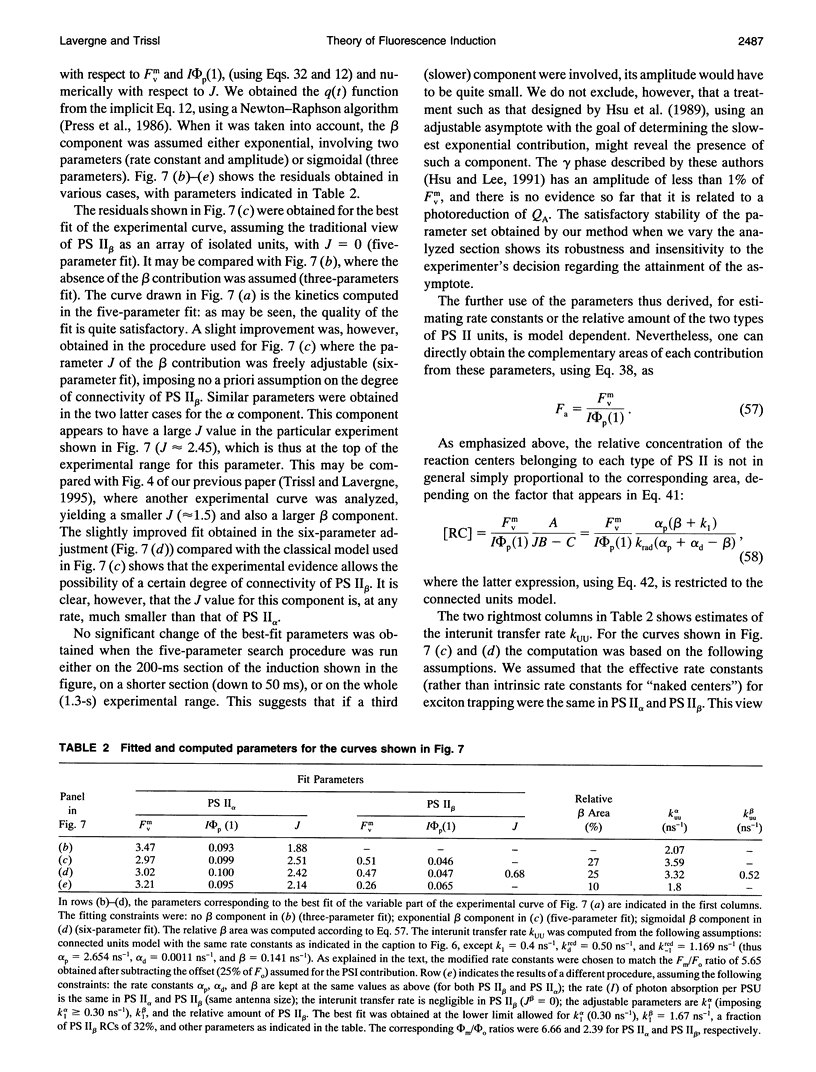
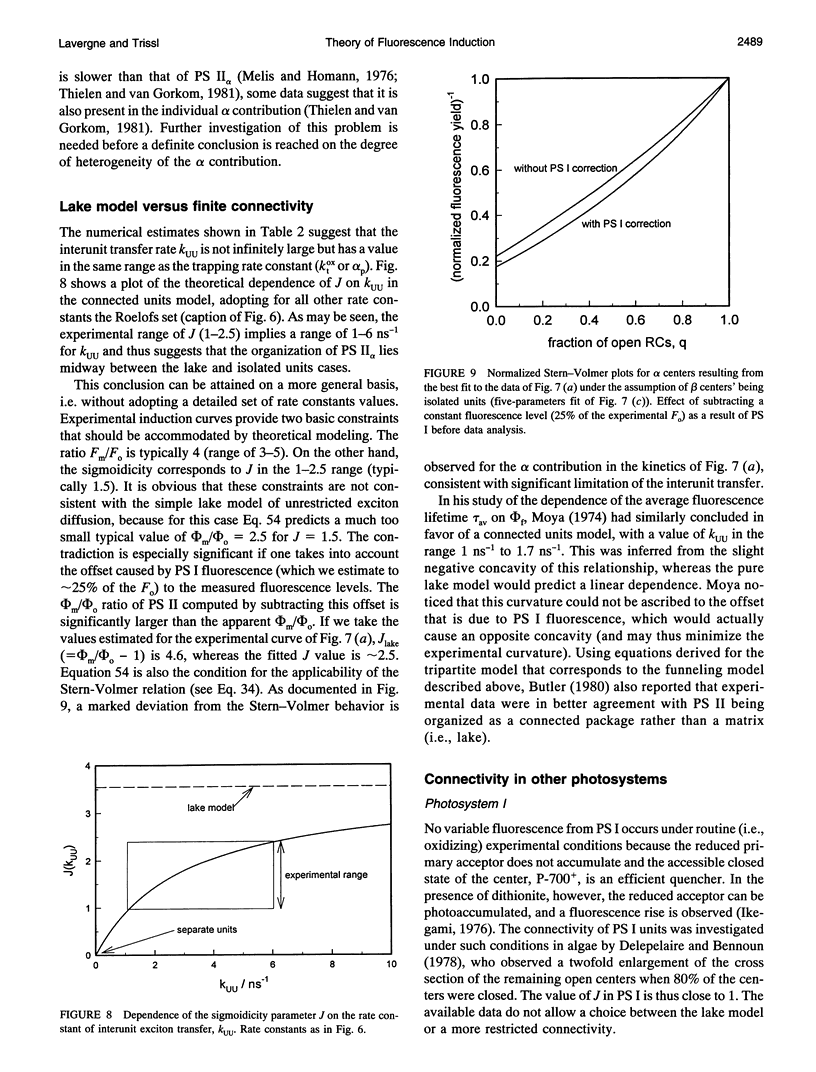
Abstract
The theoretical relationships between the fluorescence and photochemical yields of PS II and the fraction of open reaction centers are examined in a general model endowed with the following features: i) a homogeneous, infinite PS II domain; ii) exciton-radical-pair equilibrium; and iii) different rates of exciton transfer between core and peripheral antenna beds. Simple analytical relations are derived for the yields and their time courses in induction experiments. The introduction of the exciton-radical-pair equilibrium, for both the open and closed states of the trap, is shown to be equivalent to an irreversible trapping scheme with modified parameters. Variation of the interunit transfer rate allows continuous modulation from the case of separated units to the pure lake model. Broadly used relations for estimating the relative amount of reaction centers from the complementary area of the fluorescence kinetics or the photochemical yield from fluorescence levels are examined in this framework. Their dependence on parameters controlling exciton decay is discussed, allowing assessment of their range of applicability. An experimental induction curve is analyzed, with a discussion of its decomposition into alpha and beta contributions. The sigmoidicity of the induction kinetics is characterized by a single parameter J related to Joliot's p, which is shown to depend on both the connectivity of the photosynthetic units and reaction center parameters. On the other hand, the relation between J and the extreme fluorescence levels (or the deviation from the linear Stern-Volmer dependence of 1/phi f on the fraction of open traps) is controlled only by antenna connectivity. Experimental data are consistent with a model of connected units for PS II alpha, intermediate between the pure lake model of unrestricted exciton transfer and the isolated units model.
Full text
PDF












Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albertsson P. A., Andreasson E., Svensson P. The domain organization of the plant thylakoid membrane. FEBS Lett. 1990 Oct 29;273(1-2):36–40. doi: 10.1016/0014-5793(90)81045-p. [DOI] [PubMed] [Google Scholar]
- Beekman L. M., van Mourik F., Jones M. R., Visser H. M., Hunter C. N., van Grondelle R. Trapping kinetics in mutants of the photosynthetic purple bacterium Rhodobacter sphaeroides: influence of the charge separation rate and consequences for the rate-limiting step in the light-harvesting process. Biochemistry. 1994 Mar 22;33(11):3143–3147. doi: 10.1021/bi00177a001. [DOI] [PubMed] [Google Scholar]
- Bennoun P., Li Y. New results on the mode of action of 3,-(3,4-Dichlorophenyl)-1,1-dimethylurea in spinach chloroplasts. Biochim Biophys Acta. 1973 Jan 18;292(1):162–168. doi: 10.1016/0005-2728(73)90260-0. [DOI] [PubMed] [Google Scholar]
- Breton J., Geacintov N. E., Swenberg C. E. Quenching of fluorescence by triplet excited states in chloroplasts. Biochim Biophys Acta. 1979 Dec 6;548(3):616–635. doi: 10.1016/0005-2728(79)90069-0. [DOI] [PubMed] [Google Scholar]
- Butler W. L. Energy transfer between photosystem II units in a connected package model of the photochemical apparatus of photosynthesis. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4697–4701. doi: 10.1073/pnas.77.8.4697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler W. L., Kitajima M. Fluorescence quenching in photosystem II of chloroplasts. Biochim Biophys Acta. 1975 Jan 31;376(1):116–125. doi: 10.1016/0005-2728(75)90210-8. [DOI] [PubMed] [Google Scholar]
- Butler W. L. On the primary nature of fluorescence yield changes associated with photosynthesis. Proc Natl Acad Sci U S A. 1972 Nov;69(11):3420–3422. doi: 10.1073/pnas.69.11.3420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton R. K. An analysis of the relations between fluorescence and photochemistry during photosynthesis. J Theor Biol. 1967 Feb;14(2):173–186. doi: 10.1016/0022-5193(67)90112-9. [DOI] [PubMed] [Google Scholar]
- Dainese P., Bassi R. Subunit stoichiometry of the chloroplast photosystem II antenna system and aggregation state of the component chlorophyll a/b binding proteins. J Biol Chem. 1991 May 5;266(13):8136–8142. [PubMed] [Google Scholar]
- Delepelaire P., Bennoun P. Energy transfer and site of energy trapping in photosystem I. Biochim Biophys Acta. 1978 May 10;502(2):183–187. doi: 10.1016/0005-2728(78)90040-3. [DOI] [PubMed] [Google Scholar]
- Falkowski P. G., Kolber Z., Mauzerall D. A comment on the call to throw away your fluorescence induction apparatus. Biophys J. 1994 Mar;66(3 Pt 1):923–926. doi: 10.1016/s0006-3495(94)80868-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groot M. L., Peterman E. J., van Kan P. J., van Stokkum I. H., Dekker J. P., van Grondelle R. Temperature-dependent triplet and fluorescence quantum yields of the photosystem II reaction center described in a thermodynamic model. Biophys J. 1994 Jul;67(1):318–330. doi: 10.1016/S0006-3495(94)80483-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haberkorn R., Michel-Beyerle M. E. On the mechanism of magnetic field effects in bacterial photosynthesis. Biophys J. 1979 Jun;26(3):489–498. doi: 10.1016/S0006-3495(79)85266-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoff A. J. Magnetic field effects on photosynthetic reactions. Q Rev Biophys. 1981 Nov;14(4):599–665. doi: 10.1017/s0033583500002481. [DOI] [PubMed] [Google Scholar]
- Holzwarth A. R. Is it time to throw away your apparatus for chlorophyll fluorescence induction? Biophys J. 1993 Apr;64(4):1280–1281. doi: 10.1016/S0006-3495(93)81461-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikegami I. Fluorescence changes related in the primary photochemical reaction in the P-700-enriched particles isolated from spinach chloroplasts. Biochim Biophys Acta. 1976 Nov 9;449(2):245–258. doi: 10.1016/0005-2728(76)90137-7. [DOI] [PubMed] [Google Scholar]
- JOLIOT A., JOLIOT P. ETUDE CIN'ETIQUE DE LA R'EACTION PHOTOCHIMIQUE LIB'ERANT L'OXYG'ENE AU COURS DE LA PHOTOSYNTH'ESE. C R Hebd Seances Acad Sci. 1964 May 4;258:4622–4625. [PubMed] [Google Scholar]
- Joliot P., Bennoun P., Joliot A. New evidence supporting energy transfer between photosynthetic units. Biochim Biophys Acta. 1973 May 30;305(2):317–328. doi: 10.1016/0005-2728(73)90179-5. [DOI] [PubMed] [Google Scholar]
- Joliot P., Joliot A., Kok B. Analysis of the interactions between the two photosystems in isolated chloroplasts. Biochim Biophys Acta. 1968 Apr 2;153(3):635–652. doi: 10.1016/0005-2728(68)90191-6. [DOI] [PubMed] [Google Scholar]
- Kitajima M., Butler W. L. Quenching of chlorophyll fluorescence and primary photochemistry in chloroplasts by dibromothymoquinone. Biochim Biophys Acta. 1975 Jan 31;376(1):105–115. doi: 10.1016/0005-2728(75)90209-1. [DOI] [PubMed] [Google Scholar]
- Laible P. D., Zipfel W., Owens T. G. Excited state dynamics in chlorophyll-based antennae: the role of transfer equilibrium. Biophys J. 1994 Mar;66(3 Pt 1):844–860. doi: 10.1016/s0006-3495(94)80861-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malkin S., Kok B. Fluorescence induction studies in isolated chloroplasts. I. Number of components involved in the reaction and quantum yields. Biochim Biophys Acta. 1966 Nov 8;126(3):413–432. doi: 10.1016/0926-6585(66)90001-x. [DOI] [PubMed] [Google Scholar]
- Melis A., Homann P. H. Heterogeneity of the photochemical centers in system II of chloroplasts. Photochem Photobiol. 1976 May;23(5):343–350. doi: 10.1111/j.1751-1097.1976.tb07259.x. [DOI] [PubMed] [Google Scholar]
- Moya I. Durée de vie et rendement de fluorescence de la chlorophylle in vivo. Leur relation dans différents modèreles d'unités photosynthetiques. Biochim Biophys Acta. 1974 Nov 19;368(2):214–227. doi: 10.1016/0005-2728(74)90150-9. [DOI] [PubMed] [Google Scholar]
- Murata N., Nishimura M., Takamiya A. Fluorescene of chlorophyll in photosynthetic systems. II. Induction of fluorescence in isolated spinach chloroplasts. Biochim Biophys Acta. 1966 May 12;120(1):23–33. doi: 10.1016/0926-6585(66)90273-1. [DOI] [PubMed] [Google Scholar]
- Paillotin G. Capture frequency of excitations and energy transfer between photosynthetic units in the photosystem II. J Theor Biol. 1976 May 7;58(1):219–235. doi: 10.1016/0022-5193(76)90149-1. [DOI] [PubMed] [Google Scholar]
- Paillotin G., Geacintov N. E., Breton J. A master equation theory of fluorescence induction, photochemical yield, and singlet-triplet exciton quenching in photosynthetic systems. Biophys J. 1983 Oct;44(1):65–77. doi: 10.1016/S0006-3495(83)84278-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peloquin J. M., Williams J. C., Lin X., Alden R. G., Taguchi A. K., Allen J. P., Woodbury N. W. Time-dependent thermodynamics during early electron transfer in reaction centers from Rhodobacter sphaeroides. Biochemistry. 1994 Jul 5;33(26):8089–8100. doi: 10.1021/bi00192a014. [DOI] [PubMed] [Google Scholar]
- Rademaker H., Hoff A. J., Duysens L. N. Magnetic field-induced increase of the yield of (bacterio)chlorophyll emission of some photosynthetic bacteria and of Chlorella vulgaris. Biochim Biophys Acta. 1979 May 9;546(2):248–255. doi: 10.1016/0005-2728(79)90043-4. [DOI] [PubMed] [Google Scholar]
- Roelofs T. A., Lee C. H., Holzwarth A. R. Global target analysis of picosecond chlorophyll fluorescence kinetics from pea chloroplasts: A new approach to the characterization of the primary processes in photosystem II alpha- and beta-units. Biophys J. 1992 May;61(5):1147–1163. doi: 10.1016/s0006-3495(92)81924-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schatz G. H., Brock H., Holzwarth A. R. Kinetic and Energetic Model for the Primary Processes in Photosystem II. Biophys J. 1988 Sep;54(3):397–405. doi: 10.1016/S0006-3495(88)82973-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonneveld A., Rademaker H., Duysens L. N. Chlorophyll a fluorescence as a monitor of nanosecond reduction of the photooxidized primary donor P-680 Of photosystem II. Biochim Biophys Acta. 1979 Dec 6;548(3):536–551. doi: 10.1016/0005-2728(79)90063-x. [DOI] [PubMed] [Google Scholar]
- VREDENBERG W. J., DUYSENS L. N. Transfer of energy from bacteriochlorophyll to a reaction centre during bacterial photosynthesis. Nature. 1963 Jan 26;197:355–357. doi: 10.1038/197355a0. [DOI] [PubMed] [Google Scholar]
- Volk M., Gilbert M., Rousseau G., Richter M., Ogrodnik A., Michel-Beyerle M. E. Similarity of primary radical pair recombination in photosystem II and bacterial reaction centers. FEBS Lett. 1993 Dec 27;336(2):357–362. doi: 10.1016/0014-5793(93)80837-k. [DOI] [PubMed] [Google Scholar]
- Wolff C., Witt H. T. On metastable states of carotenoids in primary events of photosynthesis. Registration by repetitive ultra-short-flash photometry. Z Naturforsch B. 1969 Aug;24(8):1031–1037. doi: 10.1515/znb-1969-0818. [DOI] [PubMed] [Google Scholar]
- Woodbury N. W., Parson W. W. Nanosecond fluorescence from isolated photosynthetic reaction centers of Rhodopseudomonas sphaeroides. Biochim Biophys Acta. 1984 Nov 26;767(2):345–361. doi: 10.1016/0005-2728(84)90205-6. [DOI] [PubMed] [Google Scholar]
- Woodbury N. W., Peloquin J. M., Alden R. G., Lin X., Lin S., Taguchi A. K., Williams J. C., Allen J. P. Relationship between thermodynamics and mechanism during photoinduced charge separation in reaction centers from Rhodobacter sphaeroides. Biochemistry. 1994 Jul 5;33(26):8101–8112. doi: 10.1021/bi00192a015. [DOI] [PubMed] [Google Scholar]
- Xiao W., Lin S., Taguchi A. K., Woodbury N. W. Femtosecond pump-probe analysis of energy and electron transfer in photosynthetic membranes of Rhodobacter capsulatus. Biochemistry. 1994 Jul 12;33(27):8313–8322. doi: 10.1021/bi00193a019. [DOI] [PubMed] [Google Scholar]


