Abstract
We investigated the contribution of Candida albicans ALS1, which encodes a candidal adhesin, to the pathogenesis of experimental murine oropharyngeal candidiasis. Our results indicate that the ALS1 gene product is important for the adherence of the organism to the oral mucosa during the early stage of the infection.
Candida albicans causes both hematogenously disseminated and mucosal infections. The ability of C. albicans to adhere to various host constituents plays an important role in colonizing the host and then initiating and maintaining an infection. Several adherence genes have been characterized (6, 7, 12). The C. albicans ALS1 (agglutinin-like sequence) gene encodes an adhesin that mediates attachment to endothelial cells (4, 9). In this study, we used C. albicans mutants in which one or both alleles of ALS1 were disrupted to investigate the role of Als1p in adherence to epithelial cells during experimental murine oropharyngeal candidiasis (OPC).
We used strains constructed from C. albicans CAI4 (3), as described previously (5), and SC5314, the wild-type parent strain of CAI4 (Table 1). The growth rates of all strains in vitro are similar (5).
TABLE 1.
Strains
| Strain | Designation | Genotype |
|---|---|---|
| SC5314 | Wild-type | |
| I-13 | ALS1/als1 | Δals1::hisG-URA3-hisG/ALS1 Δura3::imm434/Δura3::imm434 |
| 3-13-22 | als1/als1 | Δals1::hisG/Δals1::hisG-URA3-hisG Δura3::imm434/Δura3::imm434 |
| 13R11 | als1/als1+ ALS1 | Δals1::hisG/Δals1::hisG-ALS1-URA3-hisG Δura3::imm434/Δura3::imm434 |
Experimental OPC was induced as described previously (10). All animal experiments were carried out according to the guidelines of the Institutional Animal Care and Use Committee of Sankyo Co., Ltd. Specific pathogen-free male ddY mice (5 weeks old; Japan SLC, Inc., Shizuoka, Japan) were immunosuppressed with 4 mg of cortisone acetate administered subcutaneously on the day before and 1 day after inoculation. Mice received tetracycline hydrochloride (Achromycin V; Lederle Japan Ltd., Tokyo, Japan) in their drinking water (0.5 mg/ml), starting the day before inoculation. Before inoculation, mice were anesthetized by intraperitoneal injection with 26 μg of dimorpholamine (Theraptique; Eisai Co., Ltd., Tokyo, Japan), 207 μg of xylazine (Bayer Yakuhin Ltd., Osaka, Japan), and 1.04 mg of pentobarbital sodium (Nembutal; Dainippon Pharmaceutical Co., Ltd., Osaka, Japan), and cotton wool balls (3-mm diameter) containing 104 blastospores were placed sublingually in the oral cavity for 2 h.
To determine the viable counts in the oral tissue, each mouse was sacrificed and the mandibular soft tissue, including the tongue, was dissected free of the teeth and bone. The excised tissue was homogenized in saline, after which serial dilutions were plated onto Sabouraud dextrose agar containing 10 μg of chloramphenicol/ml for colony counting. For histopathological study, the excised tissue was fixed with formalin and embedded in paraffin, after which thin sections were prepared and stained with periodic acid-Schiff (PAS) stain.
The adherence of C. albicans to the tongue ex vivo was determined as follows. Tongues were excised from sacrificed mice, added to each well of a 24-well culture plate containing 104 C. albicans cells in phosphate-buffered saline, and incubated at 35°C for 30 min with gentle rotation. Next, the tongues were washed three times with phosphate-buffered saline and homogenized in saline, and serial dilutions were plated onto Sabouraud dextrose agar containing chloramphenicol. The viable counts adhering to each tongue were determined by colony counting.
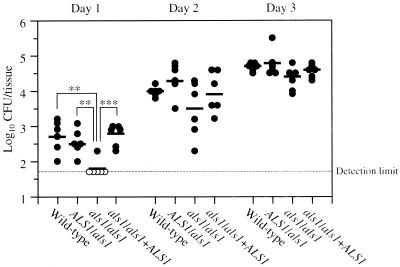
We first investigated the contribution of ALS1 to the virulence of C. albicans in experimental OPC. Figure 1 shows the viable counts in the oral tissue on days 1, 2, and 3 postinoculation. On day 1, the als1/als1 mutant was the least virulent among the strains used. In five out of six mice inoculated with the als1/als1 mutant, the viable counts were below the limit of detection (<1.7 log10 CFU/tissue). The levels of viable counts were similar for the wild-type, ALS1/als1, and als1/als1+ALS1 strains. On days 2 and 3, the viable counts increased progressively, and there was a trend towards a reduced viable count in mice inoculated with the als1/als1 mutant compared to mice inoculated with the other strains, but these differences were not significant.
FIG. 1.
Viable C. albicans counts in oral tissue of mice inoculated with C. albicans strains on days 1, 2, and 3. Each circle represents the result for an individual mouse. The closed circles indicate results that were above the limit of detection (1.7 log10 CFU/tissue), and the open circles represent results that were below the limit of detection. When the culture of the oropharynx was sterile, the value of the detection limit was used for the statistical analysis. The bars indicate the mean value (n = 6). ∗∗, P < 0.01; ∗∗∗, P < 0.001(Tukey's test).
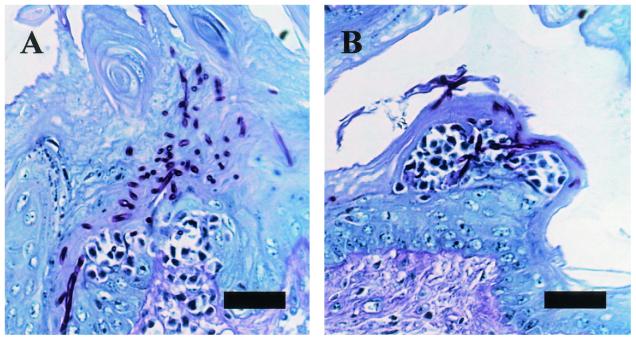
ALS1 is important for hyphal formation as well as adherence (5, 8). Therefore, we examined the oral tissues microscopically to determine the morphology of the different strains. Figure 2 shows representative micrographs of PAS-stained sections of the oral tissues of mice inoculated with the wild-type strain (Fig. 2A) and the als1/als1 mutant (Fig. 2B) on day 1. The infectious foci in mice inoculated with the wild-type strain were larger than those in mice inoculated with the als1/als1 mutant. However, both strains formed filaments of similar length. The histopathology results with the ALS1/als1 and the als1/als1+ALS1 mutants were similar to those of mice inoculated with the wild-type strain (data not shown).
FIG. 2.
Histopathological analysis of oral tissues of mice inoculated with the wild-type strain (A) or als1/als1 strain (B) on day 1 (PAS stain). Bar, 30 μm.
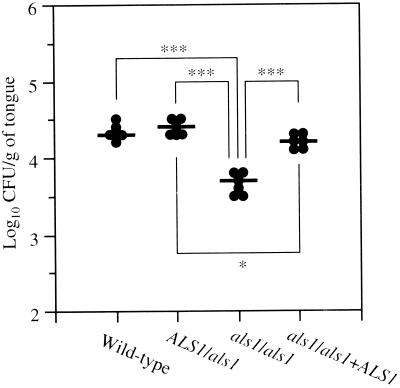
Next, we investigated the adherence of the various strains to the tongues of mice after a 30-min incubation ex vivo (Fig. 3). The als1/als1 mutant was significantly less adherent to the tongue than the other strains. Although the als1/als1+ALS1 mutant was significantly less adherent than the ALS1/als1 mutant, the absolute difference in adherence was small compared with the differences between the als1/als1 mutant and the other strains and it was probably not biologically significant. From these experiments, there does not appear to be a gene dosage effect. In general, the relative difference in adherence among the various strains in the ex vivo assay parallels the oral fungal burden of the mice infected with these strains on day 1 in the in vivo experiment. Therefore, the reduced virulence of the als1/als1 mutant early in the infection may have been due to reduced adherence to the oropharyngeal mucosa.
FIG. 3.
Adherence of C. albicans strains to tongues of mice ex vivo. The closed circles indicate data from individual mice and each bar indicates the mean value (n = 6). ∗, P < 0.05; ∗∗∗, P < 0.001 (Tukey's test).
Our results demonstrate that ALS1 is important during the early stages of OPC in the mouse model. Previously, we found that ALS1 also contributes to virulence in the mouse model of hematogenously disseminated candidiasis (5). In both models, the tissue fungal burden of mice infected with the als1/als1 mutant was significantly less than that of mice infected with the wild-type strain on day 1, but the number of organisms at the infection site subsequently returned to wild-type levels afterward (5). These results suggest that there may be compensatory upregulation of a gene(s) that can eventually substitute for the lack of the ALS1 gene product. It is possible that these genes are other members of the ALS gene family (6, 9, 12).
In this OPC model, the homozygous ALS1 null mutant filamented normally in the oropharynx. On the other hand, in the disseminated infection model, this mutant produced shorter filaments than wild-type or als1/als1+ALS1 strains in the kidney after a similar duration of infection, but the lengths of filaments were similar for all strains by 40 h of infection (5). Multiple pathways have been identified that regulate filamentation in C. albicans (1, 2, 11). These results suggest that a filamentous pathway other than the ALS1 pathway may operate in the oropharynx earlier than in the kidney, or a filamentous pathway that operates in the oropharynx may be different from that in the kidney. The lack of an observed filamentation defect of the als1/als1 mutant in the oropharynx combined with its impaired ability to adhere to the murine tongue ex vivo suggest that the reduced early virulence of this mutant is predominantly the result of reduced adherence. Collectively, these results support the concept that adherence to the oropharynx is important for induction of OPC and that methods to block this adherence are likely to be efficacious in preventing and/or treating this infection.
Acknowledgments
S.G.F. was supported in part by U.S. Public Health Service grants R01 DE13974, R01 AI19990, and P01 AI37194.
Editor: T. R. Kozel
REFERENCES
- 1.Braun, B. R., and A. D. Johnson. 2000. TUP1, CPH1 and EFG1 make independent contributions to filamentation in Candida albicans. Genetics 155:57-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brown, A. J., and N. A. Gow. 1999. Regulatory networks controlling Candida albicans morphogenesis. Trends Microbiol. 7:333-338. [DOI] [PubMed] [Google Scholar]
- 3.Fonzi, W. A., and M. Y. Irwin. 1993. Isogenic strain construction and gene mapping in Candida albicans. Genetics 134:717-728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fu, Y., G. Rieg, W. A. Fonzi, P. H. Belanger, J. E. Edwards, Jr., and S. G. Filler. 1998. Expression of the Candida albicans gene ALS1 in Saccharomyces cerevisiae induces adherence to endothelial and epithelial cells. Infect. Immun. 66:1783-1786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fu, Y., A. S. Ibrahim, D. C. Sheppard, Y.-C. Chen, S. W. French, J. Cutler, S. G. Filler, and J. E. Edwards, Jr. 2002. Candida albicans Als1p: an adhesin that is a downstream effector of the EFG1 filamentation pathway. Mol. Microbiol. 44:61-72. [DOI] [PubMed]
- 6.Gale, C. A., C. M. Bendel, M. McClellan, M. Hauser, J. M. Becker, J. Berman, and M. K. Hostetter. 1998. Linkage of adhesion, filamentous growth, and virulence in Candida albicans to a single gene INT1. Science 279:1355-1358. [DOI] [PubMed] [Google Scholar]
- 7.Gaur, N. K., and S. A. Klotz. 1997. Expression, cloning, and characterization of a Candida albicans gene, ALA1, that confers adherence properties upon Saccharomyces cerevisiae for extracellular matrix proteins. Infect. Immun. 65:5289-5294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hoyer, L. L., S. Scherer, A. R. Shatzman, and G. P. Livi. 1995. Candida albicans ALS1: domains related to Saccharomyces cerevisiae sexual agglutinin separated by a repeated motif. Mol. Microbiol. 15:39-54. [DOI] [PubMed] [Google Scholar]
- 9.Hoyer, L. L. 2001. The ALS gene family of Candida albicans. Trends Microbiol. 9:176-180. [DOI] [PubMed] [Google Scholar]
- 10.Kamai, Y., M. Kubota, Y. Kamai, T. Hosokawa, T. Fukuoka, and S. G. Filler. 2001. New model of oropharyngeal candidiasis in mice. Antimicrob. Agents Chemother. 45:3195-3197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lengeler, K. B., R. C. Davidson, C. D'souza, T. Harashima, W. C. Shen, P. Wang, X. Pan, M. Waugh, and J. Heitman. 2000. Signal transduction cascades regulating fungal development and virulence. Microbiol. Mol. Biol. Rev. 64:746-785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Staab, J. F., S. D. Bradway, P. L. Fidel, and P. Sundstorm. 1999. Adhesive and mammalian transglutaminase substrate properties of Candida albicans Hwp1. Science 283:1535-1538. [DOI] [PubMed] [Google Scholar]