Abstract
Trypanosoma cruzi proteinases are involved in host cell invasion in human patients and in mouse models. In mice, murine α2-macroglobulin (MAM) and murinoglobulin are circulating plasma proteinase inhibitors that also have important roles in inflammation and immune modulation. To define their role in experimental Chagas disease, we investigated the susceptibility to T. cruzi infection of mice that are deficient only in α2-macroglobulins (AM-KO) or in both MAM and monomeric murinoglobulin-1 (MM-KO), relative to the wild type (WT). Despite the high parasite load, parasitemia was lower in AM-KO and MM-KO mice than in WT mice. Nevertheless, we observed a significantly higher parasite load in the hearts of AM-KO and MM-KO mice, i.e., more amastigote nests and inflammatory infiltrates than in WT mice. This result demonstrates a protective role for MAM in the acute phase of murine T. cruzi infection. We further demonstrated in vitro that human α2-macroglobulins altered the trypomastigote morphology and motility in a dose-dependent way, and that also impaired T. cruzi invasion in cardiomyocytes. Finally, we demonstrated that the levels of transforming growth factor β in AM-KO mice increased significantly in the third week postinfection, concomitant with high amastigote burden and important fibrosis. Combined, these in vivo and in vitro findings demonstrate that the MAM contribute to the resistance of mice to acute myocarditis induced by experimental T. cruzi infection.
α-Macroglobulins (AM) are physiological proteinase inhibitors with important roles in inflammation and immune modulation, with some isoforms behaving as acute-phase proteins in some animals (14, 34). In mice, two main AM are present as plasma proteins, the tetrameric murine α2-macroglobulin (MAM) equivalent to human α2-macroglobulin (A2M) and the monomeric murinoglobulin-1 (MUG) (24, 35). The ability of AM to bind to a wide range of proteinases as well as of several physiologically important molecules, including cytokines and mitogens (13), would enable AM to contribute to the homeostasis of proteolytic activities (14) and to increase the half-life of cytokines (13) and other proteinase-sensitive molecules. Proteolytic cleavage of the bait region in AM breaks the cysteinyl-glutamyl internal thiolesters, leading to a major conformational change in the native molecule (N-A2M) to the transformed or fast form (F-A2M) in which the proteinase is trapped and hindered in its access to substrates (34). F-A2M exposes a cryptic receptor-binding domain that binds specifically to the multifunctional A2M receptor to clear the AM-proteinase complexes from the circulation (34). The major A2M receptor is identical to the low-density lipoprotein receptor-related protein (LRP) that is responsible for the clearance of many other unrelated ligands in addition to the A2M-proteinases complexes (for review, see references 7 and 31).
We are studying the role of AM in an experimental mouse model of Trypanosoma cruzi infection, the parasite that causes Chagas disease (6, 26). Parasite proteinases are very likely involved in host cell invasion (reviewed in reference 3), and we proposed that A2M could protect the host. We have shown that in BALB/c mice increased AM levels correlated with survival to acute T. cruzi infection (4) and also that there is a heterogeneity of AM responses in outbred and inbred mouse strains (15, 16), indicating the involvement of additional mechanisms. More recently, we observed that the time course of expression of liver mRNA coding for MAM, MUG, and AM receptor systems was very different in C57BL/6 and in C3H-infected mice (29). Results obtained with human individuals lend further support to the hypothesis of the protective role of AM, since we observed that asymptomatic Bolivian children acutely infected by T. cruzi had higher plasma levels of A2M than age-matched symptomatic patients (18).
To define the role of AM, we have now investigated the course of T. cruzi infection in mice deficient in MAM (AM-KO) or deficient in MAM and MUG combined (MM-KO) compared to wild-type (WT) mice (32, 33). AM-KO has normal levels of MUG and expresses normal levels of LRP while being more resistant to endotoxin.
Our present results show conclusively that AM plays a protective role in early T. cruzi infection, especially by controlling the parasite load in tissues, such as the heart, and by regulating transforming growth factor β (TGF-β)-mediated fibrotic effects. However, other mechanisms contribute to the overall resistance of the KO animals, which indeed may successfully face sublethal parasite challenges and survive acute infection.
MATERIALS AND METHODS
Knock-out mice.
Mice with a targeted disruption of the MAM gene (AM-KO, n = 30) or of the MAM and MUG genes combined (double knock-out) (MM-KO, n = 29) were generated as previously described (32, 33). Both deficient strains were backcrossed into the C57BL/6 background for at least seven generations. All experiments were carried out with female mice 8 weeks old on the day of infection (T. cruzi or sham). Mice were housed five per cage and kept in a conventional animal room maintained at 20 to 25°C under a 12/12-h light/dark cycle. Sterile water and food were provided ad libitum. Following infection and euthanasia by cervical dislocation, the organs were removed and weighted. All procedures were carried out in accordance with the guidelines established by the animal facilities of Pasteur Institute and following international guidelines and ethics for the handling of experimental animals.
Infection with T. cruzi and analysis.
Bloodstream trypomastigotes of T. cruzi CL/Brener clone F11F5 were isolated from the blood of infected mice obtained by cardiac puncture at the peak of parasitemia (28). Mice were infected by intraperitoneal injection of 103 bloodstream trypomastigotes, while control (uninfected) mice were injected with vehicle only and further maintained in the same conditions. The level of parasitemia was determined on individual blood samples by direct microscopic counting of 5 μl of blood collected from the tail vein (15) on different days postinfection (dpi). Serum or EDTA-plasma samples were collected from the retro-orbitary plexus before infection and on 3, 6, 15, and 22 dpi. At 15 and 22 dpi, mice were euthanized and the heart and liver were quickly removed, cut longitudinally, rinsed in ice-cold phosphate-buffered saline (10 mM sodium phosphate, 0.015 M NaCl, pH 7.4), and fixed in Millonig-Rosman solution (10% formaldehyde in phosphate-buffered saline). At least four infected and four uninfected mice were analyzed at each time point.
Histopathology.
Fixed tissue was dehydrated and embedded in paraffin. Sections (3 μm) stained by routine hematoxylin-eosin (HE) were analyzed by light microscopy. The extent of necrosis and fibrosis, the number of amastigote nests and of inflammatory infiltrates (more than 10 mononuclear cells), was determined in 15 microscopical fields/slide (11.5 mm2; total magnification, 400×). The mean number of amastigotes or inflammatory infiltrates per field was obtained from at least five infected mice (15 and 22 dpi), with three sections per mouse per time point and per genotype. Since data displayed a non-normal distribution, the non-parametric Mann-Whitney U test was applied to ascertain the significance of the observed differences. Fibrosis was further studied by Masson trichrome and picrosirius red staining (10). Staining with 4′,6′-diamidino-2-phenylindole (DAPI; Sigma, St. Louis, Mo.) was performed to reveal nuclear and kinetoplast DNA of intracellular amastigotes.
Cytokine measurements.
We measured total TGF-β on acid-treated plasma samples by enzyme-linked immunosorbent assay (Promega, Madison, Wis.), according to the manufacturer's instructions. Gamma interferon (IFN-γ), interleukin-2 (IL-2), IL-4, and IL-10 serum levels were measured by enzyme-linked immunosorbent assay as described previously (28).
In vitro studies.
Primary cultures of cardiomyocytes were prepared from mouse embryos as previously described (19) and maintained in medium supplemented with 5% fetal calf serum, 5% horse serum, 2% chicken embryo extract, 1 mM glutamine, and 2.5 mM CaCl2, plated in 24-well culture plates, and maintained at 37°C in 5% CO2 atmosphere. After 24 h, cells were infected with bloodstream trypomastigotes of T. cruzi obtained as described above. For some in vitro assays, the T. cruzi Y strain was used to obtain higher yields of infected cells (19).
In vitro assays using F-A2M.
Native human plasma α2-macroglobulin (HuA2M) was prepared from pooled citrated plasma using Zn affinity chromatography (generous gift from F. Pochon and E. Delain) and inactivated by methylamine, thus generating F-A2M as previously described (34). Parasites or cardiomyocytes were incubated for 5 or 24 h with F-A2M at concentrations varying from 1 to 100 μg/ml, followed by analysis for motility (parasites), morphology, and rate of contraction (heart cells). Parasite motility was scored on a scale from 0 (not mobile) to 3 (highly mobile). A similar semiquantitative scale was defined for heart cell rate of contraction, ranging from 0 (not contracting) to 3 (normal beating cells). The effect of F-A2M on parasite-host cell interaction was determined by adding it during infection at the concentrations indicated. In alternative experiments, parasites and/or cardiomyocytes were pretreated with F-A2M for 1 h at 37°C. After 5 and 24 h of parasite-cell interaction, cells were fixed with Bouin solution and stained with Giemsa. The percentage of myofibers containing parasites and the mean number of intracellular parasites per infected cell were determined (19).
RESULTS
Time course of T. cruzi infection.
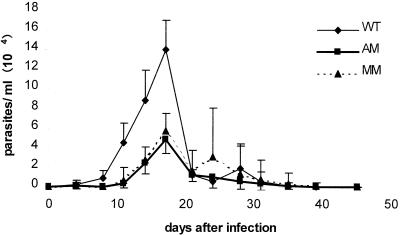
We first established the conditions in which AM-KO and MM-KO mice survived an acute infection with different inocula: lethal (10,000 parasites/mice, 50% mortality) or sublethal (1,000 parasites/mice, 0 to 10% mortality) as used for wild-type C57BL/6 mice. The 104 inoculum was similarly lethal for the AM-KO and MM-KO mice and resulted in high parasitemia in all mice, ranging from 2 × 105 to 10 × 105 parasites/ml and increasing until the death of the animals after 18 to 24 days (data not shown). All AM-KO mice and 75% of the MM-infected mice survived the 103 inoculum and developed a lower peak of parasitemia in two independent experiments (data not shown). Mean parasitemia curves were recorded for eight mice/genotype, infected with 103 parasites (Fig. 1). Similar kinetics of parasitemia were recorded for the three genotypes, with ascending and descending phases and peak levels attained at 17 dpi; still, parasitemia of the AM-KO and MM-KO mice were about 50% lower than that of WT mice over the entire period studied.
FIG. 1.
Parasitemia curves of WT, AM-KO, and MM-KO infected mice. Animals were infected with 103 parasites from the CL-Brener clone, clone F11F5. Results are the means of eight mice/group in the same experiment.
Histopathological analysis.
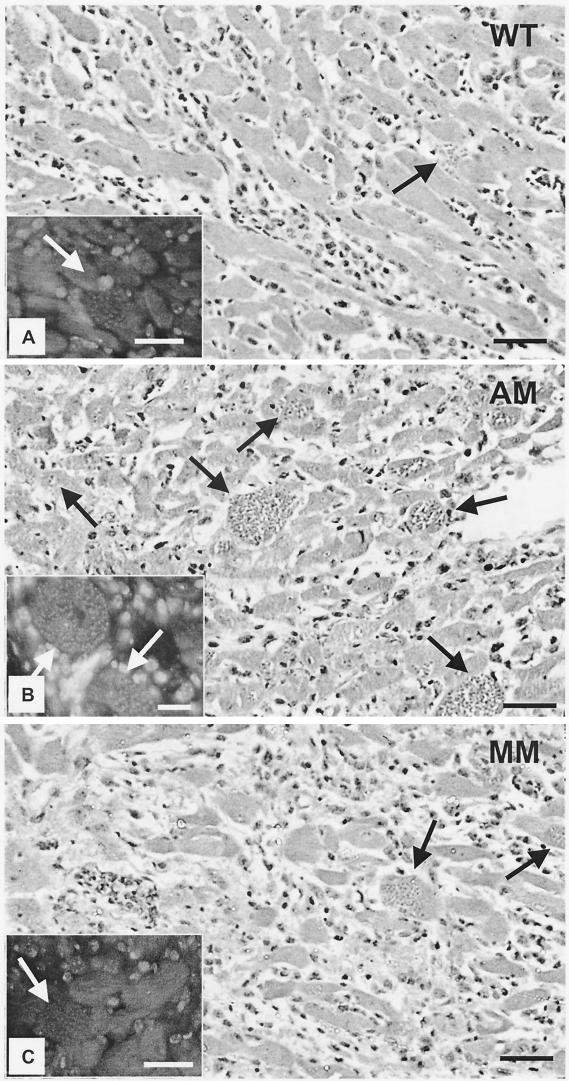
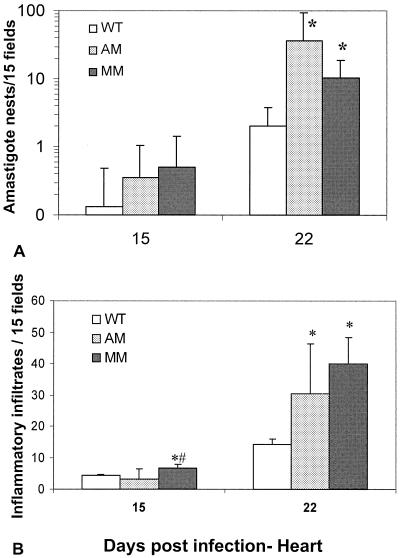
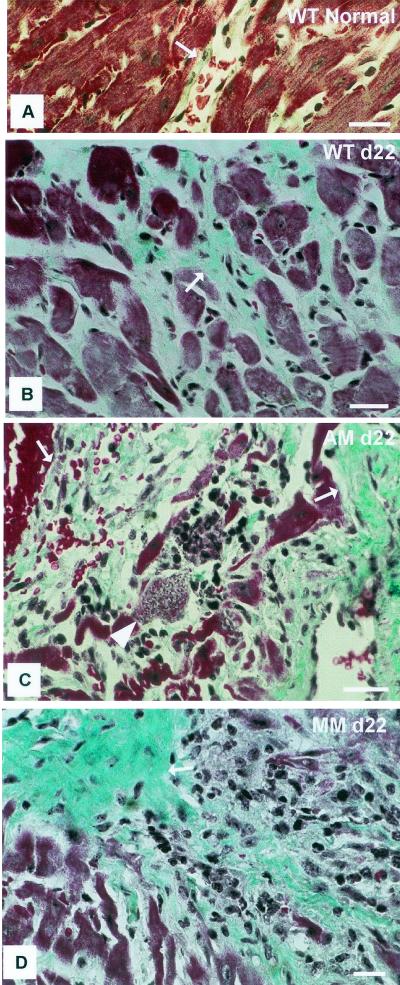
The unexpected finding of lower parasitemias in AM-KO and MM-KO mice prompted us to investigate the parasitic load in the hearts and livers of the infected animals. Both HE- and DAPI-stained sections clearly showed the occurrence of parasite nests to be different in different tissues (Fig. 2 to 4). The hearts of AM-KO (Fig. 2B) and MM-KO mice (Fig. 2C) contained several larger amastigote nests 22 dpi than did WT mice (Fig. 2A). The parasite load was already different 15 dpi and became statistically significant 22 dpi (Fig. 3A, log scale), which is after the peak of plasma parasitemia. In the AM-KO mice, infection 22 dpi was about 50 times higher than 15 dpi. Concomitant with high parasitism 22 dpi, the number of inflammatory foci and of interstitial mononuclear infiltrates (Fig. 2 and 3B) was significantly higher in AM-KO and MM-KO mice than in WT mice. HE-stained sections demonstrated that fibrosis and necrosis were also higher in AM-KO and MM-KO mice than in WT mice.
FIG. 2.
Histopathology of HE-stained heart sections from WT (A), AM-KO (B), and MM-KO (C) mice 22 dpi with T. cruzi. Note mononuclear infiltrating cells and amastigote nests (black arrows), which were much more frequent in AM-KO- and MM-KO-infected mice. Insets show fluorescent DNA stained with DAPI, with clear detection of amastigote nests (white arrows). Bars, 50 μm.
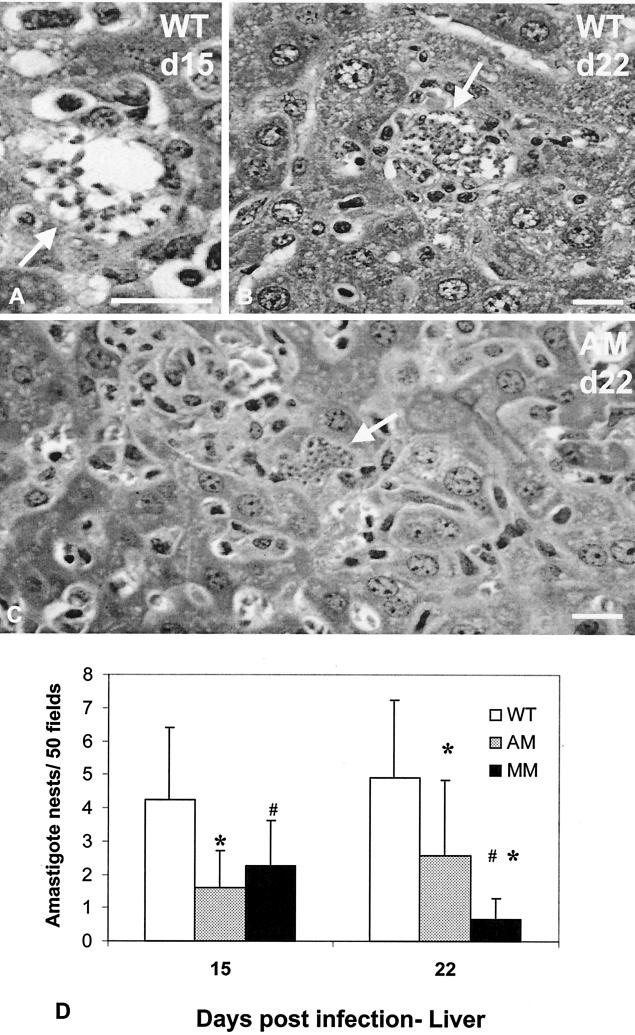
FIG. 4.
Histopathology of HE-stained liver sections from WT (A and B) and KO-AM (C) mice 15 (A) and 22 (B and C) dpi with T. cruzi. Note amastigote nests (white arrows) that are seen in macrophages (A) or in hepatocytes (C). Amastigote nests were quantified (mean and standard deviation) in 50 microscopic fields for WT, AM-KO, and MM-KO mice (D), where statistically significant differences (P < 0.05, Mann-Withney test) are marked for WT (*) and AM-KO (#). Bars, 50 μm.
FIG. 3.
Parasite (amastigote nests) (A) and inflammatory (cell infiltrates with more than 10 cells/infiltrate) (B) loads in hearts of WT, AM-KO, and MM-KO infected mice 15 or 22 dpi with T. cruzi. Means and standard deviations are shown. Statistically significant differences (P < 0.05, Mann-Whitney test) are marked for the WT (*) and KO-AM (#).
Since the results did not show a direct correlation between tissue and blood parasitism and since organ compartmentalization of parasite invasion and growth was dependent on the genetic background and associated with basal levels of AM (27), we also analyzed the parasite load in the liver (Fig. 4). Considerably more nests were found in the WT mice (Fig. 4A and B), mainly in Kupffer cells. In AM-KO mice, the rarely observed amastigotes were detected mainly in hepatocytes (Fig. 4C), which were not found in infected WT mice. The analysis for amastigote nests in liver sections was extended to 50 microscopic fields, but even then fewer than 10 nests were observed in infected WT mice (Fig. 4D). It must be noted that a significantly lower amastigote load was consistently evident in AM-KO- and MM-KO-infected mice both 15 and 22 dpi, compared to WT mice (Fig. 4D), with a better correlation with the parasitemia data.
In vitro studies of infection of cardiomyocytes by T. cruzi.
The results revealed that the myocardium of infected AM-KO and MM-KO mice contained about 10 times more amastigote nests than infected WT mice. This and the fact that A2M can inhibit T. cruzi infection in vitro (2) prompted us to assay the infection of primary cultures of cardiomyocytes. The protective role of AM for invasion of cardiomyocytes was approached directly by using the fast form of A2M (F-A2M). The rationale was that plasma A2M accumulating in the inflamed myocardium (8) will inhibit both T. cruzi and tissue-derived proteinases (23), leading to high levels of F-A2M in a microenvironment that is low in A2M-receptor (LRP; 29). Methylamine-inactivated human A2M was therefore assayed for its direct effect on parasites and heart cells and on T. cruzi/host-cell interaction.
Morphological alteration of the bloodstream trypomastigotes was evidenced when treated with a low concentration (25 μg/ml) of F-A2M (Table 1). Also, the motility of the parasites decreased at this concentration but became markedly different, mainly at higher concentrations of F-A2M (50 and 100 μg/ml). Trypomastigotes collected from the serum-free supernatant of infected cell cultures (72 h postinfection) were found to display altered morphology and decreased motility, even at lower F-A2M concentrations (1 to 10 μg/ml). The higher concentration of F-A2M (100 μg/ml) lysed the parasites after 24 h of incubation, indicating that they were affected by F-A2M in a dose- and time-dependent manner (Table 1).
TABLE 1.
Effect of F-A2M on trypomastigotes of T. Cruzi
| Concn of F-A2M (μg/ml) in: | Trypomastigote characteristicsa
|
|||
|---|---|---|---|---|
| Morphology
|
Motilityb
|
|||
| 5 h | 24 h | 5 h | 24 h | |
| Blood | ||||
| 0 | Slender | Slender | 3 | 3 |
| 1 | Slender | Slender | 3 | 3 |
| 10 | Slender | Slender | 3 | 3 |
| 25 | Stout | Stout | 2 | 2 |
| 50 | Stout | Round | 2 | 2 |
| 100 | Stout | Round | 2 | 1 |
| Culture | ||||
| 0 | Slender | Slender | 3 | 2 |
| 1 | Stout | Stout | 3 | 2 |
| 10 | Stout | Stout | 3 | 2 |
| 25 | Round | Round | 2 | 1 |
| 50 | Round | Round | 1 | 0 |
| 100 | Round | Lysed | 1 | —c |
T. cruzi Y strain.
Classification ranges from 0 to 3 to score the motility of parasites: 0 = immobile; 3 = highly mobile. Results shown are the means of four independent experiments.
—, impossible to evaluate.
F-A2M also affected cultured cardiomyocytes (Table 2), as evidenced by changed cell morphology, characterized by less refringent membranes and lower contractility after 20 h incubation with the high concentration of 100 μg of F-A2M/ml. However, changes in the ability of the heart muscle cells to contract was noted even when a lower dose (10 μg/ml) of F-A2M was used.
TABLE 2.
Effect of F-A2M on morphology and contractility of heart cells
| F-A2M concn (μg/ml) | Time (h)a | Morphology | Contractionb |
|---|---|---|---|
| 0 | 1 to 4 | Normal | + |
| 1 | 1 to 4 | Normal | + |
| 10 | 1 to 4 | Normal | + |
| 100 | 1 to 4 | Normal | + |
| 0 | 20 | Normal | + |
| 1 | 20 | Normal | + |
| 10 | 20 | Normal | − |
| 100 | 20 | Altered | − |
The cultures were observed under the inverted optical microscope for 1 to 4 hours, and after 20 h incubation.
Indicated are the presence (+) or absence (−) of heart cell contraction. Results shown are the means of four independent experiments.
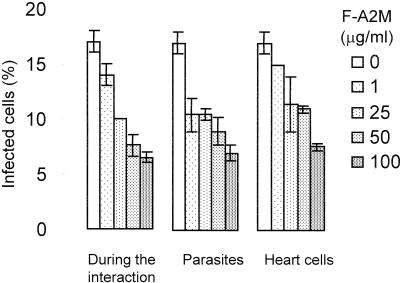
The effect of F-A2M on T. cruzi invasion in cardiomyocytes was assayed after 5 h of parasite-cell interaction, a condition in which neither the host cells nor the parasites were directly affected by F-A2M. F-A2M decreased the percentage of infected cells in a dose-dependent way (Fig. 5). To test if this effect was due to the action of F-A2M upon the parasites or upon the cardiomyocytes, we separately pretreated each of them with F-A2M at 37°C for 1 h and, after washing, combined and observed them for 5 h of parasite-cell interaction. In both conditions, cell invasion was dose-dependently inhibited by F-A2M (Fig. 5), but the effect was more pronounced when the parasites were pretreated, resulting in 38 and 60% inhibition of cell invasion at 1 and 100 μg of F-A2M/ml, respectively. The follow-up of these cultures for up to 72 h showed that the few parasites that succeeded invasion did develop the entire cycle, transforming into amastigotes, proliferating, and differentiating in trypomastigotes (data not shown). Taken together, these results confirmed that AM impairs cell invasion by T. cruzi but not amastigote proliferation and strongly indicate that the high parasite loads observed in the heart of infected AM-KO and MM-KO mice were the result of higher T. cruzi cell invasion in the absence of MAM.
FIG. 5.
Effect of F-A2M (1 to 100 μg/ml) on T. cruzi interaction with cardiomyocites (5 h) with three different systems: adding A2M during interaction (a), pretreating the parasites (b), and pretreating the host cells (c). The parasite-to-cell ratio was 10:1, and data were obtained by counting 400 cells/slide in three experiments performed in duplicate.
Fibrosis and TGF-β levels in serum of noninfected and infected mice.
Since A2M is a physiological carrier for TGF-β (36) and since signaling through TGF-β receptors is crucial in cell invasion by T. cruzi (22, 11), we examined whether, in the absence of MAM, TGF-β contributed to the higher parasite load and increased fibrosis.
Noninfected mice of the three genotypes did not contain any signs of fibrosis (Fig. 6A). Infected WT mice presented moderate staining with both Masson and Picrosirius red (Fig. 6B) as opposed to AM-KO (Fig. 6C) and MM-KO (Fig. 6D) infected mice that showed large fibrotic areas (stained in blue by affinity of the collagen fibers in extracellular matrix with Masson staining), especially at 22 dpi, when the parasite load was most important.
FIG. 6.
Extracellular matrix staining with Masson trichrome in heart sections from WT (A and B), AM-KO (C), and MM-KO (D) mice 22 dpi with T. cruzi. Note the areas of blue staining (arrows) in the hearts of infected mice (B to D), larger in AM-KO (C) and MM-KO (D) than in the WT (B), contrasting to the slight blue area in sections from noninfected mice (WT- 6A), which was similar and representative from noninfected AM-KO and MM-KO mice (not shown). Bars, 50 μm.
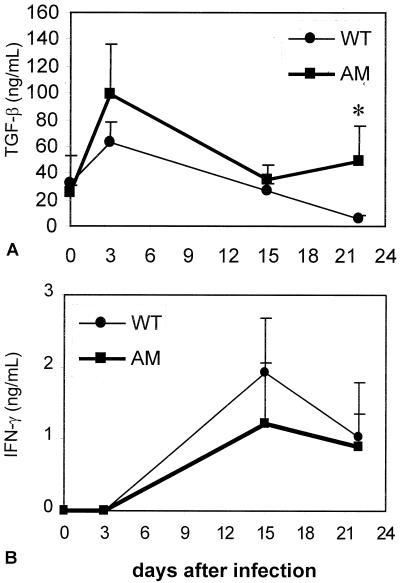
Since MUG does not bind to TGF-β (35), total TGF-β levels in serum of WT and AM-KO mice were compared (Fig. 7A) and were shown to be increased soon after the infection in both WT and AM-KO mice but were significantly higher in AM-KO mice 22 dpi, concomitantly with high tissue amastigote numbers and larger areas of fibrosis (Fig. 3A and 6C). IL-2, IL-4, and IL-10 levels were not detected in any group of mice (data not shown), but IFN-γ levels showed a similar kinetics of increase 15 dpi with a decrease 22 dpi (Fig. 7B) in both WT and AM-KO-infected mice.
FIG. 7.
TGF-β (A) and IFN-γ (B) circulating levels in WT (circles) and AM-KO (squares) mice. *, statistically significant difference in TGF-β levels between WT and AM-KO 22 dpi with T. cruzi (P < 0.05).
DISCUSSION
The data presented here demonstrate directly, by using a gene knock-out mouse model, that A2M and MAM are involved in the control of T. cruzi invasion into heart and other tissue cells. The in vivo data were further complemented and supported by experiments on cultured cells.
Heart myofibers are important targets for parasite invasion and inflammatory cell infiltration (21). Chronic myocarditis is the main pathological finding associated with Chagas' disease morbidity and is probably triggered by parasite-host interaction during the initial acute phase (17). Parasite proteases, i.e., cruzipain, were shown to be important for parasite-host interaction (20), and the levels of MAM and A2M, important endogenous plasma proteinase inhibitors, rise in the acute phase of both experimental (12, 6,16) and human (18) Chagas disease. In vivo studies of BALB/c mice indicated that the ability to increase AM levels was positively associated with the rate of survival to the acute phase (4). Earlier observations of heterogeneity among mouse strains (15, 16) could be explained by recent work demonstrating different genetic backgrounds to be associated with different basal levels of MAM and MUG (29). In addition, the kinetics of MAM, MUG, and LRP mRNA expression were profoundly different between C57BL/6 and C3H mice (30). The latter study also showed that parasitemia did not reflect directly the heart tissue parasitism in those mouse strains. Such findings could help to explain why the AM-KO and MM-KO mice could have lower parasitemia than WT mice despite the fact that their levels of heart parasitism was about 5 to 10 times higher. It is possible that in the absence of MAM or of both MAM and MUG, the parasites released from disrupted infected cells in the first cycles are not hindered by the normal physiological proteinase inhibitors and infect neighboring cells more easily. This may explain the high number of amastigote nests in the heart of the AM- or MM-deficient mice, but not the lower parasitemia. Moreover, since the CL clone F11F5 is characteristically myotropic, developing poorly in the liver and better in the heart (21), the higher number of circulating parasites in WT mice could have originated in the liver. Indeed, after 15 dpi, more amastigote nests were found in the liver of WT mice than in those of AM-KO mice. A differential effect on heart and liver parasitism was reported in T. cruzi-infected mice treated with anti-CD8 monoclonal antibodies (27). Besides, the in vitro treatment of resident macrophages with A2M increases the levels of phagocytosis through different receptors and activates uptake and killing of T. cruzi (5). It is then also possible that A2M exert different effects in distinct tissues, depending on their enrichment or not in professional phagocytes (such as Kupffer cells in the liver), thus contributing to the compartmentalized infection that we presently observed.
Mononuclear infiltration in the heart is a common feature in experimental acute infection by T. cruzi. The expression of chemokines and cytokines that mediate this process (1) was shown to correlate with the intensity of T. cruzi invasion and proliferation in the inflamed heart. Then, the increased inflammatory cell recruitment observed in the infected AM- and MM-deficient mice is probably due to a larger parasite load. This further attests to the important role of A2M (in humans) and MAM (in mice) during T. cruzi invasion, adding to the previous findings that MAM can be immunocytochemically detected in infected heart (8), binds to the parasite surface (9), and impairs parasite uptake (reference 2 and present results).
Another original and important finding resulting from the present work was that complexed (or fast-form) A2M can impair host cell invasion by T. cruzi and alter parasite morphology. A2M that leak out of the plasma through increased vascular permeability (25) will form complexes with the proteinases derived from the parasites or the damaged tissue cells to generate F-A2M. Local synthesis of MAM by recruited and activated monocytes and macrophages is unlikely, given the absence of MAM mRNA in the heart of infected mice (29). Our current findings that F-A2M inhibits invasion but also caused direct damage to the heart cells leave in dubio the interpretation of decreased expression of LRP mRNA in the heart of infected C57BL/6 mice (29). This will result in slower clearance of the locally formed F-A2M complexes in the heart microenvironment and allow them to further accumulate locally. Some of the tissue necrosis observed histopathologically could be caused or promoted by this excess of F-A2M that can damage heart cells, although other factors could contribute. The mechanism involved in the observed morphological alteration of the parasites induced by F-A2M is still unidentified; however, we cannot discard the possibility that in the inflamed tissue, the released parasites could be in direct contact with the activated form of A2M, which could be the result of host and parasite protease activation. Thus, this possible altered morphology and motility of the released parasites could impair/or difficult the host re-infection. Biochemical and ultrastructural approaches are under way in order to better clarify this point.
The third relevant finding of the present study refers to the ability of A2M to bind cytokines and growth factors that could provide local modulation of the immune response (7). A2M binds or neutralizes TGF-β and helps to sustain IFN-γ-mediated macrophage activation (36). The requirement of signaling through TGF-β receptors for T. cruzi invasion would imply that latent TGF-β needs to be activated locally. The finding of increased levels of serum TGF-β in AM-KO mice 22 dpi is suggestive of the involvement of this cytokine in the higher tissue parasitic burden. The important role of the enhancement of TGF-β levels in the fibrotic process of AM-KO-infected mice is reinforced by the finding of similar IFN-γ levels in WT and AM-KO mice, concomitant with undetectable/low levels of IL-4 and IL-10, which might directly link the observations of increased TGF-β and fibrosis. These are characteristic features of the Th1-type of immune response that occurs in the C57BL/6 mice infected with the CL-Brener clone. Although the source(s) of this TGF-β increase is (are) unknown, the finding of a concomitant higher intensity of fibrotic lesions in these mice, which indicates in vivo TGF-β activity, is of interest.
Evidently, other functions of A2M could play protecting roles in host survival to the acute phase of T. cruzi infection and for the establishment of a benign form of Chagas' disease. The possible involvement of A2M as an immune regulator for T and B-cell responses, through its activity as scavenger and/or regulator of TNF-α, IL-2 or other important cytokines is being addressed elsewhere. Combined, the presented in vivo and in vitro findings already demonstrate convincingly that A2M is an essential contributor to the resistance of mice (and humans) to the acute myocarditis induced by T. cruzi infection, through its ability to scavenge for proteinases and cytokines.
Acknowledgments
We thank Mauricio R. M. P. Luz, Solange L. de Castro, and Sabine Bailly for critical review of the manuscript, Alexandre H. Oliveira and Marcos M. Batista for excellent technical support, and Bruno Avila for helping with image processing.
This work was financed by grants from Conselho Nacional de Desenvolvimento Científico e Tecnológico do Brasil (CNPq), Fundação de Amparo a Pesquisa no Estado do Rio de Janeiro FAPERJ, Fundação Oswaldo Cruz (PAPES, Pasteur-FIOCRUZ and INSERM-FIOCRUZ accords), by FWO-Vlaanderen, by the IUAP program of the Belgian Government, and by the Special Research Fund of the KULeuven.
Editor: J. M. Mansfield
REFERENCES
- 1.Aliberti, J. C. S., J. T. Souto, A. P. M. P. Marino, J. Lannes-Vieira, M. M. Teixeira, J. Farber, R. T. Gazzinelli, and J. S. Silva. 2001. Modulation of chemokine production and inflammatory responses in interferon-gamma- and tumor necrosis factor-R1-deficient mice during Trypanosoma cruzi infection. Amer. J. Pathol. 158:1433-1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Araújo-Jorge, T. C., E. P. Sampaio, and W. De Souza. 1986. Inhibition of host cell uptake of infective bloodstream forms by alpha-2-macroglobulin. Z. Parasitenkd. 72:323-329. [DOI] [PubMed] [Google Scholar]
- 3.Araújo-Jorge, T. C., H. S. Barbosa, and M. N. L. Meirelles. 1992. Trypanosoma cruzi: recognition by macrophages and muscle cells: opening perspectives after a 15-years study. Mem. Inst. Oswaldo Cruz 87(Suppl.V):43-56. [DOI] [PubMed] [Google Scholar]
- 4.Araújo-Jorge, T. C., M. J. F. Lage, M. T. Rivera, Y. Carlier, and F. Van Leuven. 1992. Trypanosoma cruzi: enhanced alpha-macroglobulin levels correlate with the resistance of Balb/cj mice to acute infection. Parasitol. Res. 78:215-221. [DOI] [PubMed] [Google Scholar]
- 5.Araújo-Jorge, T. C., M. N. L. Meirelles, and L. Isaac. 1990. Trypanosoma cruzi: killing and enhanced uptake by resident peritoneal macrophages treated with alpha-2-macroglobulin. Parasitol. Res. 76:545-552. [DOI] [PubMed] [Google Scholar]
- 6.Araújo-Jorge, T. C., M. R. M. P. Luz, C. M. L. M. Coutinho, N. Medrano, M. N. C. Soeiro, M. N. L. Meirelles, L. Isaac, and F. Van Leuven. 1994. α2-macroglobulin in experimental and human Chagas' disease. Ann. N. Y. Acad. Sci. 10:453-455. [DOI] [PubMed] [Google Scholar]
- 7.Chu, C. T., and S. V. Pizzo. 1994. Biology of disease. α2-macroglobulin, complement, and biologic defense: antigens, growth factors, microbial proteases, and receptor ligation. Lab. Investig. 71:792-812. [PubMed] [Google Scholar]
- 8.Coutinho, C. M. L. M., G. Cavalcanti, F. Van Leuven, and T. C. Araújo-Jorge. 1999. Detection of alpha-macroglobulin in the heart of mice infected with Trypanosoma cruzi. Parasitol. Res. 85:249-255. [DOI] [PubMed] [Google Scholar]
- 9.Coutinho, C. M. L. M., G. H. Cavalcanti, F. Van Leuven, and T. C. Araújo-Jorge. 1997. Alpha-2-macroglobulin binds to the surface of Trypanosoma cruzi. Parasitol. Res. 83:144-150. [DOI] [PubMed] [Google Scholar]
- 10.Gomes, R. M., C. G. Castagnino, and M. I. Berria. 1992. Extracellular matrix remodelling after coxsackievirus B3-induced murine myocarditis. Int. J. Exp. Pathol. 73:643-653. [PMC free article] [PubMed] [Google Scholar]
- 11.Hall, B. S., and M. A. Pereira. 2000. Dual role for transforming growth factor beta-dependent signaling in Trypanosoma cruzi infection of mammalian cells. Infect. Immun. 68:2077-2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Isaac, L., M. Pereira, M. Santos, E. P. Sampaio, N. R. Lima, M. J. F. Lage, and T. C. Araújo-Jorge. 1990. Trypanosoma cruzi: plasma levels of alpha-2-macroglobulin during experimental murine infections with reticulotropic and myotropic strains. Parasitol. Res. 76:726-728. [DOI] [PubMed] [Google Scholar]
- 13.James, K. 1990. Interactions between cytokines and alpha-2-macroglobulin. Immunol. Today 11:163-166. [DOI] [PubMed] [Google Scholar]
- 14.Koj, A., D. Magielska-Zero, A. Kurdowska, and J. Bereta. 1988. Proteinase inhibitors as acute phase reactants: regulation of synthesis and turnover, p. 171-183. In W. H. Horl and A. Heidland (ed.), Proteases II: potential role in health and disease. Plenum, New York, N.Y. [DOI] [PubMed]
- 15.Luz, M. R. M. P., F. Van Leuven, and T. C. Araújo-Jorge. 1994. Heterogeneity in the plasma levels of two acute-phase proteins in mice from inbred strains infected with Trypanosoma cruzi. Parasitol. Res. 80:439-441. [DOI] [PubMed] [Google Scholar]
- 16.Luz, M. R. M. P., F. Van Leuven, and T. C. Araújo-Jorge. 1995. Heterogeneity in the synthesis of alpha-macroglobulins in outbred swiss albino mice acutely infected with Trypanosoma cruzi. Parasitol. Res. 81:662-667. [DOI] [PubMed] [Google Scholar]
- 17.Marinho, C. R., M. R. D'Imperio Lima, M. G. Grisotto, and J. M. Alvarez. 1999. Influence of acute-phase parasite load on pathology, parasitism, and activation of the immune system at the late chronic phase of Chagas' disease. Infect. Immun. 67:308-318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Medrano-Mercado, N., M. R. M. P. Luz, F. Torrico, G. T. Tapia, F. Van Leuven, and T. C. Araújo-Jorge. 1996. Acute-phase proteins and serologic profiles of chagasic children from an endemic area in Bolivia. Am. J. Trop. Med. Hyg. 54:154-161. [DOI] [PubMed] [Google Scholar]
- 19.Meirelles, M. N. L., T. C. Araújo-Jorge, C. Miranda, W. De Souza, and H. S. Barbosa. 1986. Interaction of Trypanosoma cruzi with heart muscle cells: ultrastructural and cytochemical analysis of endocytic vacuole formation and effect upon myogenesis in vitro. Eur. J. Cell Biol. 41:198-206. [PubMed] [Google Scholar]
- 20.Meirelles, M. N., L. Juliano, E. Carmona, S. G. Silva, E. M. Costa, A. C. Murta, and J. Scharfstein. 1992. Inhibitors of the major cysteinyl proteinase (GP57/51) impair host cell invasion and arrest the intracellular development of Trypanosoma cruzi in vitro. Mol. Biochem. Parasitol. 52:175-184. [DOI] [PubMed] [Google Scholar]
- 21.Melo, R. C., and Z. Brener. 1978. Tissue tropism of different Trypanosoma cruzi strains. J. Parasitol. 64:475-482. [PubMed] [Google Scholar]
- 22.Ming, M., M. E. Ewen, and M. E. A. Pereira. 1995. Trypanosome invasion of mammalian cells requires activation of the TGFβ signaling pathway. Cell 82:287-296. [DOI] [PubMed] [Google Scholar]
- 23.Morrot, A., D. K. Strickland, M. L. Higuchi, M. Reis, R. Pedrosa, and J. Scharfstein. 1997. Human T cell response against the major cysteine proteinase (cruzipain) of Trypanosoma cruzi: role of the multifunctional α2-macroglobulin receptor in antigen presentation by monocytes. Int. Immun. 9:825-834. [DOI] [PubMed] [Google Scholar]
- 24.Overbergh, L., S. Torrekens, F. Van Leuven, and H. Van den Berghe. 1991. Molecular characterization of the murinoglobulins. J. Biol. Chem. 266:16903-16910. [PubMed] [Google Scholar]
- 25.Petersen, C. M., J. V. Povesen, and J. Ingerslev. 1985. Enzyme- linked immunosorbent assay (ELISA) for the measurement of small quantities of alpha-2-macroglobulin. Scand. J. Clin. Lab. Investig. 45:735-740. [DOI] [PubMed] [Google Scholar]
- 26.Rossi, M. A., and R. B. Bestetti. 1995. The challenge of chagasic cardiomyopathy. Cardiology 86:1-7. [DOI] [PubMed] [Google Scholar]
- 27.Russo, M., N. Starobinas, M. C. Marcondes, P. Minoprio, and M. Honteyberie-Joskowicz. 1996. The influence of T cell subsets on Trypanosoma cruzi multiplication in different organs. Immunol. Lett. 49:163-168. [DOI] [PubMed] [Google Scholar]
- 28.Santos-Lima, E. C., and P. Minoprio. 1996. Chagas' disease is attenuated in mice lacking γδ T cells. Infect. Immun. 64:215-221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Soeiro, M. N. C., M. M. Paiva, M. C. Waghabi, M. N. S. L. Meirelles, K. Lorent, A. Henriques-Pons, C. M. L. M. Coutinho, F. Van Leuven, andT. C. Araújo-Jorge. 2000. Acute Trypanosoma cruzi infection affects expression of α-2-macroglobulin and A2MR/LRP receptor differently in C3H and C57BL/6 mice. Exp. Parasitol. 96:97-107. [DOI] [PubMed] [Google Scholar]
- 30.Soeiro, M. N. C., M. M. Paiva, M. C. Waghabi, M. N. S. L. Meirelles, K. Lorent, T. C. Araújo-Jorge, and F. Van Leuven. 2001. Differential expression of mRNA coding for the alpha-2-macroglobulin family and the LRP receptor system in C57BL/6 and C3H mice. Cell Struct. Func. 25:337-350. [DOI] [PubMed] [Google Scholar]
- 31.Strickland, D. K., M. Z. Kounnas, S. E. Williams, and W. S. Argraves. 1994. LDL Receptor-related protein (A2MR/LRP): a multiligand receptor. Fibrinolysis 8:204-215. [Google Scholar]
- 32.Umans, L., L. Serneels, L. Overbergh, K. Lorent, F. Van Leuven, and H. Van den Berghe. 1995. Targeted inactivation of the mouse α2-macroglobulin gene. J. Biol. Chem. 270:19778-19785. [DOI] [PubMed] [Google Scholar]
- 33.Umans, L., L. Serneels, L. Stas, and F. Van Leuven. 1999. Alpha-2-Macroglobulin and Murinoglobulin-1 deficient mice: a mouse model for acute pancreatitis. Am. J. Pathol. 155:983-993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Van Leuven, F. 1982. Human alpha-2-macroglobulin: structure and function. Trends Biochem. Sc. 7:185-187. [Google Scholar]
- 35.Van Leuven, F., S. Torrekens, L. Overbergh, K. Lorent, B. De Strooper, and H. Van Der Berghe. 1992. The primary sequence and the subunit structure of mouse α-2-macroglobulin, deduced from protein sequencing of the isolated subunits and from molecular cloning of cDNA. Eur. J. Biochem. 210:319-327. [DOI] [PubMed] [Google Scholar]
- 36.Webb, D. J., A. M. Weaver, T. L. Atkins-Brady, and S. L. Gonias. 1996. Proteinases are isoform-specific regulators of the binding of transforming growth factor β to α2-macroglobulin. Biochem. J. 320:551-555. [DOI] [PMC free article] [PubMed] [Google Scholar]