Abstract
Costimulation through the B7-CD28 interaction is an important second signal for T-cell activation, and previous studies have shown that CD28−/− mice infected with Toxoplasma gondii generate suboptimal CD4+ T-cell responses, associated with a defect in production of the T-cell growth factor interleukin-2 (IL-2). To address the role of IL-2 in the expansion of T cells during toxoplasmosis, IL-2−/− mice were infected with T. gondii and their ability to generate a protective T-cell response was assessed. Although IL-2−/− mice produced normal levels of IL-12p40, they had reduced levels of gamma interferon (IFN-γ) in serum, had an increased parasite burden, and succumbed to infection with T. gondii within 20 days. Fluorescence-activated cell sorter analysis revealed that, although uninfected IL-2−/− mice had an increased number of activated T cells compared with uninfected IL-2+/+ mice, following infection they were unable to further upregulate this population. Examination of the ability of splenocytes from uninfected and infected mice to produce IFN-γ revealed that IL-2−/− mice were hyporesponsive to stimulation with anti-CD3 or parasite antigen compared with wild-type mice, and the addition of IL-2 alone or in combination with IL-12 or stimulation with phorbol myristate acetate and ionomycin did not restore the production of IFN-γ. Together, these studies reveal that IL-2−/− mice are unable to generate a protective IFN-γ response following infection with T. gondii and suggest that IL-2−/− mice have an intrinsic defect in their ability to activate and expand IFN-γ-producing T cells required for resistance to T. gondii.
The two-signal hypothesis of T-cell activation proposes that signal one is provided by the interaction of the T-cell receptor with appropriate major histocompatibility complex-antigen complexes, while the second signal is provided by costimulatory molecules which lead to the production of the T-cell growth factor interleukin-2 (IL-2) and increased expression of the IL-2 receptor (IL-2R) (8, 24). Although there are many different molecules that have been identified and possess costimulatory properties, the interaction of CD28 on T cells with B7 molecules on accessory cells represents one of the most important costimulatory pathways (2). Signaling through CD28 enhances T-cell production of IL-2, an autocrine growth factor (17, 26), and enhances the expression of the bcl family of proteins which protect against apoptosis (3). The generation of CD28−/− mice and the demonstration that CD28−/− T cells had defects in their ability to proliferate, produce cytokines, and resist activation induced cell death confirmed the importance of the CD28-B7 interaction in the regulation of T-cell responses (4, 16). These defects were primarily attributed to the reduced production of IL-2 by CD28−/− mice (16).
Recent studies from this laboratory involved in understanding the role of costimulatory molecules in the regulation of immunity to Toxoplasma gondii demonstrated that CD28−/− mice have a reduced capacity to produce IL-2 following infection with T. gondii, correlating with a reduced number of parasite-specific memory CD4+ T cells. As a consequence, splenocytes from chronically infected CD28−/− mice produced reduced levels of IFN-γ when stimulated with parasite antigen, and these mice were susceptible to rechallenge with the virulent RH strain of T. gondii. Moreover, the addition of exogenous IL-2 to splenocytes from chronically infected CD28−/− mice resulted in the emergence of antigen-specific gamma interferon (IFN-γ)-producing CD8+ T cells (27). These studies indicated the importance of CD28 in providing the costimulatory signal for the production of IL-2, which is required for optimal T-cell production of IFN-γ, which is required for resistance to rechallenge. However, these studies did not distinguish between the possibility that the reduced numbers of parasite-specific CD4+ T cells observed in chronically infected CD28−/− mice was due to a failure of these cells to expand (18, 27) (likely IL-2 dependent) and/or due to increased susceptibility to apoptosis associated with decreased CD28-dependent expression of antiapoptotic proteins. Therefore, to address the mechanism whereby CD28 regulates T-cell responses, IL-2−/− mice were infected with T. gondii and their T-cell responses were assessed. These studies reveal that IL-2−/− mice are highly susceptible to acute toxoplasmosis, and this is associated with severe defects in antigen-specific IFN-γ responses and a failure to increase the numbers of T cells expressing activation markers. In addition, stimulation with IL-2 alone or in combination with IL-12 failed to restore the production of IFN-γ by splenocytes from infected IL-2−/− mice.
MATERIALS AND METHODS
Parasites and infection.
Female Swiss Webster and CBA/CaJ mice obtained from Jackson Laboratories (Bar Harbor, Maine) were used to maintain the ME49 strain of T. gondii and provided a source of tissue cysts. For infection with ME49 tissue cysts, brains of chronically infected CBA/CaJ mice were harvested and prepared as previously described (1, 6). Mice were inoculated intraperitoneally with 20 cysts in a volume of 0.2 ml. The RH strain was maintained in vitro at 37°C using human foreskin fibroblasts (HS27) cultured in modified Eagle medium (Life Technologies, Gaithersburg, Md.) supplemented with 1% fetal calf serum (HyClone Laboratories, Logan, Utah), penicillin (100 U/ml), streptomycin (100 μg/ml), amphotericin B (25 ng/ml), and gentamicin (50 μg/ml). The RH strain was used as a source of soluble toxoplasma antigen (STAg) as previously described (25). STAg activity was titrated to determine the optimal concentration for splenocyte proliferation and cytokine production (25 μg/ml).
IL-2−/− mice.
IL-2−/−mice backcrossed for more than eight generations onto the C57BL/6 background (10) were bred and maintained as heterozygotes for the mutated IL-2 gene. IL-2−/−, IL-2+/−, and IL-2+/+ mice were obtained from IL-2+/− matings and were identified by a modified PCR amplification protocol using DNA extracted from digestion of ear clip specimens as previously described (23). Briefly, ear clip specimens from mice were digested in 20 μl of ear digestion buffer containing 10 mM Tris-Cl, pH 8.0; 100 mM NaCl (Sigma Diagnostics, St. Louis, Mo.); 25 mM EDTA; 0.5% (wt/vol) sodium dodecyl sulfate (Life Technologies); and proteinase K (1 mg/ml; Roche, Mannheim, Germany) overnight at 55°C. Ear digests were diluted with water to a final volume of 200 μl, and 1 μl of digest solution was used as a source of DNA. Four- to six-week-old mice displaying no overt signs of disease were used for all experiments, and deaths of uninfected IL-2−/− mice during the course of the experiments were rare. Due to the difficulties associated in breeding and maintenance of these mice, mice of both sexes were used in the experiments reported. All mice used were maintained within Thoren caging units (Thoren Caging Systems, Hazelton, Pa.) at the University Laboratory Animal Resource facilities at the University of Pennsylvania.
Histological analysis.
At the time of sacrifice, samples of livers, lungs, and spleens were removed from each animal and prepared for hematoxylin and eosin or immunohistochemical staining (14). Briefly, tissues were fixed overnight in Accustain 10% Formalin neutral buffered solution (Sigma Diagnostics) and then embedded in paraffin. Paraffin sections (5 μm thick) were stained with hematoxylin and eosin to visualize pathological changes. T. gondii parasites and antigens were detected in tissues of infected mice as described previously (9). Briefly, paraffin sections of heart, liver, and lung were stained with a rabbit anti-T. gondii antiserum (Transduction Laboratories, Lexington, Ky.) and bound antibody was visualized by serial incubation with biotinylated goat-anti-rabbit (Vector Laboratories Inc., Burlingame, Calif.) and avid-biotin-peroxidase complexes (ABC kit; Vector Laboratories Inc.) using diaminobenzamide (Vector Laboratories Inc.) as the chromogen. To quantitate parasite burden, peritoneal exudate cells were harvested with 5 ml of ice-cold phosphate-buffered saline without Ca2+/Mg2+ (BioWhittaker, Walkersville, Md.), and 5 × 105 cells/100 μl were used to prepare cytospins. Cells were fixed and stained using Protocol Hema3 (Biochemical Sciences, Swedesboro, N.J.) as described in the manufacturer's manual and then mounted and sealed using Cytoseal (Stephens Scientific, Kalamazoo, Mich.). Where the percentage of cells infected was less than 1% but parasites could still be observed, a value of 0.1% was assigned.
Reagents.
Complete RPMI 1640 (Life Technologies) medium contained 10% heat-inactivated fetal bovine serum (HyClone Laboratories), sodium pyruvate, nonessential amino acids, penicillin (100 U/ml), streptomycin (1 mg/ml), and amphotericin B (25 ng/ml; Life Technologies). Murine recombinant IL-12 was a gift from Joe Sypek (Genetics Institute, Boston, Mass.), IL-15 was a gift from Mary Kennedy (Immunex, Seattle, Wash.), and recombinant murine IL-18 was purchased from Peprotech Inc. (Rocky Hill, N.J.). Human recombinant IL-2 was purchased from Chiron Therapeutics (Emeryville, Calif.), and phorbol myristate acetate (PMA) and ionomycin were purchased from Sigma. Anti-CD3ɛ (145-2C11) was purified from hybridoma culture supernatants. Rat immunoglobulin G isotype control antibody was obtained from Sigma. Enzyme-linked immunosorbent assays (ELISAs) for IL-12 and IFN-γ were performed as previously described (18, 27), and the limits of detection for IL-12p40 and IFN-γ were 79 and 20 pg/ml, respectively.
Analysis of T-cell responses.
Spleens harvested from individual animals were dissociated into single cell suspension and depleted of erythrocytes using 0.83% wt/vol ammonium chloride (Sigma Chemical Co.). Cells were washed and resuspended in complete RPMI before being plated at a density of 2 × 105 cells per well in a final volume of 200 μl in 96-well flat-bottom plates (Costar, Costar, N.Y.). Cells were stimulated with soluble anti-CD3 (1 μg/ml) or STAg (25 μg/ml) for 48 h at 37°C under 5% CO2. The following exogenous cytokines were used: IL-12 (1 ng/ml), IL-2 (200 IU/ml), IL-15 (1 ng/ml), and IL-18 (10 ng/ml). Alternatively, splenocyte cultures were incubated with PMA (50 ng/ml) plus ionomycin (500 ng/ml). Levels of IFN-γ and IL-12p40 produced by splenocytes were measured using ELISA (20). To assess surface expression of activation markers on T cells, purified splenocytes were resuspended in FACS buffer (1× phosphate-buffered saline, 0.2% bovine serum albumin fraction V, 4 mM sodium azide) at a concentration of 107 cells/ml. One million cells were preincubated with saturating concentrations of Fc Block (PharMingen, San Diego, Calif.) for 10 to 20 min on ice and stained with phycoerythrin-conjugated anti-CD4; fluorescein isothiocyanate-conjugated anti-CD8; and biotinylated anti-CD69, anti-CD44, or anti-CD62L (PharMingen) for 20 min on ice. Cells were then washed with FACS buffer and then incubated with allophycacyanin- conjugated streptavidin (PharMingen) for an additional 20 min on ice, washed, resuspended in FACS buffer, and analyzed using a FACScalibur Flow cytometer (Becton Dickinson, San Jose, Calif.). Optimal antibody concentrations used were empirically determined. Results were analyzed using CELL Quest software (Becton Dickinson).
Statistics.
INSTAT software (GraphPad, San Diego, Calif.) was used for unpaired two-tailed Student t tests. A P of <0.05 was considered significant.
RESULTS
IL-2−/− mice are susceptible to acute toxoplasmosis.
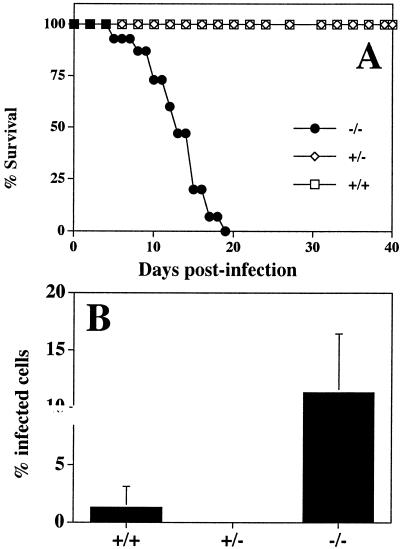
To address the role of IL-2 in the generation of protective T-cell responses during toxoplasmosis, IL-2−/− mice were infected i.p. with 20 cysts of the ME49 strain of T. gondii and survival was monitored. As expected, IL-2+/+ and IL-2+/− mice were resistant to acute toxoplasmosis (Fig. 1A). However, IL-2−/− mice infected with T. gondii succumbed to infection with 100% mortality by day 19 postinfection (Fig. 1A). This increased susceptibility to T. gondii infection correlated with increased parasite burden at the site of infection at day 7 postinfection (Fig. 1B). Immunohistochemical analyses of tissues from infected mice revealed that although the lungs of infected IL-2+/+, IL-2+/−, and IL-2−/− mice displayed a severe pneumonia, parasites were rare in IL-2+/+ and IL-2+/− mice, but parasites were readily detectable in the lungs of IL-2−/− mice (data not shown).
FIG. 1.
IL-2−/− mice are susceptible to infection with T. gondii. (A) IL-2+/+ (n = 15), IL-2+/− (n = 13), and IL-2−/− (n = 13) mice were infected intraperitoneally with 20 ME49 cysts, as described in Materials and Methods, and survival was monitored. Results presented are pooled data from three independent experiments containing three to five mice per group. (B) Peritoneal exudate cells were harvested from 7-day-infected mice (IL-2+/+, n = 2; IL-2+/−, n = 6; IL-2−/−, n = 6) and cytospins prepared to estimate the percentage of cells infected. Where the percentage of cells infected was less than 1% but parasites could still be observed, a value of 0.1% was assigned. Error bars, SD.
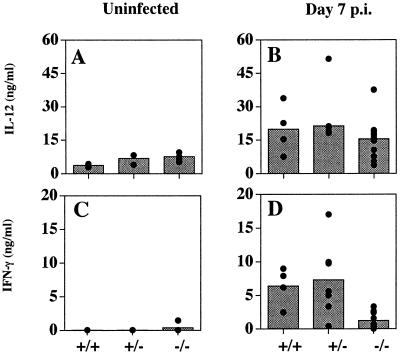
Analysis of levels of IL-12p40 and IFN-γ in serum in uninfected IL-2+/+, IL-2+/−, and IL-2−/− mice revealed similar levels of IL-12, and although the IL-2−/− mice had elevated baseline levels of IFN-γ, this did not reach statistical significance in these studies (P = 0.07) (Fig. 2A and C). Following infection for 7 days, there was a four- to fivefold increase in the levels of IL-12p40 in serum in all groups of mice and a marked increase in the serum levels of IFN-γ in IL-2+/+ and IL-2+/− mice (Fig. 2B and D). However, although there was a small infection-induced increase in levels of IFN-γ in serum infected IL-2−/− mice (P < 0.05), these levels were significantly lower than those for infected IL-2+/+ or IL-2 +/− mice (P < 0.002). Nevertheless, since IFN-γ−/− mice die within 10 days of infection with T. gondii (22), the low levels of IFN-γ produced by the IL-2−/− mice appear to provide a limited mechanism of resistance to T. gondii. The source of this IFN-γ in the IL-2−/− mice is unclear but may be derived from NK cells.
FIG. 2.
Infection of IL-2−/− mice results in normal IL-12 but reduced IFN-γ responses. Levels of IL-12p40 (A and B) and IFN-γ (C and D) in serum from uninfected (A and C) and 7-day-infected animals (B and D) were assessed by ELISA. No statistically significant differences were observed in levels of IL-12p40 and IFN-γ in serum among uninfected groups, nor were levels of IL-12p40 in serum from infected mice statistically significant. However, serum IFN-γ levels between 7-day-infected IL-2+/+ and IL-2−/− mice were statistically significant (P < 0.002). Results presented are pooled data from four independent experiments. Shaded bars represent the mean values and black circles are cytokine values of individual mice. p.i., postinfection.
T-cell expression of activation markers.
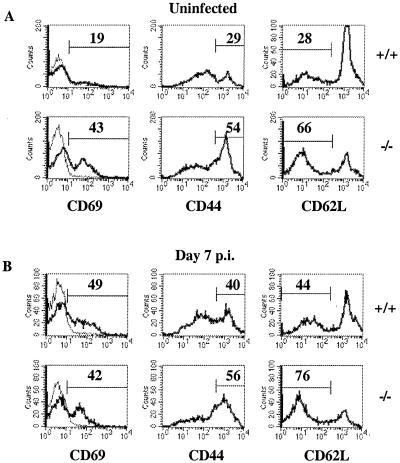
To determine whether IL-2 was required for the activation and/or expansion of T cells during toxoplasmosis, splenocytes from uninfected and infected mice were stained with antibodies and analyzed by fluorescence-activated cell sorting for expression of activation markers (CD69, CD44, and CD62L) by CD4+ and CD8+ T cells. The data shown in Fig. 3A demonstrate that approximately 20 to 25% of CD4+ T cells from uninfected IL-2+/+ mice expressed activated memory or effector phenotypes (CD69+, CD44hi, and CD62Llo). In contrast, approximately 45 to 60% of CD4+ T cells from uninfected IL-2−/− mice expressed an activated phenotype consistent with previous reports (19). At day 7 postinfection, there was an approximately twofold increase in the percentage of activated CD4+ T cells from IL-2+/+ mice (Fig. 3B), consistent with an approximately twofold increase in the total numbers of splenocytes (data not shown). In contrast, uninfected IL-2−/− mice had marked splenomegaly and infection did not result in increased numbers of splenocytes (data not shown) or the percentage of activated CD4+ T cells (Fig. 3B). Analysis of the expression of activation markers by CD8+ splenocytes from IL-2+/+, IL-2+/−, and IL-2−/− mice following infection revealed similar results (data not shown).
FIG. 3.
Expression of activation markers by CD4+ T cells from IL-2+/+ and IL-2−/− mice following infection with T. gondii. CD4+ splenocytes from uninfected (A) or 7-day-infected (B) IL-2+/+ and IL-2−/− mice were electronically gated and analyzed for expression of CD69, CD44, and CD62L as described in Materials and Methods. Numerical values shown represent the percentage of cells positive for CD69, CD44hi, and CD62Llo CD4+ splenocytes. Results shown are representative of two independent experiments containing two to four mice per group. Levels of expression of activation markers in CD4+ splenocytes from IL-2+/− mice were similar to those for IL-2+/+ mice (data not shown). Light lines represent background staining obtained with an isotype control.
Splenocytes from IL-2−/− mice have defects in the production of IFN-γ in vitro.
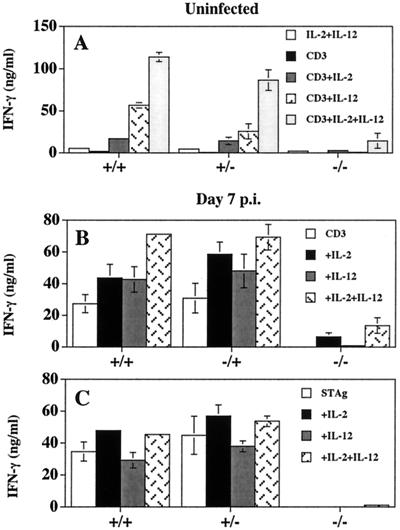
Since production of IFN-γ by T cells is the major mediator of resistance to T. gondii, and IL-2 and IL-12 have been demonstrated to be involved in the optimal production of IFN-γ by T cells (13, 22), we assessed the capacity of IL-2−/− cells to produce IFN-γ during toxoplasmosis. In a typical experiment, stimulation of splenocytes from IL-2+/+ and IL-2+/− mice with anti-CD3 resulted in the production of 1,900 ± 1,100 and 435 ± 360 pg/ml of IFN-γ, respectively, while splenocytes from uninfected IL-2−/− mice produced 53 ± 46 pg/ml of IFN-γ (values are means ± standard deviations [SD]). Furthermore, although the addition of IL-2 or IL-12 to cultures stimulated with anti-CD3 augmented IFN-γ production by splenocytes from uninfected IL-2+/+ and IL-2+/− mice, it did not restore the ability of IL-2−/− splenocytes to produce IFN-γ (Fig. 4A). Similar results were obtained using splenocytes from infected mice (Fig. 4B). Similarly, stimulation of splenocytes from IL-2+/+ and IL-2+/− mice with PMA and ionomycin, which nonspecifically activate protein kinase C and calcineurin signaling pathways, resulted in the production of IFN-γ (IL-2+/+, 80.3 ± 12.6 ng/ml; n = 5), whereas splenocytes from IL-2−/− mice given the same stimulus produced markedly less IFN-γ (2.7 ± 1.1 ng/ml; n = 3). To assess parasite-specific T-cell responses following infection with T. gondii, splenocytes from 7-day-infected IL-2+/+, IL-2+/−, and IL-2−/− mice were stimulated with STAg alone or in combination with IL-2 or IL-12 and the production of IFN-γ measured. As shown in Fig. 4C, splenocytes from IL-2+/+ and IL-2+/− mice produced high levels of IFN-γ when stimulated with STAg, but the addition of IL-2 or IL-12 did not significantly enhance the amount of IFN-γ produced. In contrast, splenocytes from IL-2−/− mice failed to produce IFN-γ and the addition of the combination of IL-2 plus IL-12 only slightly enhanced the production of IFN-γ by these cells. In additional studies, the inclusion of IL-15 or IL-18 to these cultures did not restore production of IFN-γ (data not shown). Previous studies have shown that both CD4+ and CD8+ T cells contribute to the production of IFN-γ in these types of experiments (13, 21, 27), therefore these results demonstrate that even under optimal stimulation conditions (i.e., T-cell receptor stimulation plus IL-2 and IL-12, or PMA and ionomycin), T cells from IL-2−/− mice have a profound defect in their ability to produce IFN-γ compared with T cells from IL-2+/+ and IL-2+/− mice.
FIG. 4.
Production of IFN-γ by splenocytes from IL-2+/+, IL-2+/−, and IL-2−/− mice. (A) Splenocytes, from uninfected mice, cultured with anti-CD3 in the presence of IL-2, IL-12, or IL-2 plus IL-12 for 48 h and supernatants were assayed for the production of IFN-γ. Splenocytes from mice infected 7 days previously and stimulated with anti-CD3 (B) or STAg (C) in the presence of IL-2, IL-12,or IL-2 plus IL-12 for 48 h were assayed for the production of IFN-γ. IFN-γ levels from infected splenocytes stimulated with IL-2 alone (IL-2+/+, 27.15 ± 10.51 ng/ml; IL-2+/−, 32.44 ± 9.12 ng/ml; IL-2−/−, less than 0.02 ng/ml) or IL-12 alone (IL-2+/+, 11.62 ± 8.14 ng/ml; IL-2+/−, 14.27 ± 6.03 ng/ml; IL-2−/−, less than 0.02 ng/ml) were determined. Results shown are means ± SD of a single experiment with three to four mice per experimental group and are representative of two independent experiments performed.
DISCUSSION
IL-2 is an important growth factor involved in the activation and expansion of T-cell responses during an immune response. The importance of IL-2 in T-cell-mediated immune responses is illustrated by studies in which it was shown that T cells from IL-2−/− mice have profound defects in their ability to proliferate in response to anti-CD3 or concanavalin A (15, 23). Moreover, previous studies also showed that IL-2−/− mice have a reduced capacity to generate antigen-specific CD8+ T-cell responses following infection with lymphocytic choriomeningitis virus (LCMV) (11). During infection with T. gondii it is thought that the production of IL-2 by CD4+ T cells is required for the optimal production of IFN-γ by CD8+ T cells (13, 27). Results of our study presented here demonstrate that IL-2−/− mice are susceptible to acute toxoplasmosis. Surprisingly, γc−/− mice, in which IL-2-IL-2R interactions are disrupted, are resistant to acute toxoplasmosis but susceptible to the chronic phase of infection (21). These differences in susceptibility to toxoplasmosis may be attributed to the fact that in γc−/− mice CD4+ T cells can make IFN-γ in response to infection, whereas the data presented here demonstrate that the ability of T cells from infected IL-2−/− mice to make IFN-γ was profoundly suppressed. One explanation for this difference is that CD4+ T cells from γc−/− animals express the IL-2Rβ chain which can activate mitogen-activated protein kinase and the STAT5 signaling pathways (7, 12). Thus, despite the absence of the common γ chain, T cells may still be able to respond to endogenous IL-2 through the IL-2Rβ chain whereas this pathway would be absent in IL-2−/− mice.
The studies presented here begin to address the role of IL-2 in the generation of protective antigen specific T-cell responses following infection with T. gondii and have revealed a major defect in the ability of T cells from IL-2−/− mice to produce IFN-γ. Although comparable levels of IL-12p40 production were detected in IL-2+/+, IL-2+/−, and IL-2−/− mice following infection with T. gondii, there was a major defect in the ability of splenocytes from uninfected or infected IL-2−/− mice to produce IFN-γ. There are several possible explanation to account for this defect. For example, although uninfected IL-2−/− mice had elevated numbers of activated T cells in the spleen compared with those for IL-2+/+ mice, there was only a modest increase in the number of activated T cells following infection. These data suggest that there may be an intrinsic defect in homeostatic feedback mechanisms present in the IL-2−/− mice which prevents activation and expansion of antigen specific IFN-γ producing T cells necessary for protection against T. gondii.
This is consistent with previous studies which have shown that IL-2−/− mice have defects in T-cell homeostasis, resulting in the accumulation of activated, autoreactive CD4+ T cells in secondary lymphoid organs and severe lymphoid hyperplasia (10, 15, 19). An alternative explanation is that in the absence of IL-2, T cells become anergic following activation. Previous studies have shown that T-cell activation in the absence of CD28 costimulation results in T-cell anergy associated with the lack of endogenous IL-2 necessary for T activation and expansion (5). However, this anergic state is transient and T-cell function can be restored by exogenous IL-2. In contrast, the addition of IL-2, alone or in combination with other cytokines, to splenocytes from uninfected or infected IL-2−/− mice did not restore IFN-γ production suggesting that anergy is not the cause of the defect in the ability of IL-2−/− T cells to produce IFN-γ.
Of relevance to the data presented here, Cousens and others have reported that IL-2−/− mice cannot generate antigen specific production of IFN-γ and CD8+ CTL responses during LCMV infection (11). In their studies, LCMV-specific CD8+ CTL responses and production of IFN-γ were restored to wild-type levels following exogenous addition of IL-2. However, the data presented here show that the addition of IL-2 did not restore the production of IFN-γ by splenocytes from IL-2−/− mice whether or not they were infected. Moreover, stimulation with PMA and ionomycin, which bypasses receptor-mediated signaling events and activates protein kinase C and calcium/calcineurin signaling pathways, had little effect on the production of IFN-γ by splenocytes from IL-2−/− mice, suggesting that T cells from IL-2−/− mice have defects in the activation of transcription factors necessary for optimal T-cell function. The basis for this defect is unclear and whether it relates to the underlying autoimmune condition of the IL-2−/− mice is open to question. Nevertheless, because of the presence of large numbers of activated T cells in uninfected IL-2−/− mice, the studies presented here do not resolve the role of IL-2 in the expansion of parasite specific CD4+ T cells and alternative strategies are needed to address this issue. However, although these studies do not tell us whether IL-2 is important in the CD28-dependent expansion of parasite-specific CD4+ T cells, our findings show that the increased susceptibility of IL-2−/− mice to toxoplasmosis correlates with the inability of these mice to produce IFN-γ.
Acknowledgments
This work was supported by Public Health Service grants AI 42334 and AI 41158 awarded to C.A.H. and by grants AI31972, AI45993, AI41562, and PO1RR12211 awarded to S.R.C. E.N.V. was supported by an NIH minority predoctoral fellowship (AI 09562).
Editor: J. M. Mansfield
REFERENCES
- 1.Blewett, D. A., J. K. Miller, and J. Harding. 1983. Simple technique for the direct isolation of toxoplasma tissue cysts from fetal ovine brains. Vet. Rec. 112:98-100. [DOI] [PubMed] [Google Scholar]
- 2.Bluestone, J. A. 1995. New perspectives of CD28-B7-mediated T cell costimulation. Immunity 2:555-559. [DOI] [PubMed] [Google Scholar]
- 3.Boise, L. H., A. J. Minn, P. J. Noel, C. H. June, M. A. Accavitti, T. Lindsten, and C. B. Thompson. 1995. CD28 costimulation can promote T cell survival by enhancing the expression of BCl-xL. Immunity 3:87-98. [DOI] [PubMed] [Google Scholar]
- 4.Boise, L. H., P. J. Noel, and C. B. Thompson. 1995. CD28 and apoptosis. Curr. Opin. Immunol. 7:620-625. [DOI] [PubMed] [Google Scholar]
- 5.Boussiotis, V. A., F. J. Freeman, J. G. Gribben, and L. E. Nadler. 1996. The role of B7-1/B7-2:CD28/CTLA-4 pathways in the prevention of anergy, induction of productive immunity and down-regulation of the immune response. Immunol. Rev. 153:5-25. [DOI] [PubMed] [Google Scholar]
- 6.Brinkmann, V., S. D. Sharma, and J. S. Remington. 1986. Differential regulation of the L3T4-T cell subset by B-cells in different mouse strains bearing the H-2k haplotype. J. Immunol. 137:2991-2997. [PubMed] [Google Scholar]
- 7.Cacalano, N. A., and J. A. Johnston. 1999. Interleukin-2 signaling and inherited immunodeficiency. Am. J. Hum. Genet. 65:287-293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cerdan, C., Y. Martin, M. Courcoul, C. Mawas, F. Birg, and D. Olive. 1995. CD28 costimulation regulates long-term expression of the three genes (alpha, beta, gamma) encoding the high-affinity IL2 receptor. Res. Immunol. 146:164-168. [DOI] [PubMed] [Google Scholar]
- 9.Conley, F. K., and K. A. Jenkins. 1981. Immunohistochemical study of the anatomic relationship of toxoplasma antigen to the inflammatory response in the brains of mice chronically infected with Toxoplasma gondii. Infect. Immun. 31:1184-1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Contractor, N. V., H. Bassiri, T. Reya, A. Y. Park, D. C. Baumgart, M. A. Wasik, S. G. Emerson, and S. R. Carding. 1998. Lymphoid hyperplasia, autoimmunity, and compromised intestinal intraepithelial lymphocyte development in colitis-free gnotobiotic IL-2-deficient mice. J. Immunol. 160:385-394. [PubMed] [Google Scholar]
- 11.Cousens, L. P., J. S. Orange, and C. A. Biron. 1995. Endogenous IL-2 contributes to T cell expansion and IFN-γ production during lymphocytic choriomeningitis virus infection. J. Immunol. 155:5690-5699. [PubMed] [Google Scholar]
- 12.Friedmann, M. C., T. S. Migone, S. M. Russell, and W. J. Leonard. 1996. Different interleukin 2 receptor β-chain tyrosines couple to at least two signaling pathways and synergistically mediate interleukin 2-induced proliferation. Proc. Natl. Acad. Sci. USA 93:2077-2082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gazzinelli, R. T., F. T. Hakim, S. Hieny, G. M. Shearer, and A. Sher. 1991. Synergistic role of CD4+ and CD8+ T lymphocytes in IFN-γ production and protective immunity induced by an attenuated Toxoplasma gondii vaccine. J. Immunol. 146:286-292. [PubMed] [Google Scholar]
- 14.Hunter, C. A., J. S. Abrams, M. H. Beaman, and J. S. Remington. 1993. Cytokine mRNA in the central nervous system of SCID mice infected with Toxoplasma gondii importance of T-cell-independent regulation of resistance of T. gondii. Infect. Immun. 61:4038-4044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kundig, T. M., H. Schorle, M. F. Bachmann, H. Hengartner, R. M. Zinkernagel, and I. Horak. 1993. Immune responses in interleukin-2-deficient mice. Science 262:1059-1061. [DOI] [PubMed] [Google Scholar]
- 16.Lenschow, D. J., T. L. Walunas, and J. A. Bluestone. 1996. CD28/B7 system of T cell costimulation. Annu. Rev. Immunol. 14:233-258. [DOI] [PubMed] [Google Scholar]
- 17.Lindstein, T., C. H. June, J. A. Ledbetter, G. Stella, and C. B. Thompson. 1989. Regulation of lymphokine messenger RNA stability by a surface-mediated T cell activation pathway. Science 244:339-343. [DOI] [PubMed] [Google Scholar]
- 18.Reichmann, G., E. N. Villegas, L. Craig, R. Peach, and C. A. Hunter. 1999. The CD28/B7 interaction is not required for resistance to Toxoplasma gondii in the brain but contributes to the development of immunopathology. J. Immunol. 163:3354-3362. [PubMed] [Google Scholar]
- 19.Sadlack, B., H. Merz, H. Schorle, A. Schimpl, A. C. Feller, and I. Horak. 1993. Ulcerative colitis-like disease in mice with a disrupted interleukin-2 gene. Cell 75:253-261. [DOI] [PubMed] [Google Scholar]
- 20.Sander, B., I. Hoiden, U. Anderson, E. Moller, and J. S. Abrams. 1993. Similar frequencies and kinetics of cytokine producing cells in murine peripheral blood and spleen. J. Immunol. Methods 166:201-214. [DOI] [PubMed] [Google Scholar]
- 21.Scharton-Kersten, T., H. Nakajima, G. Yap, A. Sher, and W. J. Leonard. 1998. Infection of mice lacking the common cytokine receptor γ-chain (γc) reveals an unexpected role for CD4+ T lymphocytes in early IFN-γ-dependent resistance to Toxoplasma gondii. J. Immunol. 160:2565-2569. [PubMed] [Google Scholar]
- 22.Scharton-Kersten, T. M., T. A. Wynn, E. Y. Denkers, S. Bala, E. Grunvald, S. Hieny, R. T. Gazzinelli, and A. Sher. 1996. In the absence of endogenous IFN-γ, mice develop unimpaired IL-12 responses to Toxoplasma gondii while failing to control acute infection. J. Immunol. 157:4045-4054. [PubMed] [Google Scholar]
- 23.Schorle, H., T. Holtschke, T. Hunig, A. Schimpl, and I. Horak. 1991. Development and function of T cells in mice rendered interleukin-2 deficient by gene targeting. Nature 352:621-624. [DOI] [PubMed] [Google Scholar]
- 24.Shahinian, A., K. Pfeffer, K. P. Lee, T. M. Kundig, K. Kishihara, A. Wakeham, K. Kawai, P. S. Ohashi, C. B. Thompson, and T. W. Mak. 1993. Differential T cell costimulatory requirements in CD28-deficient mice. Science 261:609-612. [DOI] [PubMed] [Google Scholar]
- 25.Sharma, S. D., J. Mullenax, F. G. Auaujo, A. A. Erlich, and J. S. Remington. 1983. Western blot analysis of the antigens of Toxoplasma gondii recognized by human IgM and IgG antibodies. J. Immunol. 131:977-983. [PubMed] [Google Scholar]
- 26.Thompson, C. B., T. Lindsten, J. A. Ledbetter, S. L. Kunkel, H. A. Young, S. G. Emerson, J. M. Leiden, and C. H. June. 1989. CD28 activation pathway regulates the production of multiple T-cell-derived lymphokines/cytokines. Proc. Natl. Acad. Sci. USA 86:1333-1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Villegas, E. N., M. M. Elloso, G. Reichmann, R. Peach, and C. A. Hunter. 1999. Role of CD28 in the generation of effector and memory responses required for resistance to Toxoplasma gondii. J. Immunol. 163:3344-3353. [PubMed] [Google Scholar]