The introduction of highly active antiretroviral therapy (HAART), with either a protease inhibitor or a non-nucleoside reverse transcriptase inhibitor in combination with two or more nucleoside reverse transcriptase inhibitors, was a landmark event in the history of HIV treatment. Since 1996, HAART has been widely adopted in the USA and Western Europe, with profound impact on HIV-related morbidity and mortality1,2,3,4,5,6. In April 2001, fifteen antiretroviral drugs had been licensed for use in Europe and the USA and an additional eight were in development. In Europe, the USA and Canada, the mortality of AIDS (Figure 1) and the incidence of different AIDS-defining illnesses (Figure 2) have declined over the past decade. In the UK, new AIDS diagnoses dropped from 1187 in 1994 to 507 in 1999, the decline being steepest between 1996 and 1998 (from 941 to 522) and greatest in those infected through sex with men1. The biggest percentage reductions in incidence of AIDS diagnoses occurred with Pneumocystis carinii pneumonia (PCP) (35-80%), Kaposi's sarcoma (34-75%), cryptosporidiosis (60-88%), oesophageal candidiasis (29-81%) and cytomegalovirus (CMV) (70%)2,3,4,5,6, and discontinuation of primary and even secondary prophylaxis has proved possible for several of these infections7. The use of HAART has also been associated with remission of Kaposi's sarcoma, refractory cryptosporidiosis, and progressive multifocal leukoencephalopathy, in addition to improvement in many of the common HIV-related symptoms such as night sweats, fatigue, weight loss, and seborrhoeic dermatitis. Some unusual manifestations of infections have been reported as a result of the heightened immune response or immune reconstitution associated with antiretroviral therapy. These include lymphadenitis due to Mycobacterium avium complex, vitritis associated with CMV, flare-up of hepatitis due to hepatitis B, and an aggressive course of multicentric Castleman's disease associated with human herpesvirus-8.
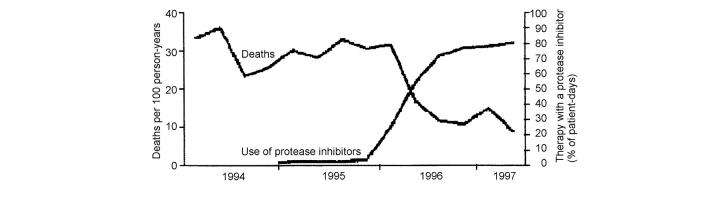
Figure 1.
Impact of protease inhibitor use on mortality. Reproduced by permission from Palella et al. (Ref. 3)
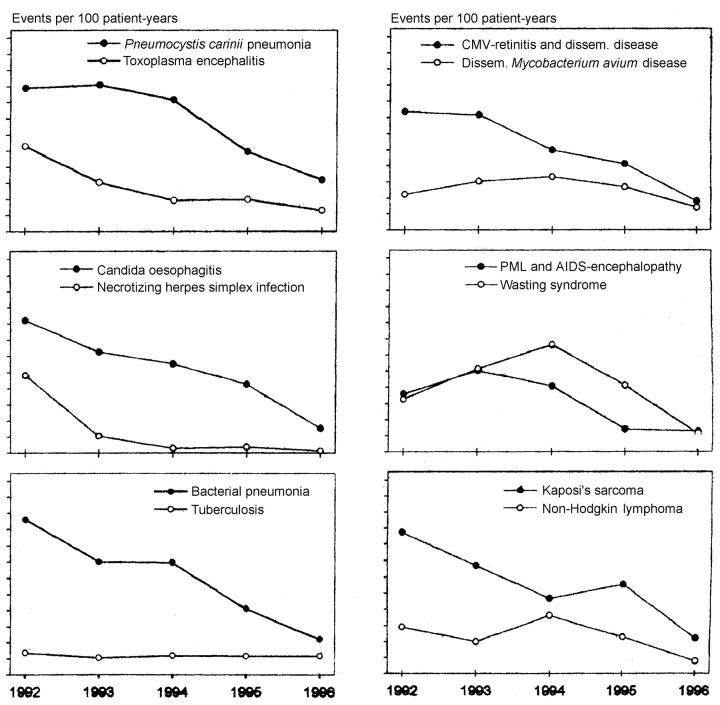
Figure 2.
Annual events of different AIDS-defining diseases calculated as events per 100 patient-years. Reproduced by permission from Brodt et al. (Ref. 2). PML=progressive multifocal leucoencephalopathy
The spectrum of paediatric HIV disease has also changed dramatically—not only because of the availability of HAART but also because of prompt early diagnosis and treatment and the development of more reliable surrogate markers of disease progression. In addition, because of a widespread increase in screening of pregnant women for HIV, more patients are identified as HIV-infected and receive antiretroviral therapy during pregnancy. This in turn has resulted in a sharp decline in the prevalence of childhood infection in developed countries. Since 1994 in various parts of Western Europe, mother-to-child HIV transmission rates have decreased from 25% to less than 8%.
CHALLENGES IN THE MANAGEMENT OF HIV DISEASE
Treatment failure
Despite the successes of antiretroviral treatment new diagnoses of HIV infection have not decreased, so the number of patients living with HIV continues to grow. In addition, it is becoming evident that sustained viral suppression with HAART may not be feasible in all patients receiving HAART. Several studies have found that 40-50% of patients experience virological failure within 12 to 24 months of starting HAART8,9. Contributory factors include poor adherence to treatment because of high pill burdens and dietary restrictions, unacceptable side-effects, drug interactions resulting in suboptimal drug levels, and pre-existing drug resistance. Although viral rebound does not seem often to cause clinical progression of disease, the long-term outlook for these patients is uncertain.
Toxicities
About 40% of patients develop one or more adverse reactions within 18 months of starting protease inhibitors, and side-effects specific to nucleoside and non-nucleoside reverse transcriptase inhibitors have also been recognized. The spectrum, overall, includes gastrointestinal problems, metabolic effects (hyperlipidaemia, hyperglycaemia, lactic acidosis, osteopenia), lipodystrophy or fat redistribution syndrome, hepatic dysfunction, peripheral neuropathy and renal toxicities10.
Peripheral neuropathy, which affects 10-35% of HIV-infected patients and 11-55% of those using didanosine (ddI), dideoxycytidine (ddC), lamivudine or stavudine, has emerged as one of the most difficult HIV-related symptoms or drug side-effects to manage. Neuropathy can greatly impair quality of life, particularly in patients without any remaining antiretroviral treatment options and in whom the neuropathy has progressed.
Role of palliative care in HIV
Although HIV has been transformed by HAART into a chronic rather than a uniformly fatal illness, palliative care continues to have an important evolving role. Increasingly, the WHO palliative care definition ‘active total care’ includes use of antiretroviral therapy along with prevention or management of opportunistic infections, and the palliative approach of offering symptomatic and supportive care is seen as important at all stages of HIV disease13,14,15.
PALLIATIVE CARE ISSUES SPECIFIC TO HIV INFECTION
Common symptoms encountered in advanced HIV disease are summarized in Table 1. Overall, the palliative-care needs and symptom-control difficulties are similar to those in patients with metastatic disease or degenerative neurological conditions, but several are specific to HIV disease.
Table 1.
Common symptoms in HIV disease
| Symptoms | Cause |
|---|---|
| Due to HIV | |
| Pain in limbs | Neuropathy |
| Disfiguring skin lesions | Kaposi's sarcoma, seborrhoeic dermatitis, molluscum contagiosum |
| Nausea, vomiting | GI infections, intracranial pathology |
| Diarrhoea | HIV, cryptosporidiosis, MAI, salmonella |
| Abdominal pain | Sclerosing cholangitis, MAI, lymphadenopathy, TB peritonitis |
| Breathlessness | Pneumocystis pneumonia, other chest infections, TB lung, Kaposi's sarcoma, lymphoid interstitial pneumonitis in children |
| Headache | Cerebral lymphoma, toxoplasma, TB |
| Lymphadenopathy | HIV, lymphoma, TB |
| Drenching sweats | HIV, TB, lymphoma |
| Blindness | Cytomegalovirus, toxoplasma, progressive multifocal leucoencephalopathy |
| Mouth discomfort | Candida, herpes simplex, herpes zoster, aphthous ulcers, gingivitis, peridontitis, malignancy, Kaposi's sarcoma |
| Tiredness | HIV, anaemia due to HIV |
| Due to therapy | |
| Neuropathy | Nucleoside analogues, especially ddI, ddC, d4T |
| Pancreatitis | Nucleoside analogues |
| Lactic acidosis, liver failure | Nucleoside analogues |
| Diarrhoea | ddI, ritonavir, nelfinavir |
| Nausea | ddI, ritonavir, 3TC, ZDV |
| Anaemia | ZDV |
| Rash | Nevirapine, abacavir |
| Agitation, euphoria | Efavirenz |
| Lipodystrophy | Protease inhibitors, d4T |
| Renal colic | Indinavir |
| MAI=Mycobacterium avium intracellulare; ZVD=zidovudine | |
Polypharmacy: chronic antiretroviral therapy and continued prophylaxis
Long-term virological control with HAART requires a high level of adherence. Experience from other chronic diseases, such as diabetes and hypertension indicates that patients who feel well find it extraordinarily difficult to take their medications. In many HIV clinics, 20% of patients do not comply with therapy at all, and the remaining 80% probably comply only 60% of the time. Paterson and colleagues found that, even among patients with 90-95% adherence, the failure rate was 32% at three months, and of patients with 80-90% adherence about half experienced virological failure12. Since 95% adherence is a near impossible goal for many patients, there is a great need for more potent and simplified regimens, with compact easy-to-swallow tablets or capsules, that are well tolerated and forgiving of occasional non-compliance.
Polypharmacy for patients with advanced HIV disease is common. Many medications may be continued for reasons that are not clear, and hospice admission can offer an opportunity to redefine the goals of care and address the main symptoms. Here is an example:
A man aged 52 with advanced HIV disease and a recent history of PCP, CMV retinitis, wasting and chronic diarrhoea was transferred for hospice care from the hospital HIV inpatient unit after admission for low-grade fever and general debility. No underlying infection had been found but antibiotics had been started empirically. On leaving hospital he was taking, daily, the following: amoxycillin 500 mg × 3; erythromycin 500 mg × 4; co-trimoxazole 480 mg × 1; fluconazole 50 mg × 1; multivitamins × 1; acyclovir 200 mg × 3; megestrol 80 mg × 3; prochlorperazine 10 mg 6 hourly as required; sustained-release morphine 60 mg × 4; immediate-release morphine 10 mg as required; lorazepam 1 mg 6 hourly as required.
He was having difficulty with oral intake. Since fatigue, anorexia, pain and anxiety were the main symptoms, after careful discussion with the patient and his HIV physician only the following medications were continued: co-trimoxazole 480 mg for PCP prophylaxis; acyclovir 400 mg × 2 for suppression of herpes; sustained-release morphine 60 mg × 2; immediate-release morphine 10 mg as required; senna 2 tablets at bedtime; lorazepam 1 mg as required.
Multiple medical problems and coexisting diagnoses
A substantial proportion of persons with HIV have other disorders such as asthma, diabetes, tuberculosis and hepatitis B or C that can complicate their management. Patients who become infected through injection drug use are particularly likely to be infected with hepatitis B and C infection, and HIV co-infection is a strong risk factor for progression to end-stage liver disease. Treatment of both these viral infections is also associated with greater risk of adverse events, and management of co-infection poses challenges for palliative care. In addition, such patients are likely to have limited support networks, to be estranged from their families and not to be accessing healthcare.
A woman of 29 had been a windsurfing instructor abroad for many years. She had never injected drugs or had a blood transfusion. In May 1995 she was diagnosed HIV-1 positive; CD4 cell count 100 × 106/L, oral candida.
Phase 1
April 1996—Antiretroviral therapy with zidovudine and ddc May 1996—Nausea and poor adherence. Antiretroviral therapy discontinued
June 1996—Right-sided weakness and incontinence; magnetic resonance scan (MRI) consistent with progressive multifocal leucoencephalopathy (PML)
June 1997—Generalized seizures; progression of white matter lesions on MRI
July 1997—Generic hospice for terminal care; depressed, agitated, expressing wish to die
Phase 2
August 1997—Admitted to Mildmay Hospital brain impairment unit; regular eating and sleeping routine established; able to comply with PCP prophylaxis
December 1997—Referred to start HAART; progresses from directly observed therapy to supervised dosette-based therapy to independent self-medication
December 1998—Discharged to live independently; self-caring, hygienic, smartly dressed, keeping appointments
December 2000—Well at home, taking HAART.
Fluctuation in condition—difficulty in identifying terminal phase
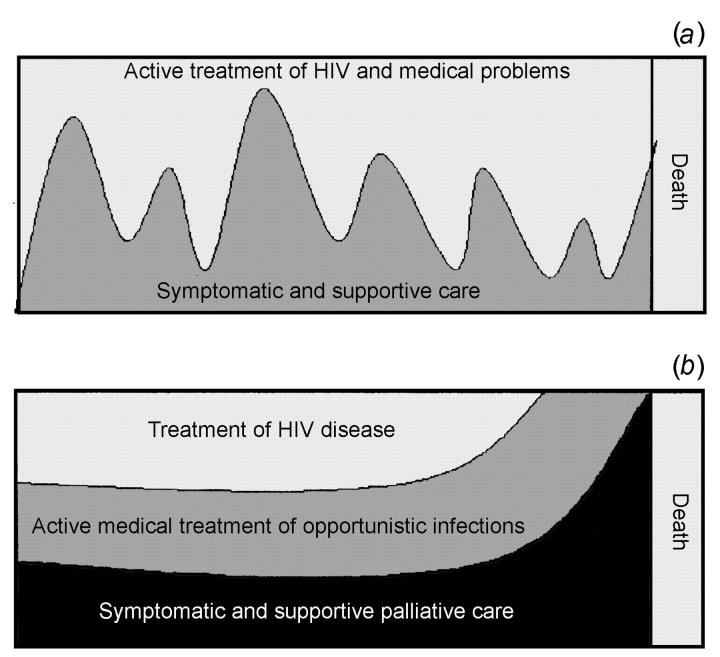
In cancer, the transition from active management to palliative care was originally thought to be a clearly defined cut-off, but in reality there is a gradual progression12,13. With HIV disease the pattern is neither a sharply defined cut-off nor a gradual progression, but may be characterized by episodes requiring a considerable degree of symptom control, alternating with periods where little or none is required (Figure 3). At times, combination antiretroviral therapy is the most effective management of HIV-related symptoms.
Figure 3.
Two models of HIV care
A 31-year-old woman from Francophone west Africa with limited English was an illegal immigrant living in the house of friends in London. She had no family in the UK, but was an active member of the local church.
She initially indicated that she had a child with her, but subsequently denied this. She remained adamant that no one should be informed of her diagnosis.
March 2000—Presented with a four-day history of diplopia and weakness of the left arm and leg. MRI showed multiple cerebral and brain stem lesions consistent with toxoplasmosis. Confirmed HIV-1 positive.
April 2000—Management problems:
Spasticity and intractable disabling intention tremor of left arm. Spasticity managed with codydramol and dothiepin, and the tremor with baclofen, benzhexol, tetrabenazine tinozide and sodium valproate
Depression
Non-compliance with antiretroviral therapy. Consistently low or undetectable antiretroviral drug levels
August 2000—Discharged to neurorehabilitation unit at Homerton Hospital.
Patients can be restored to independence and a good quality of life even after being considered terminal by their HIV specialist, nurses, and family14,15. However, the unpredictable response to therapy in the setting of advanced HIV disease means that determining prognosis is often difficult. This may lead to the apparent paradox whereby the patient receives simultaneous active treatment for one HIV-related condition and palliation for another. For example, acute opportunistic infections, superimposed on a chronic debilitated state, may require short-term aggressive interventions even though the overall goal may be one of palliation. This up-and-down course represents a constant challenge to healthcare staff and family.
Increased risk of drug reactions and drug interactions
The expanding number of approved drugs to treat HIV infection increases options for patients but also presents challenges for the HIV physician. Since many of these drugs are substrates for cytochrome P450 drug-metabolizing enzymes in the liver, and since some of them are inhibitors or inducers of these enzymes, physicians need to be aware of the potential for pharmacokinetic drug interactions. Some undesirable drug interactions, such as those produced by rifabutin (used in the treatment of mycobacterial infection) and protease inhibitors, may be circumvented by modifying the dose of one or both drugs. P450 inducers such as efavirenz, nevirapine and nelfinavir can reduce methadone concentrations, so HIV-infected patients taking methadone who are started on antiretroviral therapy should be monitored carefully and may require an increase in methadone dose. Finally patients should be cautioned that complementary medicines can interact with prescription drugs. St John's wort, a p450 enzyme inducer, substantially reduces indinavir concentrations and should be avoided by patients on antiretroviral therapy.
Isolation, stigma and lack of social support
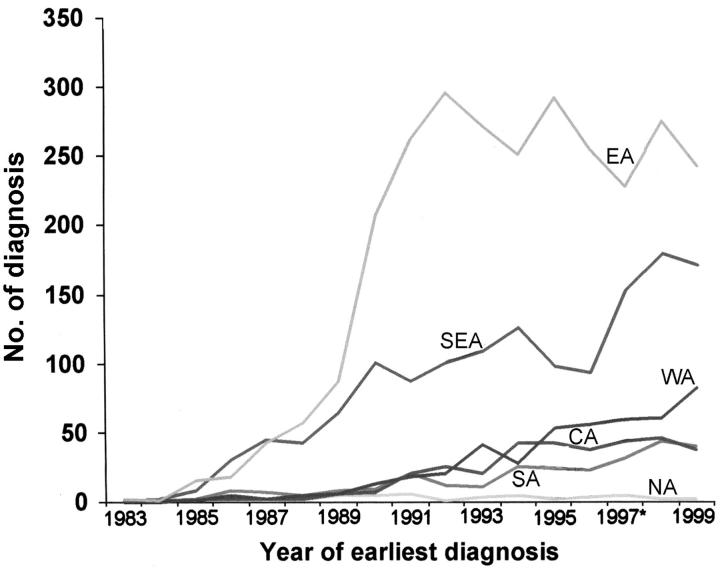
In the past 5 years in the UK, there has been a pronounced rise in the number of new HIV infections in persons from West, South and East Africa (Figure 4); in South London, 40-50% of our patients are from sub-Saharan Africa. Patients from these communities tend not to have the HIV-1 infection diagnosed until they are symptomatic and already have advanced disease16. A reluctance to come forward for HIV testing is explained partly by lack of knowledge about the benefits of therapy but also by the continuing stigma associated with HIV in many of these communities, such that patients even after diagnosis may avoid disclosing their infection to anyone else. The stigma can result in reduced uptake of services that are identifiably HIV-related (such as HIV hospices and wards). Continuing reassurance about confidentiality is therefore important. Uncertain immigration status can also cause great anxiety and prevent family members from entering the UK to visit or care for their dying relative. The situation for asylum-seekers with HIV is particularly difficult. Unwillingness to inform an unsympathetic official about their HIV status may mean that the patient is transferred outside London in accordance with the Immigration and Asylum Act, 1999. As an example, a recent resident at the Mildmay Hospital who was also an asylum-seeker was transferred from London to Bournemouth and then on to Hastings. On each occasion, links established with HIV professionals and cultural groups were disrupted, and the patient was relocated far from family and friends from the same ethnic group.
Figure 4.
HIV infections in the UK where the probable country of infection was a named country in Africa (Source: HIV and STI Division, PHLS Communicable Diseases Surveillance Centre).
NA=Northern Africa; SA=Southern Africa; CA=Central Africa; WA=Western Africa; SEA=South Eastern Africa; EA=Eastern Africa.
ORGANIZATION AND DELIVERY OF HIV PALLIATIVE CARE
In the UK, most people who are infected with HIV obtain their care from specialist hospital clinics. These clinics usually provide access to antiretroviral drugs as well as specialized services such as walk-in and day-care facilities, counselling, complementary treatments, peer support and advice about services and benefits.
Hospital
Patient surveys in the past have shown that less than 10% of HIV-infected patients who expressed a preference wished to die in hospital17. However, since the late 1980s there has been little change in the approximately 60% of patients dying in hospital, largely because dying in hospital is usually a fait accompli rather than an active decision. Drawbacks of hospitals include the variable quality of palliative care in acute medical settings and the focus on management of acute problems in a person who may be slowly dying.
Hospice
Another environment for palliative care is a residential hospice. Many district hospitals and community areas in the UK provide specialist palliative care and advice to patients, their families and GPs in association with a local hospice. The relative merits of the specialized hospice versus the non-specialized hospice for the delivery of HIV palliative care have been reviewed previously18,19. Because of specific issues in caring for people with HIV, several HIV hospices were established—such as the Mildmay Hospital in East London, which when opened in 1987 was Europe's first HIV/AIDS hospice. Staff at such hospices are experienced in the management of HIV-related problems, and as a result are particularly sensitive to the social and psychological issues associated with the disease. In addition, there is the opportunity for patients to meet and gain encouragement from other patients in similar settings. However, since stigma is still very troublesome (even dying patients with HIV may be rejected by family, community or church group), difficulties can arise if the patient has not been open about the diagnosis to friends and relatives.
As the pattern of HIV disease has changed so also has the type of care provided at the Mildmay. In 1987, 90% of patients were male and 50% died in their first admission. In 1999, only 3% of patients died during their first admission and a sizeable number of admissions were for respite care or to the family care centre. There are also dedicated beds for symptom control in patients with brain impairment, and a rehabilitation programme offers help in returning to a reasonable quality of life.
Home (UK)
Patients in the later stages of disease commonly express the desire to die at home, and the extensive experience of community-based physicians, GPs and primary care teams in looking after all patients requiring palliative care can be adapted for people with HIV. However, many patients do not have a GP and are reluctant to register with one. Even if they do register, they may still not disclose their HIV status, because of concerns over confidentiality and the fear of discrimination20. As a result, GPs often become involved with HIV care only at a late stage in the disease course. Hospital support teams and community HIV nurse specialists where available can be helpful, but many patients in our area lack a stable home environment or do not want to access local services, so that dying at home is not a realistic option.
HIV palliative care in the developing world
In less developed countries the picture is very different. Many antiretroviral drugs and other therapies that would prevent disease progression and are part of the continuum of care in developed countries are not available in these resource-poor areas21 and even the most basic care and symptom control may be unavailable. However, there are a few successful models of palliative care initiatives in these settings. The AIDS Support Organization (TASO) in Uganda was initially founded in 1987 as a self-help support group, but has now developed an outpatient clinic providing basic clinical care—although, with the lack of availability of anti-herpes agents or even fluconazole or nystatin for candida, and such a high proportion of destitute patients, a bar of soap, a bag of flour, or some bedding may be the best symptom control on offer. In recognition of the often poorquality care at home that results from stigma, TASO has also begun training and supervision programmes for families and community members in basic home care.
The Mildmay Centre for AIDS palliative care in Kampala, Uganda, which opened to patients in 1998, developed as a joint project between the Ugandan Ministry of Health, the UK Department for International Development and Mildmay International, to provide specialist outpatient palliative care and rehabilitation to HIV-infected patients for a small fee, or free of charge to the destitute. The centre's philosophy of symptom control is disseminated throughout sub-Saharan Africa by a programme of ‘training the trainers’.
References
- 1.Communicable Diseases Surveillance Centre. AIDS and HIV infection in the United Kingdom; monthly report. The effect of highly active antiretroviral therapy (HAART) on progression to AIDS. Commun Dis Rep CDR Weekly 2000;10: 123-4 [Google Scholar]
- 2.Brodt HR, Kamps BS, Gute P, Knupp B, Staszewski S, Helm EB. Changing incidence of AIDS-defining illnesses in the era of antiretroviral combination therapy. AIDS 1997;11: 1731-8 [DOI] [PubMed] [Google Scholar]
- 3.Palella FJ, Delaney KM, Moorman AC, et al. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. N Engl J Med 1998;338: 853-60 [DOI] [PubMed] [Google Scholar]
- 4.Forrest DM, Seminari E, Hogg RS, et al. The incidence and spectrum of AIDS-defining illnesses in persons treated with antiretroviral drugs. Clin Infect Dis 1998;27: 1379-85 [DOI] [PubMed] [Google Scholar]
- 5.Moore RD, Chaisson RE. Natural history of HIV infection in the era of combination antiretroviral therapy. AIDS 1999;13: 1933-42 [DOI] [PubMed] [Google Scholar]
- 6.Ives NJ, Gazzard BG, Easterbrook PJ. The changing pattern of AIDS-defining diagnoses with the introduction of HAART in a London clinic. J Infection 2001;42: 1-6 [DOI] [PubMed] [Google Scholar]
- 7.Wevering GJ, Mocroft A, Ledergerber B, et al. Discontinuation of Pneumocystis carinii pneumonia prophylaxis after start of highly active antiretroviral therapy in HIV-1 infection. Lancet 1999;353: 1293-8 [DOI] [PubMed] [Google Scholar]
- 8.Ledergerber B, Egger M, Opraveil M, et al. Clinical progression and virological failure on highly active antiretroviral therapy in HIV-1 patients: a prospective cohort study. Lancet 1999;353: 863-8 [DOI] [PubMed] [Google Scholar]
- 9.D'Arminio Monforte A, Testa L, Adorni F, et al. Clinical outcome and predictive factors of failure of highly active antiretroviral therapy in antiretroviral-experienced patients in advanced stages of HIV-1 infection. AIDS 1998;12: 1631-7 [DOI] [PubMed] [Google Scholar]
- 10.Max B, Sherer R. Management of the adverse effects of antiretroviral therapy and medication adherence. Clin Infect Dis 2000;30: 596-616 [DOI] [PubMed] [Google Scholar]
- 11.Paterson DL, Swindells S, Mohr J, et al. Adherence to protease inhibitor therapy and outcomes in patients with HIV infection. Ann Intern Med 2000;133: 21-30 [DOI] [PubMed] [Google Scholar]
- 12.WHO. World Health Report 1995: Bridging the Gap. Report of the Director General. Geneva:WHO, 1995
- 13.Foley F, Flannery S. AIDS palliative care: challenging the palliative paradigm. J Pall Care 1995;11: 34-7 [PubMed] [Google Scholar]
- 14.Stephenson J, Woods S, Scott B, Meadway J. HIV-related brain impairment: from palliative care to rehabilitation. Int J Pall Nursing 2000;6: 6-11 [DOI] [PubMed] [Google Scholar]
- 15.Rackstraw S, Conley A, Meadway J. Recovery from progressive multifocal leukoencephalopathy following directly observed highly active antiretroviral therapy (HAART) in a specialized brain impairment unit. AIDS 2000;14(suppl. 4): S12911086856 [Google Scholar]
- 16.Erwin J, Peters B. Treatment issues for HIV positive Africans. Soc Sci Med 1999;49: 1519-38 [DOI] [PubMed] [Google Scholar]
- 17.Goldstone I, Kuhl D, Johnson A, et al. Patterns of care in advanced HIV disease in a tertiary treatment centre. AIDS Care 1995;7(suppl. 1): S47-S56 [DOI] [PubMed] [Google Scholar]
- 18.Schofferman J. Hospice care of the patient with AIDS. J Hospice 1987;3: 51-74 [DOI] [PubMed] [Google Scholar]
- 19.Mansfield S, Barter G, Singh S. AIDS and palliative care. Int J STD AIDS 1992;3: 248-50 [DOI] [PubMed] [Google Scholar]
- 20.Shaw M, Tomlinson D, Higginson I. Survey of HIV patient's views on confidentiality and non-discrimination policies in general practice. BMJ 1996;312: 1463-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sanei L. Palliative Care for HIV/AIDS in Less Developed Countries. Arlington, VA: Health Technical Services (HTS) Project for USAID, 1998