Abstract
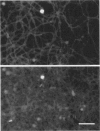
We have analyzed the dependence of actin filament sliding movement on the mode of myosin attachment to surfaces. Monoclonal antibodies (mAbs) that bind to three distinct sites were used to tether myosin to nitrocellulose-coated glass. One antibody reacts with an epitope on the regulatory light chain (LC2) located at the head-rod junction. The other two react with sites in the rod domain, one in the S2 region near the S2-LMM hinge, and the other at the C terminus of the myosin rod. This method of attachment provides a means of controlling the flexibility and density of myosin on the surface. Fast skeletal muscle myosin monomers were bound to the surfaces through the specific interaction with these mAbs, and the sliding movement of fluorescently labeled actin filaments was analyzed by video microscopy. Each of these antibodies produced stable myosin-coated surfaces that supported uniform motion of actin over the course of several hours. Attachment of myosin through the anti-S2 and anti-LMM mAbs yielded significantly higher velocities (10 microns/s at 30 degrees C) than attachment through anti-LC2 (4-5 microns/s at 30 degrees C). For each antibody, we observed a characteristic value of the myosin density for the onset of F-actin motion and a second critical density for velocity saturation. The specific mode of attachment influences the velocity of actin filaments and the characteristic surface density needed to support movement.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cooke R. The mechanism of muscle contraction. CRC Crit Rev Biochem. 1986;21(1):53–118. doi: 10.3109/10409238609113609. [DOI] [PubMed] [Google Scholar]
- Cuda G., Fananapazir L., Zhu W. S., Sellers J. R., Epstein N. D. Skeletal muscle expression and abnormal function of beta-myosin in hypertrophic cardiomyopathy. J Clin Invest. 1993 Jun;91(6):2861–2865. doi: 10.1172/JCI116530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duke T, Holy TE, Leibler S. "Gliding assays" for motor proteins: A theoretical analysis. Phys Rev Lett. 1995 Jan 9;74(2):330–333. doi: 10.1103/PhysRevLett.74.330. [DOI] [PubMed] [Google Scholar]
- Eisenberg E., Hill T. L. Muscle contraction and free energy transduction in biological systems. Science. 1985 Mar 1;227(4690):999–1006. doi: 10.1126/science.3156404. [DOI] [PubMed] [Google Scholar]
- Ey P. L., Prowse S. J., Jenkin C. R. Isolation of pure IgG1, IgG2a and IgG2b immunoglobulins from mouse serum using protein A-sepharose. Immunochemistry. 1978 Jul;15(7):429–436. doi: 10.1016/0161-5890(78)90070-6. [DOI] [PubMed] [Google Scholar]
- Finer J. T., Simmons R. M., Spudich J. A. Single myosin molecule mechanics: piconewton forces and nanometre steps. Nature. 1994 Mar 10;368(6467):113–119. doi: 10.1038/368113a0. [DOI] [PubMed] [Google Scholar]
- Harada Y., Sakurada K., Aoki T., Thomas D. D., Yanagida T. Mechanochemical coupling in actomyosin energy transduction studied by in vitro movement assay. J Mol Biol. 1990 Nov 5;216(1):49–68. doi: 10.1016/S0022-2836(05)80060-9. [DOI] [PubMed] [Google Scholar]
- Harada Y., Yanagida T. Direct observation of molecular motility by light microscopy. Cell Motil Cytoskeleton. 1988;10(1-2):71–76. doi: 10.1002/cm.970100112. [DOI] [PubMed] [Google Scholar]
- Holmes K. C., Popp D., Gebhard W., Kabsch W. Atomic model of the actin filament. Nature. 1990 Sep 6;347(6288):44–49. doi: 10.1038/347044a0. [DOI] [PubMed] [Google Scholar]
- Huxley A. F., Simmons R. M. Proposed mechanism of force generation in striated muscle. Nature. 1971 Oct 22;233(5321):533–538. doi: 10.1038/233533a0. [DOI] [PubMed] [Google Scholar]
- Huxley A. Muscle. A fine time for contractual alterations. Nature. 1992 May 14;357(6374):110–110. doi: 10.1038/357110a0. [DOI] [PubMed] [Google Scholar]
- Huxley H. E. Sliding filaments and molecular motile systems. J Biol Chem. 1990 May 25;265(15):8347–8350. [PubMed] [Google Scholar]
- Hynes T. R., Block S. M., White B. T., Spudich J. A. Movement of myosin fragments in vitro: domains involved in force production. Cell. 1987 Mar 27;48(6):953–963. doi: 10.1016/0092-8674(87)90704-5. [DOI] [PubMed] [Google Scholar]
- Kishino A., Yanagida T. Force measurements by micromanipulation of a single actin filament by glass needles. Nature. 1988 Jul 7;334(6177):74–76. doi: 10.1038/334074a0. [DOI] [PubMed] [Google Scholar]
- Kron S. J., Spudich J. A. Fluorescent actin filaments move on myosin fixed to a glass surface. Proc Natl Acad Sci U S A. 1986 Sep;83(17):6272–6276. doi: 10.1073/pnas.83.17.6272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowey S., Waller G. S., Trybus K. M. Function of skeletal muscle myosin heavy and light chain isoforms by an in vitro motility assay. J Biol Chem. 1993 Sep 25;268(27):20414–20418. [PubMed] [Google Scholar]
- Magnasco MO. Forced thermal ratchets. Phys Rev Lett. 1993 Sep 6;71(10):1477–1481. doi: 10.1103/PhysRevLett.71.1477. [DOI] [PubMed] [Google Scholar]
- Magnasco MO. Molecular combustion motors. Phys Rev Lett. 1994 Apr 18;72(16):2656–2659. doi: 10.1103/PhysRevLett.72.2656. [DOI] [PubMed] [Google Scholar]
- Pollard T. D. The myosin crossbridge problem. Cell. 1987 Mar 27;48(6):909–910. doi: 10.1016/0092-8674(87)90087-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Press J. L. The CBA/N defect defines two classes of T cell-dependent antigens. J Immunol. 1981 Apr;126(4):1234–1240. [PubMed] [Google Scholar]
- Rayment I., Rypniewski W. R., Schmidt-Bäse K., Smith R., Tomchick D. R., Benning M. M., Winkelmann D. A., Wesenberg G., Holden H. M. Three-dimensional structure of myosin subfragment-1: a molecular motor. Science. 1993 Jul 2;261(5117):50–58. doi: 10.1126/science.8316857. [DOI] [PubMed] [Google Scholar]
- Saito K., Aoki T., Aoki T., Yanagida T. Movement of single myosin filaments and myosin step size on an actin filament suspended in solution by a laser trap. Biophys J. 1994 Mar;66(3 Pt 1):769–777. doi: 10.1016/s0006-3495(94)80853-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sellers J. R., Cuda G., Wang F., Homsher E. Myosin-specific adaptations of the motility assay. Methods Cell Biol. 1993;39:23–49. doi: 10.1016/s0091-679x(08)60159-4. [DOI] [PubMed] [Google Scholar]
- Sheetz M. P., Chasan R., Spudich J. A. ATP-dependent movement of myosin in vitro: characterization of a quantitative assay. J Cell Biol. 1984 Nov;99(5):1867–1871. doi: 10.1083/jcb.99.5.1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spudich J. A. In pursuit of myosin function. Cell Regul. 1989 Nov;1(1):1–11. doi: 10.1091/mbc.1.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tesi C., Kitagishi K., Travers F., Barman T. Cryoenzymic studies on actomyosin ATPase: kinetic evidence for communication between the actin and ATP sites on myosin. Biochemistry. 1991 Apr 23;30(16):4061–4067. doi: 10.1021/bi00230a034. [DOI] [PubMed] [Google Scholar]
- Toyoshima Y. Y., Kron S. J., McNally E. M., Niebling K. R., Toyoshima C., Spudich J. A. Myosin subfragment-1 is sufficient to move actin filaments in vitro. Nature. 1987 Aug 6;328(6130):536–539. doi: 10.1038/328536a0. [DOI] [PubMed] [Google Scholar]
- Toyoshima Y. Y., Kron S. J., Spudich J. A. The myosin step size: measurement of the unit displacement per ATP hydrolyzed in an in vitro assay. Proc Natl Acad Sci U S A. 1990 Sep;87(18):7130–7134. doi: 10.1073/pnas.87.18.7130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umemoto S., Sellers J. R. Characterization of in vitro motility assays using smooth muscle and cytoplasmic myosins. J Biol Chem. 1990 Sep 5;265(25):14864–14869. [PubMed] [Google Scholar]
- Uyeda T. Q., Kron S. J., Spudich J. A. Myosin step size. Estimation from slow sliding movement of actin over low densities of heavy meromyosin. J Mol Biol. 1990 Aug 5;214(3):699–710. doi: 10.1016/0022-2836(90)90287-V. [DOI] [PubMed] [Google Scholar]
- Warrick H. M., Spudich J. A. Myosin structure and function in cell motility. Annu Rev Cell Biol. 1987;3:379–421. doi: 10.1146/annurev.cb.03.110187.002115. [DOI] [PubMed] [Google Scholar]
- Winkelmann D. A., Kinose F., Chung A. L. Inhibition of actin filament movement by monoclonal antibodies against the motor domain of myosin. J Muscle Res Cell Motil. 1993 Aug;14(4):452–467. doi: 10.1007/BF00121297. [DOI] [PubMed] [Google Scholar]
- Winkelmann D. A., Lowey S., Press J. L. Monoclonal antibodies localize changes on myosin heavy chain isozymes during avian myogenesis. Cell. 1983 Aug;34(1):295–306. doi: 10.1016/0092-8674(83)90160-5. [DOI] [PubMed] [Google Scholar]
- Winkelmann D. A., Lowey S. Probing myosin head structure with monoclonal antibodies. J Mol Biol. 1986 Apr 20;188(4):595–612. doi: 10.1016/s0022-2836(86)80009-2. [DOI] [PubMed] [Google Scholar]