Gestational diabetes mellitus (GDM), defined by the World Health Organization (WHO) as ‘carbohydrate intolerance resulting in hyperglycaemia of variable severity with onset or first recognition during pregnancy’1, is the most common metabolic disorder of pregnancy. It is evident that GDM defined in this way will include patients with undiagnosed preexisting type 2 diabetes mellitus (type 2 DM) as well as those with diabetes with first onset during pregnancy. Normal pregnancy is associated with insulin resistance similar to that found in type 2 DM. The reduction in whole-body insulin action becomes apparent in the second trimester, and insulin sensitivity declines progressively to term2,3. After delivery, whole-body insulin action returns rapidly to normal4. Hence, if the onset of diabetes is truly in pregnancy, then it is most likely to occur in the second and third trimesters and glucose tolerance may return to normal after delivery. Nonetheless, women with GDM in whom glucose tolerance becomes normal post-partum remain insulin-resistant compared with women with no history of GDM5.
Diabetes in the first trimester is more likely to be type 2 DM that was present but undiagnosed before pregnancy. In these circumstances, glycosylated haemoglobin, giving an indication of average blood glucose concentrations over the previous six to eight weeks, is likely to be raised. Occasionally, the diagnosis of GDM will identify women with type 1 diabetes mellitus6. Indeed, the incidence of type 1 DM is greater in pregnancy than in the background population7,8.
One of the aims of the St Vincent Declaration is to ‘achieve pregnancy outcome in the diabetic woman that approximates to that of the non-diabetic woman’9. It is because of the adverse outcomes of diabetic pregnancy that screening is required.
RATIONALE FOR SCREENING
Screening for a disease is recommended if the disease is common and clinically important and if a simple screening test exists that will identify the majority of diseased individuals without high rates of false-positive or false-negative results. Intervention for the disorder should affect clinical outcome and the test should be cost-effective. Thus, the purpose of screening is not to diagnose the disease but to identify those at risk to whom a diagnostic test may be offered.
In different series, GDM occurs in 1-14% of pregnancies, with an estimated prevalence in the UK of 4%10. Its clinical importance is on three levels—first, the adverse consequences of poorly controlled GDM for the fetus and neonate; second, the increased risk of type 2 DM in later life for the infant; third, the adverse consequences for the mother, especially the predisposition to type 2 DM in later life.
Adverse consequences of GDM for the fetus and neonate
The principal complication for the baby is macrosomia11. There is no agreed definition: birthweight is a continuous variable with no obvious dichotomy between normal and macrosomic. If the outcome of interest is birth trauma, absolute weight may be appropriate, since this is the best predictor of birth trauma12,13,14. If the outcome of clinical interest is neonatal metabolism or metabolism subsequently, centile weights are appropriate since these predict neonatal hypoglycaemia and later type 2 DM. In practice, an absolute weight of 4 kg or 4.5 kg is chosen, or a weight of >90th centile for gestational age (according to ethnicity).
Many of the adverse features of the fetus in GDM are thought to result from fetal hyperinsulinaemia. The features of macrosomia include birthweight >90th centile for gestational age, abdominal circumference >75th centile from 29 to 33 weeks' gestation15 and increased abdomen/head ratio in the third trimester. Abdominal circumference reflects the size of the liver and amount of abdominal subcutaneous fat, structures that are sensitive to insulin action.
Although macrosomia is the major short-term anxiety in GDM pregnancy, most macrosomic infants are born to women with no history of GDM. Maternal obesity16,17 is the strongest predictor and maternal hypertriglyceridaemia is another16,18. Nonetheless, maternal glucose levels are an important determinant.
Box 1 Screening tests for gestational diabetes mellitus
-
WHO risk factor selection (Ref. 1)
≥1 of following: older age, previous glucose intolerance, previous history of large-for-gestational-age infant, certain ethnic groups, raised fasting or casual blood glucose
-
ADA risk factor selection (Refs 57, 58)
If all the following present, no screening required: <25 years, normal body weight, no family history of type 2 diabetes, not member of high-risk ethnic group, no history of adverse obstetric outcome, no history of GDM
-
ADA glucose challenge (Ref. 57)
Plasma venous glucose 1 hour after 50 g oral glucose ≥7.8 mmol/L*
-
UK St Vincent Task Force (Ref. 70)
Random venous plasma glucose at 28 weeks' gestation >6 mmol/L fasting, >7 mmol/L within 2 hours after food
*All pregnancies screened between 24 and 28 weeks' gestation. Time and nature of last meal not taken into account. If positive screening test for GDM, women go on to have a diagnostic test.
Box 2 Diagnosis of gestational diabetes mellitus by ADA criteria (Ref. 20)
Formal testing usually at 24-28 weeks' gestation. Standard 100 g oral glucose tolerance test performed after overnight fasting (8-14 h). GDM diagnosed if ≥2 of following venous plasma glucose values are abnormal:
Fasting, ≥5.8 mmol/L
1 h, ≥10.0 mmol/L
2 h, ≥9.1 mmol/L
3 h, ≥8.0 mmol/L
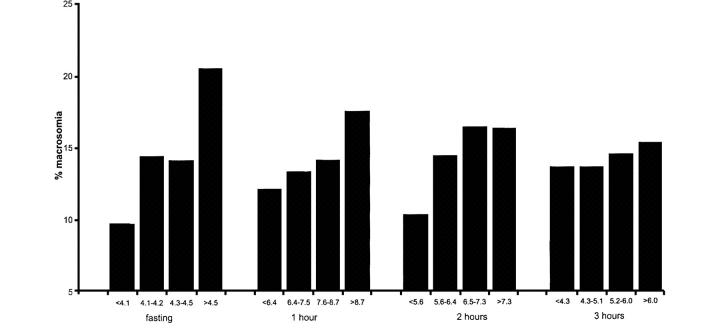
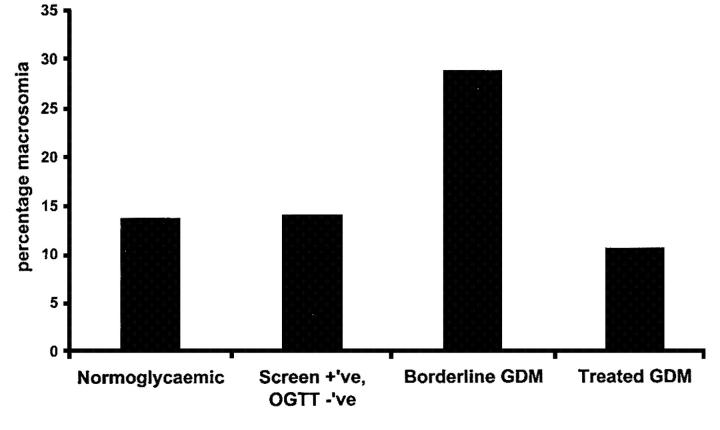
The Toronto Tri-Hospital Gestational Diabetes Project19, a large prospective study with more than 4000 women, was designed to examine pregnancy outcomes, including macrosomia, in women with varying degrees of glucose tolerance. Here the screening test was a glucose challenge test (GCT) (Box 1, discussed later), performed in the non-fasting state at 26 weeks' gestation. All women also had a 100 g 3-hour oral glucose tolerance test (OGTT) at 28 weeks' gestation. Macrosomia was defined as birth weight >4 kg. The relations of glucose values during the 100g 3-hour OGTT were examined in 3637 women without GDM according to American Diabetic Association (ADA) criteria (Box 2)20. For each time point of the test (fasting, 1 hour, 2 hours, 3 hours) the women were divided into quartiles, and the glucose interval for each quartile was recorded. Both OGTT values and GCT values by quartiles showed a graded relation to fetal size with no evidence of a glycaemic threshold. The risk gradient was strongest with the fasting plasma glucose (Figure 1), but the 1-hour and 2-hour glucose concentrations after oral glucose (and the 1 hour GCT value) were also significantly associated with macrosomia. The importance of maternal glucose concentrations is illustrated in Figure 2. The women were classified according to four grades of glucose tolerance—normoglycaemic, screen positive, ‘borderline GDM’ (Carpenter and Coustan criteria21, Box 3) and GDM (ADA criteria20, Box 2). The rate of macrosomia was higher in the untreated borderline GDM group (28.7%) than in the non-diabetic women (13.7%, p<0.001) and was also higher than in women with GDM on therapy to restore normoglycaemia (10.5%, p<0.001). No conclusions on treatment efficacy can be drawn since this was not a randomized controlled trial.
Figure 1.
Relationship of oral glucose tolerance test values by quartiles to macrosomia (Ref. 19)
Figure 2.
Percentage macrosomia (>4 kg) based on glucose test results
The Diagest study22 examined women who did not meet ADA criteria for GDM (Box 2)20. A screening test (50 g glucose challenge) was offered between 24 and 28 weeks' gestation, plasma glucose being measured at 1 hour. If this was ≥7.2 mmol/L, a 100 g 3-hour OGTT was performed. For this study ‘mild GDM’ was defined as one abnormal value on the 100 g 3-hour OGTT. Women with mild GDM received no treatment or specific advice during the pregnancy. Large-for-gestational-age babies (defined as ≥90th percentile on standard growth curves) were delivered in 21% of the 131 women in this group, compared with 11% of 108 women in whom the 50 g GCT had been negative (p<0.05). After adjustment for maternal body mass index (BMI), age, parity and educational level, the relation between macrosomia and diabetes persisted (odds ratio 2.5, confidence interval 1.16-5.40). An adverse maternal or fetal outcome of some kind occurred almost twice as frequently in this diabetic group as in the non-diabetic pregnancies.
The Hyperglycaemia and Adverse Pregnancy Outcome study (HAPO study), funded largely by the US National Institutes of Health, the ADA and the British Diabetic Association (BDA), aims to discover the glucose values on a diagnostic 75 g 2-hour OGTT at 28 weeks' gestation which predict adverse outcome23. This study will enrol 25 000 pregnant women from sixteen centres around the world, with an estimated 1000 women having ‘untreated GDM’ (2-hour plasma glucose between 7.8 and 11.1 mmol/L).
Macrosomia is the major adverse outcome for the fetus and neonate. Others, for which the evidence is less strong, include respiratory distress syndrome24, hyperbilirubinaemia25, polycythaemia25 and neonatal hypoglycaemia25,26. In addition, the likelihood of congenital malformation27 seems to be increased and admission to the special care baby unit is more likely26.
Box 3 Diagnosis of gestational diabetes mellitus by Carpenter and Coustan criteria (Ref. 21)
Formal testing usually at 24-28 weeks' gestation. Standard 100 g oral glucose tolerance test performed after overnight fasting (8-14 h). GDM diagnosed if ≥2 of following venous plasma glucose values are abnormal:
Fasting, ≥5.3 mmol/L
1 h, ≥10.0 mmol/L
2 h, ≥8.6 mmol/L
3 h, ≥7.8 mmol/L
The frequency of some of these complications, such as respiratory distress, has declined because the practice of early planned delivery for GDM women has lost favour; most patients now deliver at or near term. With good obstetric care, the perinatal mortality rate for a GDM pregnancy is similar to that in the non-diabetic population28.
Mechanisms of macrosomia in GDM
The Pedersen hypothesis29 is an attempt to explain the development of the diabetic fetus. Maternal insulin deficiency is the primary abnormality, resulting in hyperglycaemia in the mother, mainly post-prandial in the early stages. As glucose is transported across the placenta down a concentration gradient, excess glucose is transferred to the fetus in GDM, resulting in fetal hyperinsulinaemia. This excess insulin limits fetal hyperglycaemia but results in an increase in fetal triglyceride deposition, the major feature of macrosomia in a diabetic pregnancy. The macrosomia is associated with shoulder dystocia and the need for caesarean section. The fetal hyperinsulinaemia probably also contributes to increased fetal erythropoiesis and thus polycythaemia30. After delivery, the neonate is at risk of hypoglycaemia, since insulin concentrations may remain above normal at a time when maternal glucose transfer has ceased. Neonatal hypoglycaemia in the first 3 hours after delivery is therefore more frequent after GDM pregnancies, at least in some studies, than after non-diabetic pregnancies26.
The Pedersen hypothesis has been extended to include maternal aminoacid and triglyceride metabolism31,11. The relative hypoinsulinaemia and insulin resistance of GDM pregnancy is associated with an increase in maternal protein flux. Consequently there is excessive delivery of aminoacids to the fetus. From early pregnancy, some aminoacids are fetal insulin secretagogues and compound the increase in fetal insulin secretion. GDM is also associated with maternal hypertriglyceridaemia. Triglyceride is not transferred directly across the placenta, but the placenta contains abundant lipase enzyme activity32. The resultant non-esterified fatty acids may cross the placenta and contribute to fetal triglyceride synthesis and macrosomia33.
Implications of GDM for the future of the neonate
Diabetes during pregnancy may be a risk factor for diabetes and hyperglycaemia in the offspring when older. A longitudinal epidemiological study of diabetes and its complications has been conducted among the Pima Indians of Arizona since 196534. From these data the prevalence of type 2 DM was ascertained in over 1500 infants from mothers who had received a 75 g 2-hour OGTT. The infants were followed biennially from the age of 5 years with a 75 g 2-hour OGTT. Diabetes in the next generation developed in 6.9% and 30.1% of breast-fed offspring of non-diabetic and diabetic women, respectively, and in 11.9% and 43.6% of bottle-fed offspring, respectively35. It is uncertain whether this risk extends to other ethnic populations.
The offspring may also be at increased risk of obesity in adolescence. In a prospective study of 139 singleton pregnancies from 1977 to 1983, children were reviewed annually36. By 14-17 years of age, the mean BMI was 24.6 SD 5.8 kg/m2 in the offspring of diabetic mothers versus 20.9 SD 3.4 kg/m2 in controls. Obesity in adolescence was also associated with maternal weight.
Simmons and Robertson37 looked at two groups of women with GDM, one treated with insulin, the other with diet. Although the insulin-treated women were older, more obese and more hyperglycaemic (despite insulin treatment) their offspring had significantly less adiposity at 2 years and 8 months. The study, though not controlled, suggests that insulin treatment influences neonatal outcome.
Adverse consequences for the mother
During pregnancy, GDM carries an increased risk of operative delivery. This may be due partly to the frequency of macrosomia but probably also reflects tradition, with operative delivery more likely to be undertaken in a diabetic than in a non-diabetic pregnancy19,38. The frequencies of induced labour, of pre-eclampsia39,40 and of hydramnios are all above average in GDM pregnancies.
GDM also has implications for the mother's later life. Type 2 DM is a major cause of morbidity and mortality and has substantial economic implications41. Women with previous GDM constitute one of the groups at risk of developing type 2 DM in later life. Other at-risk groups for type 2 DM are shown in Box 4. For women with previous GDM and normal glucose tolerance post-partum, the lifetime risk of type 2 DM is 40-60%, with obese women having a higher prevalence in later life than lean women42,43. Furthermore, even women with previous GDM and normal post-partum glucose tolerance have abnormalities of both insulin secretion and insulin sensitivity44 and there are differences between ethnic groups45.
WHO has recommended that women with GDM have a 75 g 2-hour OGTT performed 6 weeks post-partum and be classified accordingly1. The ADA also recommends a 75 g 2-hour OGTT post-partum46. There is as yet no evidence that the long-term incidence of type 2 diabetes can be altered through case detection in gestation and subsequent intervention.
Box 4 At-risk groups for development of type 2 diabetes mellitus
Low birth weight (<2.95 kg) (Ref. 71)
Low weight at 1 year (<9.2 kg) (Ref. 72)
Non-Europids (Refs 45, 47, 73)
Risk factors for GDM
GDM is more prevalent in older than in younger women. In women over 40 years, GDM is ten times more likely to occur than in the 20-24 age-group47. GDM is also more likely in the presence of obesity42,47. It is sixteen times more likely to develop with a BMI at booking of 35-39 kg/m2 than in women with a booking BMI of 20-24 kg/m2. Ethnic origin is a major determinant. In our West London population, the adjusted odds ratio for women from the Indian subcontinent, in comparison with those of Europid origin, was 11.3 (95% confidence interval 6-8-18.8). In black women the odds ratio was 3.1 (1.8-5.5). All non-Europid ethnic groups are at greater risk than Europid women47,48.
Groups defined as at high risk for the development of GDM by WHO1 are shown in Box 1. Increased parity47,49, multiple versus singleton pregnancy50, and weight gain between pre-pregnancy and post-partum examination47 are additional risk factors. With regard to parity, it is possible that the period of hyperglycaemia during GDM leads to deterioration in maternal pancreatic β-cell function. Subsequent pregnancies appear to have an additive deleterious effect on the β-cell function, culminating in a potentially earlier onset of type 2 DM.
SCREENING POLICIES FOR GESTATIONAL DIABETES
There is no consensus as to the timing of screening, the test to be used or who to screen (universal or selected/targeted screening) (Box 1). The most widely employed screening test in the UK is based on maternal risk factors. However, use of such risk factors (maternal age ≥25 years, family history of diabetes, previous large-for gestational-age baby or stillbirth) caused 38% of gestational diabetes to go unidentified in one study51. Several groups have tried other risk factors (previous neonate >9 lb [4 kg], neonatal death, congenital anomaly, prematurity, family history of diabetes) and clinical findings during pregnancy (obesity, excessive weight gain, glycosuria, proteinuria, hypertension). About half the women with GDM were identified by these means52,53,54.
In the USA, the GCT, first proposed by O'Sullivan, is the most widely used screening test and is recommended by the ADA20. This is performed without regard to time or nature of the last meal, at 24-28 weeks' gestation. Women are given 50 g of glucose by mouth and plasma glucose is measured at 1 hour. A positive test is a plasma venous glucose concentration ≥ 7.8 mmol/L (Box 1). In O'Sullivan's original studies of 752 women, all had a 50 g GCT and a 100 g 3-hour OGTT55. The challenge test (with a screen cut-off of 7.8 mmol/L) had a sensitivity of 88% and a specificity of 82% in those ≥ 25 years. Since then, researchers have not agreed on the threshold values for the GCT, the range of suggested values being 7.2-8.3 mmol/L21. There are controversies over whether women should be tested fasting or fed56: the Toronto Tri-Hospital study showed pronounced differences of plasma glucose in the two states. Adjustment of the thresholds to 8.2 mmol, 7.9 mmol, and 8.3 mmol/L for elapsed post-prandial times of < 2, 2-3 and > 3 hours, respectively, increased the positive predictive value of the test from 14.4% to 18.7%19. The ADA has subsequently recommended that screening of a pregnant woman is unnecessary if she fulfils all the following criteria57: <25 years old; normal body weight; no family history (i.e. first degree relatives) of diabetes; and not a member of an ethnic/racial group with a high prevalence of diabetes (Hispanic, Native American, Asian-American, African-American, or Pacific Islander). The Fourth International Workshop-Conference on GDM, under the sponsorship of the ADA, further recommended that women should be screened if they have a history of GDM or poor obstetric outcome58.
The WHO recommends screening by means of maternal risk factor selection (Box 1)1. Formal systematic testing with a 75 g 2-hour OGTT for GDM between 24 and 28 weeks of gestation is recommended for older women, those with a previous history of glucose intolerance, those with a history of large-for-gestational-age babies, women from certain high-risk ethnic groups, and any pregnant woman who has a raised fasting or casual blood glucose. Also there may be a case for screening pregnant women from high-risk populations during the first trimester to detect previously undiagnosed diabetes mellitus.
In some populations risk-factor screening excludes only a small proportion of pregnant women (<20%)59, such that universal screening has been recommended. This is our policy for our multi-ethnic population in West London.
Diagnosis of GDM
For diagnosis of GDM there is no consensus on the glucose load to be used or the timing and type of blood sample. The two widely used criteria are outlined in Boxes 2 and 5.
Box 5.
Diagnosis of gestational diabetes mellitus, WHO criteria (Ref. 1)
| Formal testing usually at 24-28 weeks' gestation. Standard 75 g oral glucose tolerance test performed after overnight fast (8-14h). Glucose concentrations (mmol/L): | ||||
| Whole blood venous | Whole blood capillary | Plasma venous | Plasma capillary | |
| Fasting | ≥6.1 | ≥6.1 | ≥7.0 | ≥7.0 |
| 2h | ≥6.7 | ≥7.8 | ≥7.8 | ≥8.9 |
These tests are usually performed between 24 and 28 weeks' gestation. Early criteria for abnormal glucose tolerance in pregnancy, proposed by O'Sullivan and Mahan in 1964, were based on data obtained from the 100 g 3-hour OGTT performed on 752 pregnant women55. Abnormal glucose tolerance was defined as ≥ 2 blood glucose values, out of 4, that were greater than or equal to 2 standard deviations above the mean. The requirement for two values to be abnormal was based on a desire to ‘avoid misclassification due to laboratory error or occasional single high peaks resulting from unusually rapid absorption of glucose’55. These criteria for abnormal glucose tolerance were validated by their prediction of later non-pregnancy glucose intolerance when applied to a second group of 1013 women who had been tested during pregnancy and followed for 5-10 years post-partum. In 1979 the National Diabetes Data Group (NDDG) revised the O'Sullivan and Mahan criteria, converting the whole-blood glucose values to plasma glucose values by upward adjustment of 15%60 and the ADA later adopted this20. Carpenter and Coustan suggested that the NDDG conversion factor was too high and proposed alternative cut-off values21 (Box 3).
The WHO recommends a standard OGTT, performed after overnight fasting (8-14 hours); 75 g anhydrous glucose is given in 250-300 mL water, and plasma glucose is measured fasting and after 2 hours. Pregnant women who meet criteria for diabetes mellitus or impaired glucose tolerance in the non-pregnant state are classified as having GDM (Box 5)1. Unlike the O'Sullivan and Mahan thresholds, the WHO criteria were not developed specifically for use in pregnant women, nor were they validated by their ability to identify pregnancies at increased risk for adverse outcome. A major advantage of the WHO criteria is that the 75 g 2-hour OGTT is used as for non-pregnant individuals.
The WHO and ADA criteria for GDM have been compared; 127 Pima Indian women with no previous history of type 2 DM were studied with both protocols. It was found that 11 women who met the WHO diagnosis of GDM also included 2 women with GDM by the ADA criteria. The other 9 patients with GDM by WHO criteria had an excess of macrosomic babies and caesarean sections: 16 of the 127 women had infants > 4 kg, of whom 6 were correctly identified as abnormal by the WHO criteria compared with 1 out of the 16 by the ADA criteria. Of the 7 delivering by caesarean section, 4 had abnormal glucose tolerance by WHO criteria, none of them by ADA criteria61. Although the numbers in this study were small, there is now a move towards using the diagnostic 75 g 2-hour OGTT in pregnancy62,63 and the results from the HAPO study are awaited for definitive evidence.
Subtle differences within the range of normal glucose homoeostasis may nonetheless have clinical importance. It is known, for example, that patients with an abnormal GCT but a normal OGTT are at increased risk of adverse perinatal outcome19, as are those with one abnormal OGTT value rather than the two required by ADA criteria64.
Treatment policies
There is no consensus on treatment of those women with an abnormal GCT and normal diagnostic tests. Likewise, the implication of impaired fasting glycaemia, a term defined by WHO1 as fasting plasma glucose ≥ 6.1 < 7.0 mmol/L and 2-hour post 75 g OGTT < 7.8 mmol/L, are uncertain during pregnancy. WHO recommends a formal 75 g 2-hour OGTT in these circumstances1.
All women with GDM should receive nutritional counselling and be advised on a diet with a limited intake of sucrose.
There is no consensus on when to start insulin treatment in GDM; the ADA have recommended that treatment should begin when fasting plasma glucose is ≥ 5.8 mmol/L and the 2-hour post-prandial glucose is ≥ 6.7 mmol/L on two or more occasions within a two-week interval. There is no position statement from the WHO. It is difficult to be prescriptive with regard to insulin treatment since this is partly dependent on gestational age and the presence or absence of macrosomia. In our unit, we aim for a fasting glucose < 6 mmol/L and post-prandial glucose < 8 mmol/L.
It is noteworthy that insulin treatment during pregnancy results in a sharp reduction in macrosomia, a reduction in maternal complications, and a reduced neonatal and maternal stay19 with long-term benefit for the offspring. Other indices are being studied in the Australasian Carbohydrate Intolerance Study in Pregnancy65, which will follow 1000 women prospectively. This study aims to ascertain whether treatment improves outcome in terms of neonatal bilirubin levels, admissions to the special care baby unit, caesarean section and induction of labour. Treatment with diet plus or minus insulin will be compared with no treatment. This study, however, has not been powered for macrosomia.
COST-EFFECTIVENESS
The cost-effectiveness of screening for GDM has not been extensively studied. Savings over a 10-year period, as a result of a prevention programme aimed at reducing the onset of type 2 DM in women with GDM in the United States, have been projected65. On the assumption that 3% of pregnancies are complicated by GDM and 50% of women with GDM will develop type 2 DM over a 10-year period55, 1990 data from the National Health Centre for Statistics indicate that 62 685 women with GDM in the USA will develop type 2 DM in 10 years. The economic model suggested by the authors assumes a constant rate of progression to diabetes of 6.7% over a 10-year period. The annual healthcare cost per woman with diabetes was $2265 in 1986 dollars66. The cumulative net cost of caring for women who develop diabetes over 10 years is $81866. Taking into account the cost of counselling and evaluation of the cohort over a 10-year period and assuming a 10% reduction or delay in the onset of type 2 DM with intervention67,68,69, $71 million would be saved over this period65. This model has not been tested and the cost-effectiveness in the long term has not been studied. The ability of intervention to delay or prevent type 2 DM is unknown for an interim population but is being tested in the Diabetes Prevention Programme which has a subcategory of women with previous GDM.
CONCLUSION
Screening based on risk factors alone will pick up only 50% of the GDM population. Other screening procedures, such as the random 50 g glucose challenge test at 24-28 weeks' gestation, have been widely adopted. If the screening test is positive, a definitive test is required for diagnosis. The WHO has recommended a 75 g oral glucose tolerance test performed after an overnight fast, GDM being diagnosed according to the criteria for either impaired glucose tolerance or diabetes in the non-pregnant. This is likely to be widely adopted, even though the ADA is continuing for the moment with a 100 g oral glucose tolerance test.
References
- 1.WHO. Definition, Diagnosis and Classification of Diabetes Mellitus and its Complications. Geneva: WHO, 1999
- 2.Catalano PM, Tyzbir ED, Wolfe RR, et al. Carbohydrate metabolism during pregnancy in control subjects and women with gestational diabetes. Am J Physiol 1993;264(1 Pt 1): E60-7 [DOI] [PubMed] [Google Scholar]
- 3.Robinson S, Viira J, Learner J, et al. Insulin insensitivity is associated with a decrease in postprandial thermogenesis in normal pregnancy. Diabet Med 1993;10: 139-45 [DOI] [PubMed] [Google Scholar]
- 4.Ryan EA, MJ Os, Skyler JS. Insulin action during pregnancy. Studies with the euglycemic clamp technique. Diabetes 1985;34: 380-9 [DOI] [PubMed] [Google Scholar]
- 5.Robinson S, Niththyananthan R, Anyaoku V, Elkeles RS, Beard RW, Johnston DG. Reduced postprandial energy expenditure in women predisposed to type 2 diabetes. Diabet Med 1994;11: 545-50 [DOI] [PubMed] [Google Scholar]
- 6.Fuchtenbusch M, Ferber K, Standl E, Ziegler AG. Prediction of type 1 diabetes postpartum in patients with gestational diabetes mellitus by combined islet cell autoantibody screening: a prospective multicenter study. Diabetes 1997;46: 1459-67 [DOI] [PubMed] [Google Scholar]
- 7.Buschard K, Buch I, Molsted Pedersen L, Hougaard P, Kuhl C. Increased incidence of true type 1 diabetes acquired during pregnancy. BMJ (Clin Res Ed) 1987;294: 275-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weitzman S, Harman Boehm I, Maislos M. Gestational diabetes and risk of developing IDDM [Letter; Comment]. Diabetes Care 1990;13: 186. [DOI] [PubMed] [Google Scholar]
- 9.World Health Organization and International Diabetes Federation. Saint Vincent Declaration. WHO, IDF, 1989
- 10.King H. Epidemiology of glucose intolerance and gestational diabetes in women of childbearing age. Diabetes Care 1998;21(suppl 2): B9-13 [PubMed] [Google Scholar]
- 11.Farrell PM, Engle MJ, Frantz ID, et al. Complications of pregnancy and fetal development. Diabetes 1982;31(suppl 1 Pt 2): 89-94 [DOI] [PubMed] [Google Scholar]
- 12.Angioli R, Gomez Marin O, Cantuaria G, MJ Os. Severe perineal lacerations during vaginal delivery: the University of Miami experience. Am J Obstet Gynecol 2000;182: 1083-5 [DOI] [PubMed] [Google Scholar]
- 13.Wagner RK, Nielsen PE, Gonik B. Shoulder dystocia. Obstet Gynecol Clin Am 1999;26: 371-83 [DOI] [PubMed] [Google Scholar]
- 14.McFarland LV, Raskin M, Daling JR, Benedetti TJ. Erb/Duchenne's palsy: a consequence of fetal macrosomia and method of delivery. Obstet Gynecol 1986;68: 784-8 [PubMed] [Google Scholar]
- 15.Buchanan TA, Kjos SL, Montoro MN, et al. Use of fetal ultrasound to select metabolic therapy for pregnancies complicated by mild gestational diabetes. Diabetes Care 1994;17: 275-83 [DOI] [PubMed] [Google Scholar]
- 16.Merzouk H, Meghelli Bouchenak M, Loukidi B, Prost J, Belleville J. Impaired serum lipids and lipoproteins in fetal macrosomia related to maternal obesity. Bio Neonate 2000;77: 17-24 [DOI] [PubMed] [Google Scholar]
- 17.Hardy DS. A multiethnic study of the predictors of macrosomia. Diabetes Educ 1999;25: 925-33 [DOI] [PubMed] [Google Scholar]
- 18.Knopp RH, Magee MS, Walden CE, Bonet B, Benedetti TJ. Prediction of infant birth weight by GDM screening tests. Importance of plasma triglyceride. Diabetes Care 1992;15: 1605-13 [DOI] [PubMed] [Google Scholar]
- 19.Sermer M, Naylor CD, Farine D, et al. The Toronto Tri-Hospital Gestational Diabetes Project. A preliminary review. Diabetes Care 1998;21(suppl 2): B33-42 [PubMed] [Google Scholar]
- 20.ADA. Gestational diabetes mellitus. American Diabetes Association position statement. Diabetes Care 1986;9: 430-1 [PubMed] [Google Scholar]
- 21.Carpenter MW, Coustan DR. Criteria for screening tests for gestational diabetes. Am J Obstet Gynecol 1982;144: 768-73 [DOI] [PubMed] [Google Scholar]
- 22.Vambergue A, Nuttens MC, Verier Mine O, Dognin C, Cappoen JP, Fontaine P. Is mild gestational hyperglycaemia associated with maternal complications? The Diagest Study. Diabet Med 2000;17: 203-8 [DOI] [PubMed] [Google Scholar]
- 23.Hadden D. Evidence-based screening for gestational diabetes? Diabet Med 2000;17: 402-4 [DOI] [PubMed] [Google Scholar]
- 24.Tyrala EE. The infant of the diabetic mother. Obstet Gynecol Clin N Am 1996;23: 221-41 [DOI] [PubMed] [Google Scholar]
- 25.Hod M, Merlob P, Friedman S, Schoenfeld A, Ovadia J. Gestational diabetes mellitus. A survey of perinatal complications in the 1980s. Diabetes 1991;40(suppl 2): 74-8 [DOI] [PubMed] [Google Scholar]
- 26.Jensen DM, Sorensen B, Feilberg Jorgensen N, Westergaard JG, Beck Nielsen H. Maternal and perinatal outcomes in 143 Danish women with gestational diabetes mellitus and 143 controls with a similar risk profile. Diabet Med 2000;17: 281-6 [DOI] [PubMed] [Google Scholar]
- 27.Kousseff BG. Gestational diabetes mellitus (class A): a human teratogen? Am J Med Genet 1999;83: 402-8 [PubMed] [Google Scholar]
- 28.Fadel HE, Hammond SD. Diabetes mellitus and pregnancy: management and results. J Reprod Med 1982;27: 56-66 [PubMed] [Google Scholar]
- 29.Pedersen J. Weight and length at birth of infants of diabetic mothers. Acta Endocrinol 1954;16: 342-7 [DOI] [PubMed] [Google Scholar]
- 30.Perrine SP, Greene MF, Lee PD, Cohen RA, Faller DV. Insulin stimulates cord blood erythroid progenitor growth: evidence for an aetiological role in neonatal polycythaemia. Br J Haematol 1986;64: 503-11 [DOI] [PubMed] [Google Scholar]
- 31.Freinkel N. Banting Lecture 1980. Of pregnancy and progency. Diabetes 1980;29: 1023-35 [DOI] [PubMed] [Google Scholar]
- 32.Knopp RH, Warth MR, Charles D, et al. Lipoprotein metabolism in pregnancy, fat transport to the fetus, and the effects of diabetes. Biol Neonate 1986;50: 297-317 [DOI] [PubMed] [Google Scholar]
- 33.Goldstein R, Levy E, Shafrir E. Increased maternal-fetal transport of fat in diabetes assessed by polyunsaturated fatty acid content in fetal lipids. Biol Neonate 1985;47: 343-9 [DOI] [PubMed] [Google Scholar]
- 34.Knowler WC, Pettitt DJ, Saad MF, Bennett PH. Diabetes mellitus in the Pima Indians: incidence, risk factors and pathogenesis. Diabetes Metab Rev 1990;6: 1-27 [DOI] [PubMed] [Google Scholar]
- 35.Pettitt DJ, Knowler WC. Long-term effects of the intrauterine environment, birth weight, and breast-feeding in Pima Indians. Diabetes Care 1998;21(suppl 2): B138-41 [PubMed] [Google Scholar]
- 36.Silverman BL, Rizzo TA, Cho NH, Metzger BE. Long-term effects of the intrauterine environment. The Northwestern University Diabetes in Pregnancy Center. Diabetes Care 1998;21(suppl 2): B142-9 [PubMed] [Google Scholar]
- 37.Simmons D, Robertson S. Influence of maternal insulin treatment on the infants of women with gestational diabetes. Diabet Med 1997;14: 762-5 [DOI] [PubMed] [Google Scholar]
- 38.Moses RG, Knights SJ, Lucas EM, et al. Gestational diabetes: is a higher cesarean section rate inevitable? Diabetes Care 2000;23: 15-17 [DOI] [PubMed] [Google Scholar]
- 39.Berkowitz KM. Insulin resistance and preeclampsia. Clin Perinatol 1998;25: 873-85 [PubMed] [Google Scholar]
- 40.Persson B, Hanson U. Neonatal morbidities in gestational diabetes mellitus. Diabetes Care 1998;21(suppl): B79-84 [PubMed] [Google Scholar]
- 41.Amos AF, McCarty DJ, Zimmet P. The rising global burden of diabetes and its complications: estimates and projections to the year 2010. Diabet Med 1997;14(suppl 5): S1-85 [PubMed] [Google Scholar]
- 42.Dornhorst A, Bailey PC, Anyaoku V, Elkeles RS, Johnston DG, Beard RW. Abnormalities of glucose tolerance following gestational diabetes. Quart J Med 1990;77: 1219-28 [DOI] [PubMed] [Google Scholar]
- 43.O'Sullivan J. Body weight and subsequent diabetes mellitus. JAMA 1982;248: 949-52 [PubMed] [Google Scholar]
- 44.Ryan EA, Imes S, Liu D, et al. Defects in insulin secretion and action in women with a history of gestational diabetes. Diabetes 1995;44: 506-12 [DOI] [PubMed] [Google Scholar]
- 45.Dornhorst A, Chan SP, Gelding SV, et al. Ethnic differences in insulin secretion in women at risk of future diabetes. Diabet Med 1992;9: 258-62 [DOI] [PubMed] [Google Scholar]
- 46.ADA. Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care 1998;21(suppl): S5-S19 [DOI] [PubMed] [Google Scholar]
- 47.Dornhorst A, Paterson CM, Nicholls JS, et al. High prevalence of gestational diabetes in women from ethnic minority groups. Diabet Med 1992;9: 820-5 [DOI] [PubMed] [Google Scholar]
- 48.Yue DK, Molyneaux LM, Ross GP, Constantino MI, Child AG, Turtle JR. Why does ethnicity affect prevalence of gestational diabetes? The underwater volcano theory. Diabet Med 1996;13: 748-52 [DOI] [PubMed] [Google Scholar]
- 49.Kritz Silverstein D, Barret Connor E, Wingard DL. The effect of parity on the later development of non-insulin-dependent diabetes mellitus or impaired glucose tolerance. N Engl J Med 1989;321: 1214-19 [DOI] [PubMed] [Google Scholar]
- 50.Schwartz DB, Daoud Y, Zazula P, et al. Gestational diabetes mellitus: metabolic and blood glucose parameters in singleton versus twin pregnancies. Am J Obstet Gynecol 1999;181: 912-14 [DOI] [PubMed] [Google Scholar]
- 51.O'Sullivan J, Charles D, Mahan CM, Dandrow RV. Gestational diabetes and perinatal mortality rate. Am J Obset Gynecol 1973;116: 901-4 [DOI] [PubMed] [Google Scholar]
- 52.Lavin JP, Jr. Screening of high-risk and general populations for gestational diabetes. Clinical application and cost analysis. Diabetes 1985;34(suppl 2): 24-7 [DOI] [PubMed] [Google Scholar]
- 53.Marquette GP, Klein VR, Niebyl JR. Efficacy of screening for gestational diabetes. Am J Perinatol 1985;2: 7-9 [DOI] [PubMed] [Google Scholar]
- 54.Coustan DR, Nelson C, Carpenter MW, Carr SR, Rotondo L, Widness JA. Maternal age and screening for gestational diabetes: a population-based study. Obstet Gynecol 1989;73: 557-61 [PubMed] [Google Scholar]
- 55.O'Sullivan JB, Mahan CM. Criteria for the oral glucose tolerance test in pregnancy. Diabetes 1964;13: 278-85 [PubMed] [Google Scholar]
- 56.Coustan DR, Widness JA, Carpenter MW, Rotondo L, Pratt DC, Oh W. Should the fifty-gram, one-hour plasma glucose screening test for gestational diabetes be administered in the fasting or fed state? Am J Obstet Gynecol 1986;154: 1031-5 [DOI] [PubMed] [Google Scholar]
- 57.ADA. Gestational Diabetes Mellitus. Diabetes Care 1998;21(suppl 1): S61-S61 [Google Scholar]
- 58.Metzger BE, Coustan DR. Summary and recommendations of the Fourth International Workshop-Conference on Gestational Diabetes Mellitus. The Organizing Committee. Diabetes Care 1998;21(suppl 2): B161-7 [PubMed] [Google Scholar]
- 59.Williams CB, Iqbal S, Zawacki CM, Yu D, Brown MB, Herman WH. Effect of selective screening for gestational diabetes. Diabetes Care 1999;22: 418-21 [DOI] [PubMed] [Google Scholar]
- 60.NDDG. Classification and diagnosis of diabetes mellitus and other categories of glucose intolerance. National Diabetes Data Group. Diabetes 1979;28: 1039-57 [DOI] [PubMed] [Google Scholar]
- 61.Pettitt DJ, Bennett PH, Hanson RL, Narayan KM, Knowler WC. Comparison of World Health Organization and National Diabetes Data Group procedures to detect abnormalities of glucose tolerance during pregnancy. Diabetes Care 1994;17: 1264-8 [DOI] [PubMed] [Google Scholar]
- 62.Sacks DA, Greenspoon JS, Abu Fadil S, Henry HM, Wolde Tsadik G, Yao JF. Toward universal criteria for gestational diabetes: the 75-gram glucose tolerance test in pregnancy. Am J Obstet Gynecol 1995;172(2 Pt 1): 607-14 [DOI] [PubMed] [Google Scholar]
- 63.Deerochanawong C, Putiyanun C, Wongsuryrat M, Serirat S, Jinayon P. Comparison of National Diabetes Data Group and World Health Organization criteria for detecting gestational diabetes mellitus. Diabetologia 1996;39: 1070-3 [DOI] [PubMed] [Google Scholar]
- 64.Langer O, Brustman L, Anyaegbunam A, Mazze R. The significance of one abnormal glucose tolerance test value on adverse outcome in pregnancy. Am J Obstet Gynecol 1987;157: 758-63 [DOI] [PubMed] [Google Scholar]
- 65.Gregory KD, Kjos SL, Peters RK. Cost of non-insulin-dependent diabetes in women with a history of gestational diabetes: implications for prevention. Obstet Gynecol 1993;81(5 Pt 1): 782-6 [PubMed] [Google Scholar]
- 66.Huse DM, Oster G, Killen Ar, Lacey MJ, Colditz GA. The economic costs of non-insulin-dependent diabetes mellitus. JAMA 1989;262: 2708-13 [DOI] [PubMed] [Google Scholar]
- 67.Helmrich SP, Ragland DR, Leung RW, Paffenbarger RS, Jr. Physical activity and reduced occurrence of non-insulin-dependent diabetes mellitus. N Engl J Med 1991;325: 147-52 [DOI] [PubMed] [Google Scholar]
- 68.Manson JE, Rimm EB, Stampfer MJ, et al. Physical activity and incidence of non-insulin-dependent diabetes mellitus in women. Lancet 1991;338: 774-8 [DOI] [PubMed] [Google Scholar]
- 69.Eriksson KF, Lindgarde F. Prevention of type 2 (non-insulin-dependent) diabetes mellitus by diet and physical exercise. The 6-year Malmö feasibility study. Diabetologia 1991;34: 891-8 [DOI] [PubMed] [Google Scholar]
- 70.Brown DJ, Dawson A, Dodds R, et al. Report of the Pregnancy and Neonatal Care Group. Diabet Med 1996;13(9 suppl 4): S43-53 [PubMed] [Google Scholar]
- 71.Barker DJ, Hales CN, Fall CH, Osmond C, Phipps K, Clark PM. Type 2 (non-insulin-dependent) diabetes mellitus, hypertension and hyperlipidaemia (syndrome X): relation to reduced fetal growth. Diabetologia 1993;36: 62-7 [DOI] [PubMed] [Google Scholar]
- 72.Barker DJ, Winter PD, Osmond C, Margetts B, Simmonds SJ. Weight in infancy and death from ischaemic heart disease. Lancet 1989;ii: 577-80 [DOI] [PubMed] [Google Scholar]
- 73.Dornhorst A, Edwards SG, Nicholls JS, et al. A defect in insulin release in women at risk of future non-insulin-dependent diabetes. Clin Sci (Colch) 1991;81: 195-9 [DOI] [PubMed] [Google Scholar]
- 74.Robinson S, Chan SP, Spacey S, Anyaoku V, Johnston DG, Franks S. Postprandial thermogenesis is reduced in polycystic ovary syndrome and is associated with increased insulin resistance. Clin Endocrinol (Oxf) 1992;36: 537-43 [DOI] [PubMed] [Google Scholar]
- 75.Dunaif A, Segal KR, Futterweit W, Dobrjansky A. Profound peripheral insulin resistance, independent of obesity, in polycycstic ovary syndrome. Diabetes 1989;38: 1165-74 [DOI] [PubMed] [Google Scholar]
- 76.Edelstein SL, Knowler WC, Bain RP, et al. Predictors of progression from impaired glucose tolerance to NIDDM: an analysis of six prospective studies. Diabetes 1997;46: 701-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Golay A, Felber JP, Jequier E, DeFronzo RA, Ferrannini E. Metabolic basis of obesity and noninsulin-dependent diabetes mellitus. Diabetes Metab Rev 1988;4: 727-47 [DOI] [PubMed] [Google Scholar]
- 78.Lillioja S, Bogardus C. Obesity and insulin resistance: lessons learned from the Pima Indians. Diabetes Metab Rev 1988;4: 517-40 [DOI] [PubMed] [Google Scholar]
- 79.Nilsson P, Lindholm L, Schersten B. Hyperinsulinaemia and other metabolic disturbances in well-controlled hypertensive men and women: an epidemiological study of the Dalby population. J Hypertens 1990;8: 953-9 [DOI] [PubMed] [Google Scholar]
- 80.Cederholm J, Wibell L. Glucose intolerance in middle-aged subjects—a cause of hypertension? Acta Med Scand 1985;217: 363-71 [DOI] [PubMed] [Google Scholar]
- 81.Gelding SV, Niththyananthan R, Chan SP, et al. Insulin sensitivity in non-diabetic relatives of patients with non-insulin-dependent diabetes from two ethnic groups. Clin Endocrinol (Oxf) 1994;40: 55-62 [DOI] [PubMed] [Google Scholar]
- 82.Gelding SV, Andres C, Niththyananthan R, Gray IP, Mather H, Johnston DG. Increased secretion of 32,33 split proinsulin after intravenous glucose in glucose-tolerant first-degree relatives of patients with non-insulin dependent diabetes of European, but not Asian, origin. Clin Endocrinol (Oxf) 1995;42: 255-64 [DOI] [PubMed] [Google Scholar]
- 83.Gelding SV, Coldham N, Niththyananthan R, Anyaoku V, Johnston DG. Insulin resistance with respect to lipolysis in non-diabetic relatives of European patients with type 2 diabetes. Diabet Med 1995;12: 66-73 [DOI] [PubMed] [Google Scholar]