Abstract
In visual masking, visible targets are rendered invisible by modifying the context in which they are presented, but not by modifying the targets themselves. Here, we localize the neuronal correlates of visual awareness in the human brain by using visual masking illusions. We compare monoptic visual masking activation, which we find within all retinotopic visual areas, with dichoptic masking activation, which we find only in those retinotopic areas downstream of V2. Because monoptic and dichoptic masking are equivalent in magnitude perceptually, the present results establish a lower bound for maintenance of visual awareness of simple unattended targets. Moreover, we find that awareness-correlated circuits for simple targets are restricted to the occipital lobe. This finding provides evidence of an upper boundary in the visual hierarchy for visual awareness of simple unattended targets, thus constraining the location of circuits that maintain the visibility of simple targets to occipital areas beyond V1/V2.
Keywords: binocular rivalry, consciousness, feedback, functional MRI, standing wave
The search for the neural correlates of consciousness requires the localization of circuits in the brain that are sufficient to maintain awareness. To this end, brain areas have been sought within the ascending visual hierarchy that correlate, or more importantly, fail to correlate, with visual perception (1-17). In visual masking illusions, a target is rendered invisible by the action of a mask that is either in the same eye as the target (monoptic) or in the opposite eye from the target (dichoptic). Dichoptic and monoptic masking are mediated by different circuits in the visual hierarchy in monkeys; dichoptic masking is first generated by the binocular circuits of the cortex, whereas monoptic masking is generated by subcortical as well as cortical circuits (16). Moreover, the strength of visual masking (both monoptic and dichoptic) builds up iteratively within the early visual system, so that it is possible for neurons of a given area to process both dichoptic and monoptic masking, but to different degrees (16). Masking illusions, and in particular the standing wave version of masking we used here, evoke reliable blood oxygen level-dependent (BOLD) signals that correlate with perception within human visual cortex (18, 19). Because the psychophysical strengths of monoptic and dichoptic masking are equivalent (16, 20), we can find the point in the ascending visual hierarchy in which monoptic and dichoptic masking activity are both extant and thus determine the first point in the visual hierarchy at which awareness of visibility could potentially be maintained. Previous to this level, target responses will not be well inhibited during dichoptic masking: if these prior areas were sufficient to maintain visual awareness, the target would be perceptually visible during dichoptic masking conditions.
Here, we mapped the retinotopic visual areas with functional MRI (fMRI) in human subjects, and measured the BOLD signal in response to monoptic and dichoptic visual masking within each subject's individually mapped retinotopic areas. Our results show that dichoptic masking does not correlate with visual awareness in area V1 but begins only downstream of area V2, within areas V3, V3A/B, V4, and later. These results agree with previous electrophysiological results in monkeys using both visual masking and binocular rivalry stimuli (4, 5, 16), as well as with one fMRI study of binocular rivalry in humans (17).
Having determined the lower boundary in the visual hierarchy for the perception of visual masking of simple targets, we set out to determine whether there was also an upper boundary. We isolated the parts of the brain that showed both an increase in BOLD signal when nonillusory visible targets were displayed, as well as a decrease in BOLD signal when the same targets were rendered less visible by visual masking. Surprisingly, only areas within the occipital lobe showed differential activation between visible and invisible targets. Thus, the combined results of our experiments suggest that visual areas beyond V2, but within the occipital lobe, are sufficient to maintain our awareness of simple targets.
Materials and Methods
Psychophysics. Subjects (n = 5) were given a brightness matching task while in the magnet. The three target conditions [Target-Only, SWI (a visual masking illusion called “The Standing Wave of Invisibility”), and Control] were presented on the left half of the screen, whereas a luminance-adjustable Target-Only stimulus was presented on the right half of the screen. The bars along the vertical meridian were removed so that the left and right stimulus sets would not potentially mask each other. Each subject's task was to adjust the luminance of the adjustable Target-Only side to match the appearance of the target in the left half of the screen. Stimuli were black (lowest possible luminance from the display system) on a white background (maximal luminance). To generate the Target Visibility Rating, subject luminance ratings were inverted and normalized to between 0 and 1, where 1 was the highest possible visibility of a black target on the display system and 0 was the lowest possible visibility.
fMRI. Seventeen healthy right-handed volunteers (of both genders between the ages of 18 and 40) were used for the monoptic study, and 14 of these returned for the dichoptic study, which was carried out on a different day. All had normal depth perception and normal or corrected-to-normal visual acuity. Subjects were paid $20 per session. Most subjects were scanned on 3 separate days, with one session each day: monoptic study, retinotopic mapping, and dichoptic study. To be clear: “monocular” means “with respect to one eye,” “monoptic” means “monocular” or “same in both eyes,” “binocular” means “with respect to both eyes,” and “dichoptic” means “different in the two eyes.” All of our stimuli were binocular, but, in monoptic conditions, both the target and mask were presented to both eyes whereas, in the dichoptic conditions, the target was presented to one eye and the mask was presented to the other. Dichoptic presentation was accomplished by using red-cyan glasses. Targets and masks were presented in cyan or red, which made them appear black in the eye with the opposite color filter and made them match the background (i.e., disappear) in the eye with the same color filter.
A continuous whole-brain BOLD signal was acquired at the Dartmouth Brain Imaging Center on a GE 1.5-T Signa scanner by using a head coil. Different biological tissues have different NMR relaxation times. These differences can be exploited to provide image contrast that is derived from differences in T1 (spin-lattice relaxation time), T2 (spin-spin relaxation time), or T2* (relaxation time in the presence of local magnetic field inhomogeneities). These are known as T1-weighted, T2-weighted, and T2*-weighted images (40). We collected standard T2*-weighted echoplanar functional images using 25 slices [4.5-mm thickness and 3.75 × 3.75-mm in-plane voxel resolution, inter-slice distance 1 mm, repetition time (TR) = 2,500 ms, flip angle = 90°, field of view = 240 × 240 × 256 mm, interleaved slice acquisition, matrix size = 64 × 64] oriented approximately along the anterior-commissure posterior-commissure plane. These slices were sufficient to encompass the entire brain of each subject. Cushions were used to minimize head motion. A T1-weighted anatomical image with the same slice orientation as the echo-planar imaging (EPI) was collected for each subject, as was T2-weighted high-resolution anatomical scan.
Stimuli were projected onto a Plexiglas screen outside the bore of the magnet and viewed by means of a tangent mirror inside the magnet that permitted a maximum of 22° × 16° visible area. The projected image was smaller than this area and subtended ≈17° × 12°. The experiment had a block design with 11 (5 condition and 6 fixation) 20-s blocks. Each run began with 10 s of dummy scans (four volumes that were discarded) to bring spins to baseline. Each run thus lasted a total of 230 s. Condition order was randomized on each run. Subjects carried out a minimum of 10 runs each and a maximum of 15. The first and last blocks were always fixation-only, and condition blocks were always separated by a fixation-only block. An entire cortical volume was scanned eight times per 20-s block (each block consisted of 40 cycles of the stimulus, duty cycle = 500 ms). Each 500-ms trial was cycled 40 times (20 s per block). In the monoptic sessions, each run of four blocks (presented in random order with interdigitated 20-s blank periods) lasted 3 min. In the dichoptic sessions, the total number of conditions was six (target and mask were each presented to the two eyes separately, doubling the conditions). Each run of six blocks (presented in random order with interdigitated 20-s blank periods) lasted 3 min after spins were brought to baseline.
We controlled for eye movements, wakefulness, and attention to the fovea by requiring subjects to perform a demanding reaction-time task in which the subject had to respond, within 500 ms (by means of button press), to a randomly occurring and slight change in fixation point color. The fixation point was 0.2° × 0.2° and changed color on average about once every 1.5 s. This color change took place an equal number of times during each block. No motor areas were found to be activated differentially between conditions, corroborating that the motor task was equivalent across all conditions. This task could be carried out successfully only if the subject was fixating during both condition and fixation-only blocks and attending to the fixation point carefully. Subject performance was 92.5% correct or better during each run or the run was not analyzed further. Thus, subjects were permitted an average of only one miss or delayed response per block.
Data were analyzed offline by using brain voyager (bv) 4.9.6 and matlab software developed in-house. Effects of small head movements were removed by using the motion correction algorithm in bv. Slice scan time correction was carried out to correct for the fact that slices were not collected at the same time. Slices were corrected to have the same mean intensity. Functional data were not smoothed in the space domain, but low-frequency temporal fluctuations were removed through high-pass filtering by convolving a one-dimensional Gaussian kernel with the time course on a voxel-by-voxel basis. This filter did not introduce correlations between a voxel and its neighbors.
Retinotopy was carried out by using standard phase-encoding techniques (21) (4.5-mm thickness and 3.75 × 3.75 mm in-plane voxel resolution, inter-slice distance 1 mm, TR = 1,600 ms, flip angle = 90°, field of view = 240 × 240 × 256 mm, interleaved slice acquisition, matrix size = 64 × 64; 16 slices oriented along the calcarine sulcus), with the modification that two wedges of an 8-Hz flicker black and white checkerboard grating were bilaterally opposite like a bowtie, to enhance signal to noise. Wedges occupied a given location for 2 TRs (3.2 s) before moving to the adjacent location in a clockwise fashion. Each wedge subtended 18° of 360°. An amount equal to 9.6 s (6 TRs of dummy scans) was discarded before each run to bring spins to baseline. On each run, 168 volumes were collected. A minimum of seven wedge runs was collected for each subject and then averaged to minimize noise before retinotopic data analysis in bv 4.9.6. A minimum of three runs was collected per subject by using expanding 8-Hz flickering concentric rings that each spanned ≈1° of visual angle in ring width. Each ring was updated after one TR (1.6 s), after which it was replaced by its outward neighbor, except that the outermost ring was replaced by the innermost ring, whereupon the cycle was repeated.
Results
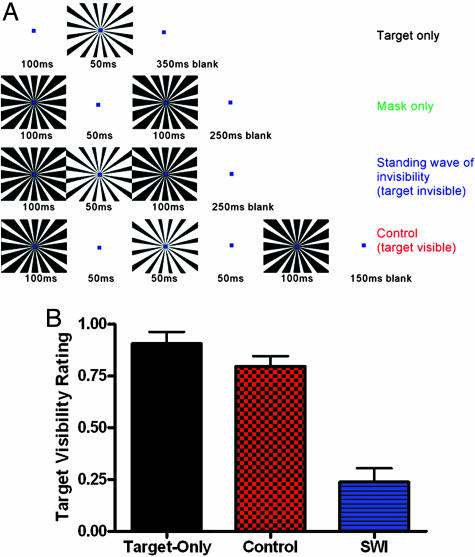
Psychophysical Analysis of Target Visibility. We presented a visual masking illusion called SWI (16, 22, 24, 25). The SWI is a combination of forward and backward masking in which the target and the mask abut but do not overlap each other spatially or temporally. Fig. 1A describes the temporal sequence during a single monoptic SWI trial, as well as the sequences from the three other conditions tested. The SWI configuration used here optimized the retinal coverage of both the target and mask stimuli to evoke the greatest possible BOLD signal. Monoptic fMRI sessions were also presented with a control condition (shown in Fig. 1A, for a total of four monoptic conditions) in which the timing of the standing wave was altered so that the targets were visible. Because the perceptual strength of monoptic masking is equivalent to that of dichoptic masking (16, 20), psychophysical testing of the new SWI configuration was conducted with monoptic stimuli only.
Fig. 1.
Stimulus layout and psychophysical results. (A) Single-trial examples of the four stimulus conditions (the layout of the stimuli varied slightly in the psychophysical experiments as described in Materials and Methods). (B) Psychophysical target visibility rating in the three target conditions.
Fig. 1B shows the perceptual visibility of the target under the various target conditions. The results quantify the experience of the subjects: The targets in the Target-Only and Control conditions were highly visible although the target in the SWI condition was low in visibility (one-way ANOVA, F = 37.62, R2 = 0.8625, P < 0.0001).
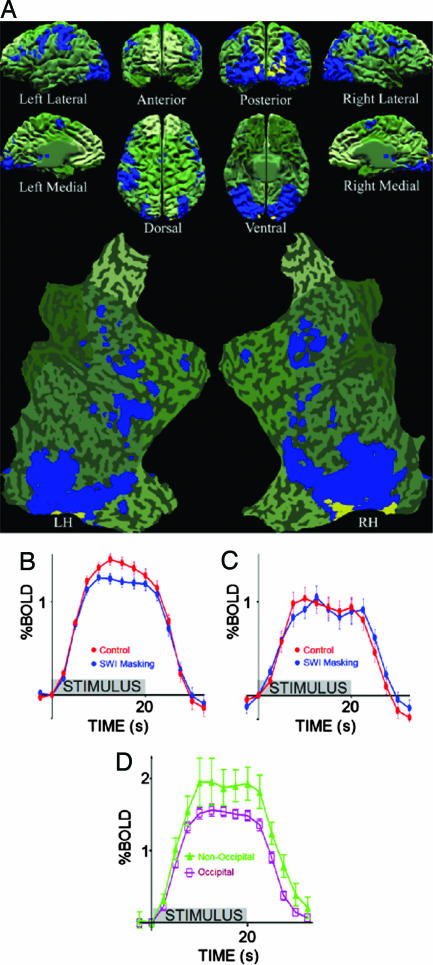
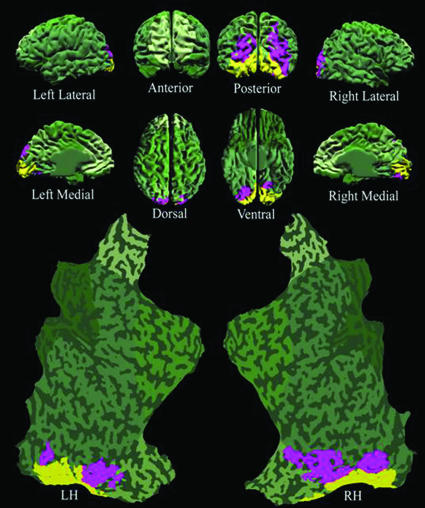
Upper Boundary for Masking of Simple Targets. Fig. 2A shows significant functional activation to the Target-Only condition (blue and yellow voxels; P < 0.05 corrected, random effects analysis), after subtracting the voxels that responded significantly in the Mask-Only condition (P < 0.05 corrected, random effects analysis). This region of interest (ROI) defines the subset of cortical voxels that responded well to the target but not to the mask. Voxels that constitute circuits responsible for the visibility of targets presumably lie within this target-responsive ROI. These visibility-correlated voxels should moreover generate greater BOLD response to target-visible rather than to target-invisible conditions. We measured the responses of the two orthogonal conditions: Control (target-visible) and SWI (target-invisible) to determine the voxels that could potentially contain the neural correlates of target visibility (yellow, P < 0.01 uncorrected, random effects analysis). We found that these target visibility-correlated (yellow) voxels overlapped a subset of the target-responsive (blue) voxels. In this initial comparison, we used uncorrected statistics to liberally specify all possible regions that could potentially correlate with target visibility, including those regions that may show spurious correlations. Despite our use of liberal thresholds, only the occipital cortex produced activation. This result suggested that the circuits that maintain the awareness of target visibility may be confined to the occipital cortex. We ascertained the statistical significance of the occipital localization by comparing the average Control (target-visible) BOLD response with the average SWI (target-invisible) BOLD response in target-responsive ROI voxels within and outside the occipital cortex. The difference between Control and SWI conditions within occipital ROI voxels was averaged, and statistical significance was verified (Fig. 2B; Wilcoxon signed rank paired two-tailed t test, P = 0.0039 corrected, random effects analysis). We tested nonoccipital ROI voxels with the same analysis (Fig. 2C) and found no significant difference.
Fig. 2.
Localization of visibility-correlated responses to the occipital lobe. (A) An individual brain model from all perspectives, including both hemispheres flat-mapped, overlaid with the functional activation from 17 subjects. The green shaded areas are those portions of the brain that did not show significant activation to Target-Only stimuli. The blue voxels exhibited significant target activation (Target-Only activation > Mask-Only activation). Yellow voxels indicate a significant difference found between Control (target-visible) and SWI (target-invisible) conditions, indicating potentially effective visual masking, and thus a correlation with perceived visibility. (B) Response time-course plots from Control versus SWI conditions in the occipital cortex (occipital masking). (C) Response time-course plots from Control versus SWI conditions in nonoccipital cortex (nonoccipital masking). (D) Response time-course plots from the nonillusory conditions (Target-Only and Mask-Only combined) in occipital versus nonoccipital cortex. (Error bars: (B, C, and D) SEM between subjects.)
Although significant target responses were found in virtually all visual areas of the cortex (Fig. 2A, blue and yellow), we found visibility-related effects only in the occipital cortex (Fig. 2A, yellow). These results suggest that awareness of simple unattended targets, such as those used in the SWI, is generated by circuits within the occipital cortex.
One alternative interpretation of this result is that, whereas there may be visibility-related activity in the occipital lobe, the absence of visibility-related activity in nonoccipital areas may be due to regional cerebral blood flow differences, which would constitute a null-result. We addressed this possibility by comparing the occipital versus nonoccipital BOLD responses with the combined nonillusory conditions (Target-Only and Mask-Only). Occipital BOLD responses to nonillusory stimuli (Fig. 2D, purple) were weaker than nonoccipital BOLD responses (Fig. 2D, green; Wilcoxon signed rank paired two-tailed t test, P = 0.0001 corrected, random effects analysis). Therefore, rather than having misinterpreted a null-result, we have instead underestimated the significance of our occipital localization findings.
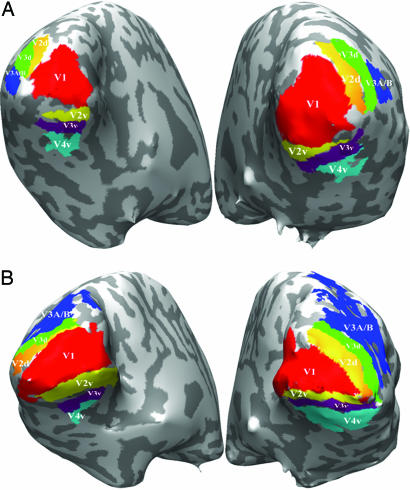
Retinotopic Analysis. To study the visual hierarchy within the occipital lobe further, we used retinotopic analysis of the occipital visual areas (21), and delineated the ROIs for areas V1, V2v, V2d, V3v, V3d, V3A/B, and V4v in each of the subjects tested. Fig. 3 shows the retinotopy results from two subjects.
Fig. 3.
Examples of retinotopy mapping from two subjects. Visual areas that have been delineated by retinotopic mapping analysis are indicated in different colors. (A) Subject 1. (B) Subject 2.
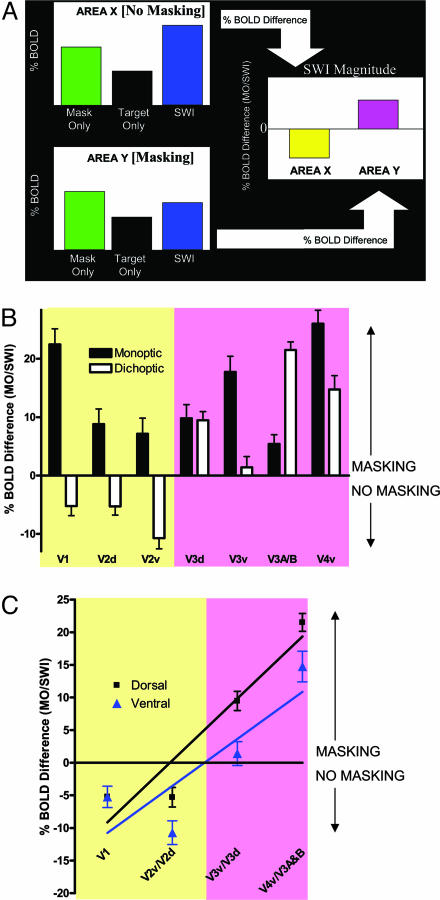
Lower Boundary for Masking of Simple Targets. Within each retinotopic area (averaged across hemispheres), we determined the average BOLD response for the Target-Only, Mask-Only, and SWI conditions (monoptic and dichoptic presentations) across subjects. To determine the amount of masking present in each area, we calculated the percentage difference of the SWI BOLD response as a function of the Mask-Only BOLD response (Fig. 4A). If the difference was <0, the target response added to the mask response. In other words, the target response was not suppressed during the SWI condition, but rather the target added to the overall BOLD response. If the increase was ≥0, the target response did not add to the mask response in the SWI condition: the target response was suppressed and therefore correlated with the perception of masking. The mask response may have been partially suppressed in the SWI condition as well [due to reciprocal masking by the target, as is commonly seen in primate physiology with this illusion (16, 24)].
Fig. 4.
Retinotopic analysis of monoptic versus dichoptic masking. (A) The logic underlying the analysis of masking magnitude for hypothetical retinotopic areas. The Mask-Only response is bigger than the Target-Only response because masks subtend a larger retinotopic angle than targets and are moreover presented twice in each cycle for 100 ms each flash, whereas the target is single-flashed for only 50 ms. If the target response adds to the mask response in the SWI condition (no-masking percept), then the SWI response will be bigger than the Mask-Only response, whereas if the target does not add (masking percept), then the SWI response will be equal to or smaller than (as the mask itself may also be reciprocally inhibited by the target) the Mask-Only response. (B) Monoptic and dichoptic masking magnitude (%BOLD difference of Mask-Only/SWI conditions) as a function of occipital retinotopic brain area, following the analysis described in A. Negative values indicate increased activation to the SWI condition (no masking), whereas values ≥0 indicate decreased or unchanged SWI activation (masking). (C) Dichoptic masking magnitude (%BOLD difference of Mask-Only/SWI conditions) as a function of occipital retinotopic brain area within the dorsal and ventral processing streams. The strength of dichoptic masking builds up as a function of level in the visual hierarchy for both the dorsal (R2 = 0.90) and ventral (R2 = 0.72) processing streams.
All of the retinotopic occipital visual areas exhibited target visibility-correlated responses when the stimuli were presented monoptically (Fig. 4B). This result agrees with and follows from previous electrophysiological data from the awake primate, showing monoptic suppression of the masked target in the lateral geniculate nucleus (LGN) and area V1, presumably due to the action of lateral inhibitory networks (16, 24, 26). As in the previous primate results, dichoptic SWI masking responses in humans were not found in V1 (16) or in dorsal or ventral V2 (V2d and V2v), but only in subsequent retinotopic areas of the visual hierarchy (Fig. 4 B and C). Because dichoptic and monoptic visual masking are perceptually equivalent in magnitude (16, 20), the current data suggest that dichoptic BOLD responses could first correlate with the perception of simple targets in areas V3d, V3v, V3A/B, and V4v.
Further analysis of the strength of dichoptic visual masking in the dorsal and ventral visual streams suggests that dichoptic visual masking builds up over successive visual areas in the hierarchy (Fig. 4C; dorsal, R2 = 0.90 and ventral, R2 = 0.72). The dorsal stream exhibited a slightly higher %BOLD increase as a function of stage within the visual hierarchy (F(1, 3) = 17.16, P < 0.0001). These results also support the previous finding from monkey single-unit recordings that, whereas monoptic inhibition of the target begins to build up subcortically and achieves full strength in the binocular neurons of V1, dichoptic inhibition of the target does not begin until area V1 and therefore achieves full strength only after several iterations of inhibitory build-up within the extrastriate visual hierarchy (16).
Fig. 5 shows the layout of visual areas (downstream of areas V1 and V2) that could potentially maintain visual awareness of simple unattended targets (pink, matching shading in Fig. 4) in a single representative subject. V1 and V2 are colored in yellow (matching shading in Fig. 4).
Fig. 5.
Layout of retinotopic areas that potentially maintain awareness of simple targets. An individual brain model from all perspectives, including both hemispheres flat-mapped, overlaid with the functional activation from one typical subject. The yellow shaded areas are those portions of the brain that did not show significant dichoptic masking and thus are ruled out for maintaining visual awareness of simple targets (as in Fig. 4 B and C). The pink-colored voxels represent the cortical areas that exhibited significant dichoptic masking and thus are potential candidates for maintaining awareness of simple targets.
Discussion
Implications for Binocular Rivalry as a Tool to Study Visual Awareness. Binocular rivalry has been used as a tool to assess the neuronal correlates of visibility but has generated controversy because of conflicting results. Some fMRI studies on humans report that BOLD activity in V1 correlates with visual awareness of binocular rivalry percepts (11, 12). In contrast, other human fMRI studies (17), and single-unit recording studies in primates (5, 27), suggest that activity in area V1 does not correlate with visual awareness. One possible reason for this discrepancy is that none of the binocular rivalry studies have determined that the visual areas tested contained the interocular suppression circuits necessary to mediate binocular rivalry. That is, when binocular rivalry is not found in a given area, there is no extant method to control for the possibility that the circuits being studied simply do not have the components necessary to cause interocular suppression, and thus binocular rivalry. A more common conclusion is that the area cannot maintain visual awareness. However, if interocular suppression circuits do not exist in that area, then this conclusion would be as inappropriate as concluding that V1 does not maintain awareness because it does not differentiate between houses and faces. Even when an indicator of neural activity in a given area is found to correlate with perceptual state during binocular rivalry, no existing study has determined whether the interocular suppression observed in that area is weak or strong. Until a control stimulus can be developed to indicate the strength of interocular suppression in any given area, unambiguous interpretation of the neural correlates of perceptual state during binocular rivalry will not be possible, no matter how high in the visual hierarchy. Thus, by using binocular rivalry stimuli alone, it is currently not possible to localize awareness circuits within the visual hierarchy.
In the present study, we measured the BOLD activity underlying visual awareness during monoptic and dichoptic masking in humans. With masking, one can compare the perceptual and neural strengths of both monoptic and dichoptic target suppression. This comparison supplies a means to quantify the strength of target suppression and also to determine whether a weak suppression of the target may be due to the weakness of interocular suppression in the circuit tested, or to the lack of masking components within the circuit.
Implications for Lateral Inhibition Models of Visual Masking. Macknik and Martinez-Conde (16) previously recorded the responses of single neurons in the lateral geniculate nucleus (LGN) and primary visual cortex (V1) of awake monkeys to monoptic and dichoptic forms of the SWI illusion, and found that neither LGN nor V1 responses correlated with perception of dichoptic targets. To investigate the role of specific areas within the human occipital lobe in maintaining awareness, the present study mapped the retinotopic regions of the occipital lobe of each subject and then presented the SWI illusion monoptically and dichoptically to compare the responses in the retinotopic visual areas. Because monoptic and dichoptic visual masking are perceptually equivalent in magnitude (16, 20), only those areas with equivalent BOLD activity in monoptic and dichoptic masking conditions can potentially maintain visual awareness. Our results show that target suppression during monoptic masking is strong in all retinotopic occipital areas, but dichoptic masking builds up within the visual cortex and becomes strong only downstream of both dorsal and ventral V2 (i.e., within areas V3v, V3d, V3A/B, and V4v). These findings match previous primate single-unit recording results for area V1 (16), suggesting that area V1 plays a similar role in monkey and human visual awareness.
The ubiquitous strength of monoptic masking in the occipital retinotopic areas and the build-up of dichoptic masking strength in the extrastriate visual hierarchy are both predicted by the previous discovery that lateral inhibition builds up iteratively within the early visual system (16, 28). Unlike monoptic lateral inhibition, which begins in the retina and builds up to full strength in the striate cortex, lateral inhibition between the eyes (interocular suppression) begins to develop only in the binocular circuits of the striate cortex, and we show here that it does not reach full strength until the areas V3A/B and V4v (which may be hierarchically equivalent levels in the respective dorsal and ventral visual processing streams). We suggest that there are both lower and upper bounds within the visual hierarchy for the processing of visual masking and the maintenance of visual awareness of simple unattended targets; the lower bound is at least as high as the border between V2 and V3, and the upper bound is within the occipital lobe, possibly somewhere downstream of V4.
The dichoptic masking results are consistent with previous conclusions drawn from recordings in primates using binocular rivalry stimuli (27), in which perception-correlated processing was concluded to begin in the visual hierarchy as late as area V4. A later fMRI study conducted with motion-based rivalry in humans furthermore supports the idea that motion-based rivalry could not be maintained until human area V3A (17). Therefore, our current results, which suggest that visual awareness may be processed as early as human V3 and V4, are consistent with previous primate results and some of the previous human results found with binocular rivalry stimuli.
Implications for Occipital vs. Nonoccipital Models of Visual Awareness. We found differing ratios of monoptic vs. dichoptic masking in occipital areas downstream of V2 (Fig. 3B), which may indicate that these areas do not maintain awareness of simple unattended targets, because dichoptic and monoptic masking are equivalent in strength psychophysically (16, 20). It is thus possible that the activation strength of monoptic versus dichoptic masking fully equilibrates only downstream of the retinotopic areas studied here, albeit somewhere within the occipital lobe. Nevertheless, the current data indicate that areas V1 and V2 fail to correlate with the perception of dichoptic masking, even though they contain circuits capable of processing visual masking (i.e., monoptically). These areas can therefore be ruled out as sufficient to maintain visual awareness, whether by themselves, or in concert with earlier subcortical areas. Our results thus suggest that visual masking of simple targets can be explained through occipital models, rather than with models of masking that require feedback or nonoccipital circuits (13, 25, 29, 30).
Care should be taken not to generalize these results to claims about the neural correlates of awareness of objects more complex than the simple targets used here. For instance, it is possible that complex visual stimuli such as faces and hands [known to be physiologically processed outside the occipital lobe (31, 32)] require activity in visual areas downstream of the occipital lobe to maintain awareness. Similarly, circuits that maintain the awareness of other types of visual processes, such as motion perception, may lie outside the occipital cortex (33). For example, Dehaene et al. (14) found neural correlates of word-priming masking outside of the occipital lobe. It is also possible that attended stimuli, including simple targets, may also invoke extraoccipital activation in a task-dependent manner (34).
Attentional Feedback and the Role of Area V1 in Human Visual Awareness. Haynes, Driver, and Rees (18, 19) have recently published two articles showing the BOLD correlates of monoptic masking in the cortex. Our study is in agreement with one of these studies, which concluded that human area V1 alone cannot maintain awareness of stimulus orientation (19). However, our results do not agree with the second study (18), which suggested that target visibility is derived by the coupling of area V1 BOLD activity with fusiform gyrus BOLD activity. The reason for this discrepancy may lie in a potential confound of this second study: the V1 activation found by Haynes et al. (18) may not indicate target visibility, but rather top-down attentional feedback. This possibility is suggested by their use of a behavioral task that demanded active attention to the target during scanning, which is known to cause increased BOLD activity in human V1 (35). Several other fMRI studies concluding that V1 maintains awareness may also be explained by uncontrolled top-down attentional feedback (11, 12, 36). Our task, on the contrary, demanded attention to the fixation point only, precluding effects of attention to the target. Lee et al. (12) found that peripheral binocularly rivalrous traveling waves could be tracked in V1 by using fMRI and concluded that V1 must thus contribute to the maintenance of awareness. However, they also found that, when the subject was attending to a different task at the fixation point, the BOLD signal from the traveling waves decreased. This finding suggests that the traveling wave BOLD signal seen by the authors was due to attentional feedback and is thus equivocal concerning a role for V1 in awareness.
Implications for Theories of Consciousness. The present results may lend support for some theories of consciousness that are modular in form and are sustainable with intermediate level processes (23, 37-39).
Acknowledgments
We thank Frank Tong for help with the retinotopic mapping analyses, Michael Beaton and Fernando Valle-Inclan for their valuable insights and discussion, and Shannon Bentz for technical assistance. This project was funded by National Institutes of Health Grant R03 MH0609660-01 (to P.U.T.), by a Dana Brain and Immuno-imaging grant (to S.M.-C.), and by Barrow Neurological Foundation grants (to S.M.-C. and S.L.M.).
Author contributions: P.U.T., S.M.-C., and S.L.M. designed research; P.U.T., S.M.-C., A.A.S., and S.L.M. performed research; P.U.T., S.M.-C., and S.L.M. contributed new reagents/analytic tools; P.U.T., S.M.-C., A.A.S., and S.L.M. analyzed data; and P.U.T., S.M.-C., and S.L.M. wrote the paper.
Conflict of interest statement: No conflicts declared.
Abbreviations: BOLD, blood oxygen level-dependent; fMRI, functional MRI; SWI, standing wave of invisibility; TR, repetition time; ROI, region of interest.
References
- 1.Crick, F. & Koch, C. (1990) Cold Spring Harbor Symp. Quant. Biol. 55, 953-962. [DOI] [PubMed] [Google Scholar]
- 2.Milner, A. D. (1995) Neuropsychologia 33, 1117-1130. [DOI] [PubMed] [Google Scholar]
- 3.He, S., Cavanagh, P. & Intriligator, J. (1996) Nature 383, 334-337. [DOI] [PubMed] [Google Scholar]
- 4.Logothetis, N. K., Leopold, D. A. & Sheinberg, D. L. (1996) Nature 380, 621-624. [DOI] [PubMed] [Google Scholar]
- 5.Sheinberg, D. L. & Logothetis, N. K. (1997) Proc. Natl. Acad. Sci. USA 94, 3408-3413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Farah, M. J. & Feinberg, T. E. (1997) Semin. Neurol. 17, 145-152. [DOI] [PubMed] [Google Scholar]
- 7.Tong, F., Nakayama, K., Vaughan, J. T. & Kanwisher, N. (1998) Neuron 21, 753-759. [DOI] [PubMed] [Google Scholar]
- 8.Zeki, S. & Ffytche, D. H. (1998) Brain 121, 25-45. [DOI] [PubMed] [Google Scholar]
- 9.Lee, S. H. & Blake, R. (1999) Vision Res. 39, 1447-1454. [DOI] [PubMed] [Google Scholar]
- 10.Lamme, V. A., Super, H., Landman, R., Roelfsema, P. R. & Spekreijse, H. (2000) Vision Res. 40, 1507-1521. [DOI] [PubMed] [Google Scholar]
- 11.Polonsky, A., Blake, R., Braun, J. & Heeger, D. J. (2000) Nat. Neurosci. 3, 1153-1159. [DOI] [PubMed] [Google Scholar]
- 12.Lee, S. H., Blake, R. & Heeger, D. J. (2005) Nat. Neurosci. 8, 22-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thompson, K. G. & Schall, J. D. (2000) Vision Res. 40, 1523-1538. [DOI] [PubMed] [Google Scholar]
- 14.Dehaene, S., Naccache, L., Cohen, L., Bihan, D. L., Mangin, J. F., Poline, J. B. & Riviere, D. (2001) Nat. Neurosci. 4, 752-758. [DOI] [PubMed] [Google Scholar]
- 15.Pascual-Leone, A. & Walsh, V. (2001) Science 292, 510-512. [DOI] [PubMed] [Google Scholar]
- 16.Macknik, S. L. & Martinez-Conde, S. (2004) J. Cognit. Neurosci. 16, 1-11. [DOI] [PubMed] [Google Scholar]
- 17.Moutoussis, K., Keliris, G., Kourtzi, Z. & Logothetis, N. (2005) Vision Res. 45, 2231-2243. [DOI] [PubMed] [Google Scholar]
- 18.Haynes, J. D., Driver, J. & Rees, G. (2005) Neuron 46, 811-821. [DOI] [PubMed] [Google Scholar]
- 19.Haynes, J. D. & Rees, G. (2005) Nat. Neurosci. 8, 686-691. [DOI] [PubMed] [Google Scholar]
- 20.Schiller, P. H. (1965) J. Exp. Psychol. 69, 193-199. [DOI] [PubMed] [Google Scholar]
- 21.Sereno, M. I., Dale, A. M., Reppas, J. B., Kwong, K. K., Belliveau, J. W., Brady, T. J., Rosen, B. R. & Tootell, R. B. (1995) Science 268, 889-893. [DOI] [PubMed] [Google Scholar]
- 22.Macknik, S. L. & Livingstone, M. S. (1998) Nat. Neurosci. 1, 144-149. [DOI] [PubMed] [Google Scholar]
- 23.Zeki, S. & Bartels, A. (1999) Conscious. Cogn. 8, 225-259. [DOI] [PubMed] [Google Scholar]
- 24.Macknik, S. L., Martinez-Conde, S. & Haglund, M. M. (2000) Proc. Natl. Acad. Sci. USA 97, 7556-7560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Enns, J. T. (2002) Psychon. Bull. Rev. 9, 489-496. [DOI] [PubMed] [Google Scholar]
- 26.Macknik, S. L. & Haglund, M. M. (1999) Proc. Natl. Acad. Sci. USA 96, 15208-15210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leopold, D. A. & Logothetis, N. K. (1996) Nature 379, 549-553. [DOI] [PubMed] [Google Scholar]
- 28.Hubel, D. H. & Wiesel, T. N. (1961) J. Physiol. (London) 155, 385-398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Enns, J. T. & Di Lollo, V. (1997) Psychol. Sci. 8, 135-139. [Google Scholar]
- 30.Thompson, K. G. & Schall, J., D. (1999) Nat. Neurosci. 2, 283-288. [DOI] [PubMed] [Google Scholar]
- 31.Desimone, R. & Gross, C. G. (1979) Brain Res. 178, 363-380. [DOI] [PubMed] [Google Scholar]
- 32.Desimone, R. (1991) J. Cognit. Neurosci. 3, 1-8. [DOI] [PubMed] [Google Scholar]
- 33.Williams, Z. M., Elfar, J. C., Eskandar, E. N., Toth, L. J. & Assad, J. A. (2003) Nat. Neurosci. 6, 616-623. [DOI] [PubMed] [Google Scholar]
- 34.Naccache, L., Blandin, E. & Dehaene, S. (2002) Psychol. Sci. 13, 416-424. [DOI] [PubMed] [Google Scholar]
- 35.Brefczynski, J. A. & DeYoe, E. A. (1999) Nat. Neurosci. 2, 370-374. [DOI] [PubMed] [Google Scholar]
- 36.Ress, D., Backus, B. T. & Heeger, D. J. (2000) Nat. Neurosci. 3, 940-945. [DOI] [PubMed] [Google Scholar]
- 37.Jackendoff, R. (1987) Cognition 26, 89-114. [DOI] [PubMed] [Google Scholar]
- 38.Crick, F. & Koch, C. (2000) in The Neuronal Correlates of Consciousness, ed. Metzinger, T. (MIT Press, Cambridge, MA), pp. 103-110.
- 39.Zeki, S. (2001) Annu. Rev. Neurosci. 24, 57-86. [DOI] [PubMed] [Google Scholar]
- 40.Jezzard, P., Matthews, P. M., Smith, S. M., eds. (2001) Functional MRI: An Introduction to Methods (Oxford Univ. Press, New York).