Trinick J. Electron cryomicroscopy of acto-myosin-S1 during steady-state ATP hydrolysis. Biophys J. 1994 May;66(5):1563–1572. doi: 10.1016/S0006-3495(94)80948-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White H. D., Taylor E. W. Energetics and mechanism of actomyosin adenosine triphosphatase. Biochemistry. 1976 Dec 28;15(26):5818–5826. doi: 10.1021/bi00671a020. [DOI] [PubMed] [Google Scholar]
- Xu S., Brenner B., Yu L. C. State-dependent radial elasticity of attached cross-bridges in single skinned fibres of rabbit psoas muscle. J Physiol. 1993 Feb;461:283–299. doi: 10.1113/jphysiol.1993.sp019514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu L. C., Brenner B. Structures of actomyosin crossbridges in relaxed and rigor muscle fibers. Biophys J. 1989 Mar;55(3):441–453. doi: 10.1016/S0006-3495(89)82838-3. [DOI] [PMC free article] [PubMed] [Google Scholar]