Abstract
Three open reading frames (ORFs) were identified by a genome walking strategy in the genomes of serotype M49 group A streptococcal (GAS) strains CS101 and 591. These ORFs were located between the mga core regulon and the dipeptide permease operon. The deduced amino acid (aa) sequences contained signature sequences indicative of a lipoprotein (306 aa), an intracellular protein (823 aa), and a secreted peptide (66 aa), respectively. ORF1 (named Lsp for lipoprotein of Streptococcus pyogenes) and ORF2 exhibited a high degree of homology to the lmb/ORF2 genes of S. agalactiae (B. Spellerberg et al., Infect. Immun. 67:871-878, 1999). The three ORFs were found to be present in each of the 27 GAS serotype strains tested. Transcription analysis revealed a polycistronic lsp/ORF2 and a monocistronic ORF3 message that were detected primarily at the transition from exponential to stationary growth phase. lsp and ORF2 mutants, ORF2- and ORF3-luciferase reporter fusions, and antiserum against recombinant Lsp were produced to examine the biological role of these genes. Although high Zn2+ and Cu2+ ion concentrations decreased lsp operon expression, Lsp did not transport divalent cations as described for other LraI-type operons. The lsp mutant had reduced fibronectin binding. Although no direct binding of Lsp to fibronectin could be demonstrated, the lsp mutant showed decreased transcription of prtF2 encoding the fibronectin-binding protein F2. Both the lsp and ORF2 mutants showed decreased laminin binding. Adherence to and internalization into A549 epithelial cells of both mutants was reduced without a detectable effect on eukaryotic cell viability. The transcription of a number of virulence factors was altered in the lsp mutants and ORF2 mutants. The changes in laminin binding and eukaryotic cell internalization could be explained by changes in transcription of speB (cysteine protease) and/or the global regulators mga, csrRS, and nra.
The characterization of streptococcal mutants defective for adhesive properties led to the identification of a specific class of surface proteins. These lipoprotein receptor antigens (LraI) as initially defined by Jenkinson (30) share the following common structural features: (i) a size of 300- to 330-amino-acid (aa) residues, (ii) a 17- to 23-aa leader sequence, (iii) a (L/I/ V)-(S/A)-(A/G)-C consensus sequence at the C terminus of the leader that serves as the recognition signal for signal peptidase II, (iv) a three-domain structure (B1-α-B2), and (v) a sequence for lipid modification of the N-terminal cysteine that anchors the protein to the cell membrane. The lipoprotein nature of LraI proteins, their association with the cell membrane of gram-positive bacteria, and the involvement of signal peptidase II in the anchoring process has been unequivocally demonstrated. The approaches used included labeling by radioactive palmitic acid and inhibition of protein secretion by the signal peptidase II-inhibitory peptide globomycin (15, 20, 22, 53). LraI proteins have been identified throughout the streptococcal family (e.g., in the Streptococcus mitis and S. mutans groups, in the group of pyogenic streptococci, and in enterococci), as well as in staphylococci, corynebacteria, and bacilli (7, 32, 47, 49, 52).
Examples of LraI protein involvement in streptococcal adhesion include the coaggregation of S. gordonii with Actinomyces naeslundii, the attachment of S. sanguinis to the salivary pellicle, the binding of S. parasanguinis to a platelet fibrin matrix, and the adherence of S. pneumoniae to type II pneumocytes (3, 16, 31).
Mutations in LraI or LraI-directed vaccination led to a reduced virulence of streptococci in mouse or rat pneumonia and endocarditis models (4, 5, 37, 56, 63). However, the direct binding of LraI proteins to target substrates has been demonstrated only once (58). In a recent publication, the involvement of LraI proteins in the attachment processes was proposed to be predominantly indirect (29).
A possible explanation for an indirect effect of LraI on adhesion was provided by the finding that some, if not the majority of LraI proteins, act as receptors for divalent cations and components of ABC-type metal ion uptake transporters. This was initially observed in Synechocystis sp. where the MntC protein was found to transport Mn2+ ions (1). Comparable functions subsequently have been shown for S. pneumoniae PsaA and AdcA, S. gordonii ScaA, S. pyogenes MtsA, S. mutans FimA, and Staphylococcus epidermidis SitC in the transport of Mn2+, Zn2+, Cu2+, or Fe2+ ions (7, 10, 28, 33, 50). Mutants in the LraI genes did not only exhibit an increased requirement for certain divalent cations but also showed impairments in various biological functions such as spore formation, response to oxidative stress, and natural competence (reviewed by Jakubovics and Jenkinson [26]).
LraI genes are generally located in three-gene operons, together with a transmembrane protein and an ATPase. In several cases, an independently transcribed gene located up- or downstream of LraI operons was found to be involved in LraI operon expression (21, 27, 50). These genes usually encode negative regulators that are activated by increased concentrations of their cognate metal ion. Due to the presence of an intraoperon transcription attenuator, the LraI protein can be hyperexpressed in relation to the other two genes in the operon (26, 28).
Among the various biological functions ascribed to LraI, matrix protein, and eukaryotic cell adhesion are also regarded as key features of S. pyogenes (group A streptococcal [GAS]) virulence. The molecular basis for GAS attachment to fibronectin and collagen I has been elucidated (17, 45, 55). These interactions are anticipated to be responsible for the tissue specificity of GAS during colonization and infection of humans. Binding of GAS to laminin is well documented, although the genetic determinants that encode specific adhesins have not been identified (54, 62).
In another species of the pyogenic group of streptococci, S. agalactiae, the Lmb lipoprotein (encoded by lmb) was recently characterized as a member of the LraI family. Lmb mutations decrease the laminin-binding capacity of this species (51). Lmb is present in human but not in the majority of animal isolates; however, its role as a virulence factor in human disease has yet to be established.
The GAS Mga regulon encodes a global positive regulator (Mga). Mga controls the transcription of both cell surface and secreted virulence factors such as the C5a peptidase (ScpA) that are involved in invasive infections (46). In order to identify the genomic boundaries of this regulon, the genomic region downstream of the scpA gene in a serotype M49 GAS strain was sequenced. In a region of 4.7 kb, we identified an open reading frame (ORF) with almost complete identity to lmb (98.7% for the deduced amino acid sequence). On the basis of the results presented below, this ORF was renamed lsp for lipoprotein of S. pyogenes. Lsp was examined for features associated with LraI proteins including (i) surface expression, (ii) ion transport functions, and (iii) a role in bacterial attachment. LraI was located on the cell surface. LraI did not have a detectable role in ion transport. Lsp mutants showed decreased binding to fibronectin; however, no direct binding of Lsp to fibronectin could be detected. Both Lsp and ORF2 mutants had decreased laminin binding and decreased eukaryotic cell attachment and internalization. These phenotypes may instead be a result of the involvement of Lsp and ORF2 in general signaling pathways, since both mutants also showed significantly altered expression of several virulence factors and virulence factor regulators.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
For the molecular analysis, S. pyogenes serotype M49 strain 591 was used. This strain is a patient isolate originally obtained from R. Lütticken, Aachen, Germany. GAS strains of M serotypes 1, 2, 3, 4, 5, 6, 8, 9, 11, 12, 13, 14, 17, 18, 19, 22, 28, 41, 42, 46, 47, 48, 49, 52, 53, 55, and 57 were as described by Podbielski et al. (41). The M-nontypeable GAS strain 64/14 was provided by M. D. P. Boyle, Toledo, Ohio. Escherichia coli DH5α was purchased from Gibco-BRL (Eggenstein, Germany) and served as hosts for plasmids pFW5 (44), PFW5-luc (45), and pMAL-c2 (New England Biolabs, Frankfurt/Main, Germany). E. coli EC101 (36) was obtained from E. Maguin, Jouy-en-Josas, France, and was employed as a host for plasmid pGhost5 (Appligen, Heidelberg, Germany).
GAS strains were cultured in Todd-Hewitt (TH) broth or on TH agar (Oxoid Unipath, Wesel, Germany), both supplemented with 0.5% yeast extract (THY), or in chemically defined medium (CDM) (61). CDM was used for measurements of lsp operon expression in the presence or absence of divalent cations. To determine the effects of Co2+, Cu2+, and Zn2+ ions, CoSO4, CuCl2, or ZnCl2 were added to complete CDM to final concentrations of 5, 10, 20, 50, and 100 μM. To determine the effect of Mg2+ and Fe2+, CDM was made without MgSO4 and FeSO4. The tested salt was then added back at a concentration of 66 and 72 μM, respectively. To determine the effect of Ca2+, CDM was prepared without Ca salt. However, unlike to other salts, to completely inhibit growth, residual Ca2+ had to be removed by addition 500 μM EGTA. CaCl2 was added back at 300, 400, or 500 μM concentrations.
The GAS mutant strains harboring pFW5 or pGhost5 insertions were maintained in medium containing 60 mg of spectinomycin or 5 mg of erythromycin liter−1, respectively. Culture conditions for GAS strains were a temperature of 37°C and a 5% CO2-20% O2 atmosphere unless otherwise indicated. Antibiotic resistance of GAS strains was assessed by using the E-test according to the manufacturer's instructions (AB Biodisk; Viva Diagnostika, Hürth/Köln, Germany). E. coli DH5α and EC101 isolates transformed with pFW5, pMAL-c2, or pGhost5 derivatives were grown on disk sensitivity agar (Oxoid Unipath) supplemented with 100 mg of spectinomycin, 50 mg of ampicillin, or 150 mg of erythromycin liter−1, respectively. E. coli Blue MRF strain infected with recombinant lambda phages was kept and processed according to the instructions of the supplier (Stratagene). All E. coli cultures, except for E. coli EC101 carrying pGhost5 derivatives, were grown at 37°C in ambient air. The EC101 strain was cultured at 30°C.
Nucleic acid techniques.
Chromosomal and plasmid DNA preparations, genetic manipulations, and other conventional DNA techniques, including electroporation of GAS strains were done as described in a previous publication (45).
PCR screening for the presence of the lsp operon in various GAS serotype strains involved primer pairs directed to lsp (5′-GCT CAT TGT CAA GAC AG 3′ and 5′ TAA GTA CTA TGA AAG AAG-3′), ORF2 (5′-ACA AGC TCA AGA GTT ATT G-3′ and 5′ TTA TTC TAA CAT GGA TAC CA-3′), and ORF3 (5′-ATT CTA ATC ATG TCA GAA GA 3′ and 5′-TTT TAA TTT TTC CTT AAC G-3′).
A previously constructed serotype M49 GAS lambda library was used for sequencing of genomic DNA according to the protocol of Podbielski et al. (43). Oligonucleotides employed for PCR and sequencing were designed with the aid of OLIGO6.0 (National Biosciences/MedLab, Oslo, Norway) and were purchased from a commercial supplier (Interactiva, Ulm, Germany). DNA sequence compilation and analysis were performed as described by Podbielski et al. (43).
For RNA preparations, serotype M49 GAS strain 591 was grown overnight in THY broth, diluted 1:5 in THY broth, and incubated for another 90 min. This mixture of stationary-phase and early-log-phase cells was subjected to RNA preparations procedures according to an established protocol (42). Denaturing gel electrophoresis, Northern blotting, generation of non-radioactively labeled probes, and semiquantitative detection of specific transcripts by hybridization was done as outlined by Podbielski and Leonard (42). Probes directed to GAS housekeeping and virulence genes were generated as described by Kreikemeyer et al. (35).
Construction of recombinant vectors and GAS strains.
The generation of a pGhost5 plasmid carrying a lsp fragment and the subsequent insertional mutagenesis into the lsp gene of M49 GAS strain 591 was done according to the protocol of Spellerberg et al. (51).
For insertional mutagenesis of ORF2, a C-terminal lsp-N-terminal ORF2 fragment was amplified by PCR with forward and reverse primers (5′-AAA GGA GTA AAA TTA ACT-3′ and 5′-TTT GAC TAC GAT CTG TTC-3′). The resulting product was cloned into pFW5 via SpeI and PstI 5′ extensions. Site-specific single-crossover insertion of the recombinant plasmid was performed according to the method of Podbielski et al. (45). Integration of pFW5 into ORF2 was confirmed by PCR.
For integration of luciferase reporter boxes downstream of the lsp operon or of ORF3, ORF2 and ORF3 fragments were amplified by PCR with forward primer (5′-ACT TGA TTT GAC TCA GAT TG-3′) and reverse primer 1 (5′-TTA TTT TAA TTT TTC CTT AAC G-3′) or reverse primer 2 (5′-GAG ATC TTC TTT TGG TTA AG-3′), respectively. The resulting fragments were cloned via NheI and XhoI extensions into pFW5-luc (45). The recombinant plasmids were integrated by site-specific single-crossover events, and PCR was used to determine whether the transcription fusion was in ORF2 or ORF3.
For Lsp expression as a recombinant maltose-binding protein (MBP) fusion, the lsp gene was amplified by PCR with the primer pair 5′-GCA GGG TGT GAT AAG TCA GC-3′ and 5′-CAC TAA TAA TCT CCT TTA CTT CAA CTG-3′. The product was cloned into pMAL-c2 via EcoRI and PstI 5′ extensions and purified from E. coli DH5α according to the manufacturer's instructions.
Recombinant plasmids and GAS strains were confirmed for correct integration of desired DNA fragments by appropriate primers and PCR, appropriate probes and Southern blot hybridizations, and/or nucleotide sequencing (data not shown).
Quantitative assays for luciferase activity.
For assessment of luciferase activity from lsp-operon-luc or ORF3-luc fusions, GAS luc reporter strains were grown in THY or CDM depleted of or supplemented with specific divalent cations as standing cultures. For measurement of luminescence, 1-ml aliquots of the cell suspensions were withdrawn at hourly intervals and processed as described by Podbielski et al. (45). All reported data are representative of at least three independent experiments.
General procedures for protein purification and characterization.
The recombinant Lsp-MBP fusion was expressed in its E. coli host, harvested, and purified by affinity chromatography utilizing an amylose column according to the pMAL manufacturer's instructions (New England Biolabs). After dialysis and a factor Xa protease digestion, the recombinant GAS protein was separated from the MBP fusion partner and factor Xa by ion-exchange chromatography. For this purpose, a DEAE-Sepharose column (Pharmacia, Freiburg, Germany) and a fast-protein liquid chromatography-high-pressure liquid chromatography workstation (BioCad Sprint; Perseptive Biosystems, Wiesbaden, Germany) was used. The recombinant GAS protein did not bind to the column, while the other proteins bound and were eluted only when using a 25 to 500 mM NaCl gradient buffer. Polyclonal rabbit antisera to the purified protein was produced by EuroGentec (Seraing, Belgium).
To identify the cellular localization of Lsp, cell cultures were fractionated into supernatant, cell envelope (cell membranes and cell walls), and cell cytoplasm according to the protocols of Spellerberg et al. (51). Proteins were separated by molecular mass by using sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) electrophoresis. Subsequent detection of the proteins was done by either Coomassie blue staining or semidry Western blotting onto polyvinylidene difluoride (PVDF) membranes (Immobilon P; Millipore, Eschborn, Germany), followed by immunodetection with polyclonal anti-Lsp rabbit antiserum and a second alkaline phosphatase-conjugated anti-rabbit immunoglobulin G (IgG) antibody (51).
Direct immunofluorescent detection of Lsp on the surface of whole GAS bacteria was done by reacting whole cells with the polyclonal anti-Lsp-antiserum or the corresponding preimmune serum, followed by reaction with a fluorescein isothiocyanate (FITC)-conjugated anti-rabbit antiserum as a secondary antibody (18). Unlabeled and labeled bacteria were detected by using an Axioplan fluorescence microscope (Zeiss, Jena, Germany). Quantitative assays were performed with a CytoFluor II fluorometer (Perseptive Biosystems) according to the manufacturer's protocols.
Human matrix protein adherence of Lsp protein and whole GAS bacteria.
For the detection of a direct interaction between Lsp and human matrix proteins, recombinant Lsp was conjugated to a peroxidase label according to the protocol of Podbielski et al. (45). Collagen I, fibronectin, and laminin (Gibco-BRL) were spotted in 0.1-, 1-, and 2-μg amounts onto PVDF membranes. Nonspecific binding was blocked with 2% blocking reagent (Roche-Boehringer, Mannheim, Germany), and then the blot was exposed to the labeled Lsp for 2 h at room temperature. A spot of the Lsp antiserum was used as a positive control for binding to the membrane. Bound peroxidase-labeled Lsp was detected as previously described (45).
To exclude the effects of conformation on the binding process, 0.1-, 1-, and 2-μg portions of recombinant unlabeled Lsp were spotted onto a PVDF membrane. The membrane was reacted with dissolved human matrix protein at 0.1 g/liter final concentration as described above. The binding of matrix proteins was detected by using polyclonal matrix protein rabbit antisera and a peroxidase-labeled secondary rabbit IgG antiserum. As a positive control, the matrix proteins were directly spotted onto the PVDF membrane.
The quantitative measurements of binding of FITC-labeled whole GAS bacteria to immobilized collagen I, fibronectin, and laminin was done as described by Podbielski et al. (43). Direct adherence inhibition by soluble recombinant Lsp was tested by preincubating the immobilized fibronectin with 0.1 g of Lsp/liter for 1 h at room temperature and washing of the wells prior to the addition of FITC-labeled whole bacteria. The results of the adherence assays are reported as the mean of at least four independent experiments. The data was statistically evaluated by using the Mann-Whitney U test.
Eukaryotic cell adherence and internalization and determination of eukaryotic cell viability.
Assessment of eukaryotic cell adherence and internalization was determined by an antibiotic protection assay according to the protocol of Molinari et al. (39). Changes to the protocol included the use of A549 cells (ATCC CCL-185). The cell culture medium consisted of RPMI 1640 medium supplemented with glutamine, nonessential amino acids, sodium bicarbonate, sodium pyruvate, and 10% inactivated fetal calf serum as recommended by American Type Culture Collection for the cultivation of A549 cells. Since the time of the assay was sufficient for GAS to double once in the test medium, only bacterial strains with equal doubling time in the test medium were compared. The results are presented as the mean of four independent experiments. The significance was determined by the Mann-Whitney U test.
The cell proliferation reagent WST-1 (Roche-Boehringer) was used according to the manufacturer's instructions to determine eukaryotic cell viability in presence of GAS strains. Eukaryotic cells were examined by transmission electron microscopy to visualize potential ultrastructural changes induced by adherent or internalized GAS bacteria (80-kV ray voltage on a EM400T electron microscope; Philips, Eindhoven, Holland, [39]).
Nucleotide sequence accession number.
The nucleotide sequence of the GAS lsp locus has been submitted to the EMBL nucleotide sequence data library under accession no. X80397
RESULTS
Sequence analysis of the lsp genomic region.
The initial lsp operon sequence was generated from a genomic library of serotype M49 GAS strain CS101 in phage Lambda ZAP Express by using a primer walking strategy. The same region from serotype M49 GAS strain 591 was sequenced by PCR with genomic DNA as a template. A 4,700-bp portion of both strains was sequenced between the upstream ORFX/fba (46, 57) and the downstream dppE (42) encoding a fibronectin-binding protein of the Mga regulon and a component of the dipeptide permease, respectively. The sequences were completely identical and, upon analysis, were found to contain three ORFs on one strand (Fig. 1). These ORFs encoded putative proteins of 306, 823, and 66 aa, which we later named Lsp, ORF2, and ORF3, respectively. Each ORF contained a signature sequence: Lsp contained an isoleucine-x-x-cysteine motif at aa 17 to 20, which is typical for leader sequences of gram-positive lipoproteins. ORF2 had an EF-hand motif at aa 695 to 707 associated with calcium binding in calmodulin-like proteins (38), and ORF3 had a double-glycine motif at aa 15 and 16, as found in leader sequences of secreted peptides in gram-positive bacteria (19). Upon comparison with sequence databases, Lsp and ORF2 exhibited 97 to 99% identity with the deduced aa sequences of Lmb and ORF2 in S. agalactiae (51). Adjacent sequences in the GAS genome, including that of ORF3, showed no homology to the genomic environment of lmb/ORF2 in S. agalactiae (14).
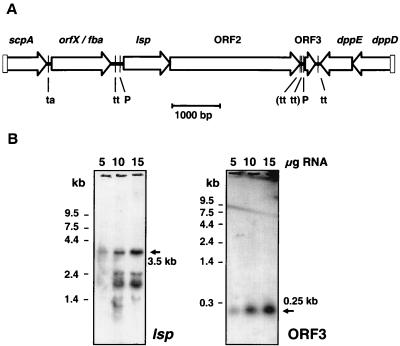
FIG. 1.
(A) Diagram of the lsp operon region in the chromosome of group A streptococcal serotype M49 strain 591. Designation of ORFs or genes was done according to Podbielski et al. (46) (C5a peptidase scpA and ORFX), Terao et al. (57) (fibronectin-binding protein fba), Podbielski and Leonard (42) (dipeptide permease dppDE), and the present study (lsp, ORF2, and ORF3). Arrows, representing the genes, indicate the direction of transcription and were drawn to scale. P, promoter; ta, transcriptional attenuator; tt, transcriptional terminator. (B) Northern blot hybridizations of lsp operon and ORF3 messages. Total RNA in amounts of 5, 10, and 15 μg was separated by denaturing agarose gel electrophoresis, transferred to membranes, and hybridized with non-radioactively labeled specific probes. The positions and sizes of the specific messages (arrows) and of a RNA size reference are marked. RNA bands of smaller sizes result from unspecific association of the mRNA with the 16S rRNA and from mRNA breakdown products.
Unlike the three genes of conventional LraI operons, ORF2 and ORF3 could not be associated with an obvious function based on sequence comparisons. However, Lsp appeared to belong to the LraI lipoprotein family since it had (i) a lipoprotein signature sequence, (ii) a potential three-domain (B1-α-B2) structure, and (iii) a high homology to the S. agalactiae Lmb. The Lsp sequence was compared to available streptococcal, enterococcal, and Synechocystis spp. LraI sequences. When the various sequences were compared to each other, two subfamilies were evident based on the percent sequence identity (Table 1).
TABLE 1.
Homology comparison of deduced amino acid sequences from selected lraI lipoprotein genes
| Organism and protein | % Identitya
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lmb | SP1002 | ScaA | ScbA | SsaB | FimA | PsaA | SloC | MtsA | EfaA | AdcA | Synechocystis sp. MntC | |
| S. pyogenes Lsp | 98.7 | 64.7 | 24.5 | 24.8 | 19.3 | 26.1 | 26.8 | 8.5 | 11.4 | 22.2 | 28.8 | 20.3 |
| S. agalactiae Lmb | 64.7 | 24.8 | 25.2 | 19.6 | 26.5 | 27.1 | 8.6 | 11.1 | 22.6 | 29.1 | 20.3 | |
| S. pneumoniae SP1002 | 21.5 | 21.8 | 21.8 | 17.7 | 18.7 | 21.1 | 24.2 | 18.3 | 33.9 | 19.4 | ||
| S. gordonii ScaA | 93.6 | 90.6 | 78.0 | 78.1 | 73.5 | 77.2 | 55.8 | 25.8 | 21.9 | |||
| S. cristae ScbA | 89.0 | 78.6 | 79.0 | 75.8 | 76.1 | 58.1 | 26.1 | 21.6 | ||||
| S. sanguinis Ssab | 78.6 | 80.3 | 74.5 | 78.2 | 57.8 | 24.9 | 22.3 | |||||
| S. parasanguinis FimA | 92.6 | 73.2 | 75.8 | 59.1 | 25.4 | 23.0 | ||||||
| S. pneumoniae PsaA | 72.2 | 75.8 | 60.6 | 26.5 | 22.6 | |||||||
| S. mutans SloC | 72.3 | 55.9 | 15.0 | 22.2 | ||||||||
| S. pyogenes MtsA | 56.4 | 19.4 | 20.1 | |||||||||
| E. faecalis EfaA | 19.8 | 23.1 | ||||||||||
| S. pneumoniae AdcA | 19.2 | |||||||||||
Values were calculated by using the PCGENE PALIGN program and are shown as percent identity. Boldfacing indicates >64% sequence homology rates between various LraI sequences and, thus, members of potential LraI subfamilies. LraI sequences originated from Lsp (this study), Lmb (51), ScaA (34), ScbA (8), SsaB (16), FimA (12), PsaA (48), SloC (32), MtsA (28), EfaA (37), AdcA (11), and MntC (1).
One subfamily (the Lmb subfamily) was comprised of Lsp, Lmb, and an uncharacterized protein (SP1002) from S. pneumoniae (59). The sequences in this subfamily had a range of 65 to 99% identity. The second subfamily (the FimA subfamily) was larger and was comprised of ScaA, ScbA, SsaB, FimA, PsaA, SloC, and MtsA. These sequences had a range of 72 to 94% identity. Within the FimA subfamily, FimA and PsaA were 92% identical and ScaA, ScbA, and SsaB were 89 to 94% identical. EfaA, AdcA, and MntC did not share high identity with any of the LraI subfamilies or with each other. With the exception of the Lmb subfamily, each LraI gene was found to be a member of a three-gene operon (27). Members of the FimA subfamily have been described to be involved in the uptake transport of divalent cations possibly as the surface-binding proteins of the transport complex.
Distribution of the lsp locus among GAS and the subcellular location of Lsp.
If, like other LraI-type proteins, Lsp is part of a general metal transporter, two predictions about the Lsp can be made: (i) the lsp locus would be expected to be present in many, if not all, GAS isolates, and (ii) the Lsp protein would be expressed on the surface of individual GAS bacteria. To determine the distribution of these genes, lsp-, ORF2-, and ORF3-specific primers were used in PCRs with genomic DNA from 27 GAS strains of different M serotypes, including one M-nontypeable strain. A product of the expected size could be amplified from all of the strains (data not shown). In all strains tested the dipeptide permease (dpp) operon was located downstream of lsp, ORF2, and ORF3. However, some strains (e.g., M1) did not harbor an ORFX/fba gene (46, 57) upstream of the three sequences.
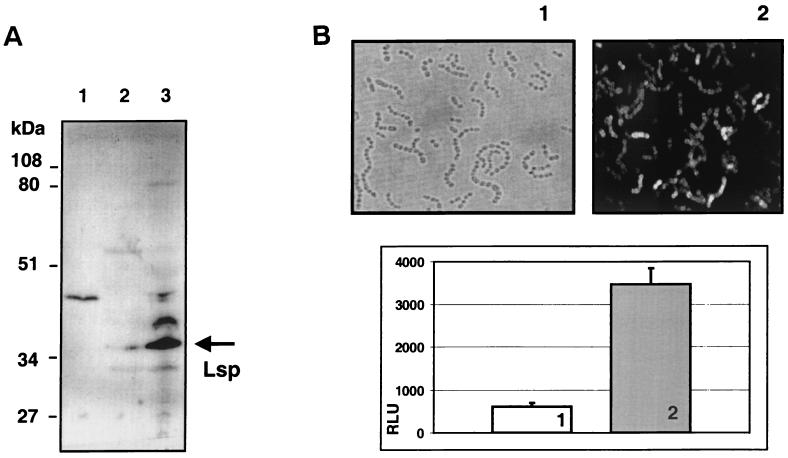
An association of Lsp with the surface of GAS bacteria was demonstrated by using two different approaches. Western immunoblot analysis of GAS culture supernatant, cell envelope, and cytoplasmic fractions revealed traces of Lsp in the cytoplasmic fraction and a majority of the protein associated with the cell envelope (Fig. 2). With the same Lsp antiserum and a second FITC-conjugated anti-rabbit IgG antiserum, the presence of the protein on the surface of whole GAS bacteria was visualized by direct immunofluorescence assays (Fig. 2). To quantitate the amount of Lsp on the surface, the levels of labeling were compared between preimmune sera and Lsp antisera. The mean fluorescence of labeled GAS was increased up to 5.7-fold over that of preimmune serum labeling levels in stationary-phase bacteria when the levels of lsp transcription were the highest (see below; Fig. 2).
FIG. 2.
(A) Western immunoblot of cell fractions with a polyclonal anti-Lsp antiserum and an anti-rabbit IgG-alkaline phosphatase conjugate. In lanes 1, 2, and 3, 30 μg of total protein from culture supernatant, cytoplasm, and the bacterial envelope fraction, respectively, were separated by SDS-PAGE, transferred to PVDF nylon membranes, and reacted with rabbit anti-Lsp antiserum. The Lsp protein band predominantly visible the cell envelope fraction is marked by an arrow. Relative molecular masses of the marker proteins are shown at the side of the blot. The minor 45-kDa bands in the culture supernatant and cell envelope fractions probably result from a surface-expressed and protease-released IgG Fc-binding GAS protein. (B) Direct immunofluorescence detection of surface expressed Lsp protein in whole GAS bacteria. Rabbit serum obtained before (panel 1) and after (panel 2) immunization with recombinant Lsp protein was for used for direct immunofluorescence detection of Lsp employing qualitative immunofluorescence microscopy (upper two panels) and quantitative measurement in a Cytofluor fluorescence reader (lower panel). RLU, relative light units.
Transcription analysis of the lsp locus.
LraI genes in streptococci have been found to be transcribed as a part of a polycistronic message with the genes for a transmembrane transporter and an ATPase. Since lsp had analogs of these genes downstream, it was predicted that lsp might also be transcribed as an operon.
Using probes directed toward the three ORFs for semiquantitative Northern blot hybridizations, two observations were made (Fig. 1): (i) the lsp gene and ORF2 were polycistronically (3.5 kb) transcribed, whereas ORF3 was expressed as a monocistronic (0.25-kb) message, and (ii) significantly more ORF3 mRNA could be detected than the lsp operon mRNA.
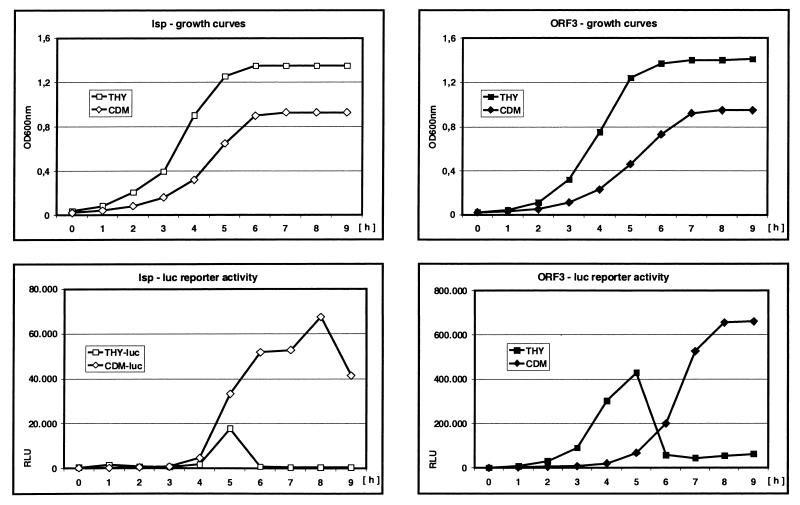
Construction of strains containing of luciferase reporter at the 3′ ends of ORF2 or ORF3 allowed for the comparison of transcription kinetics for both loci (Fig. 3). Both the lsp operon and ORF3 were maximally expressed during the same stage of the growth cycle. There were up to 20-fold differences in transcription strength between the two promoters depending on culture medium conditions. In THY, both loci were expressed at the end of the log phase, whereas in CDM, transcription rates reached their maximum during early stationary growth phase (Fig. 3)
FIG. 3.
Growth curves and lsp operon or ORF3 expression levels in GAS detected by firefly luciferase reporter activities. Promoterless luc reporter boxes were introduced at the 3′ ends of the lsp operon and ORF3 by insertion mutagenesis. The recombinant strains were grown in THY complex medium or in CDM as described in Materials and Methods. Growth was monitored by removing aliquots hourly and measuring their optical density at 600 nm. The same aliquots were utilized for measurements of luc reporter box activities as described in Materials and Methods. RLU, relative light units.
Influence of divalent cations on lsp transcription.
Since several LraI-type proteins have been found to be involved in the uptake of divalent cations in gram-positive cocci and increasing amounts of the divalent cations had a negative effect on the transcription of the corresponding LraI genes, the influence of extracellular cation concentrations on lsp operon expression was determined. The lsp operon-luciferase fusion was incubated in modified CDM depleted for or supplemented with specific divalent cations. Luciferase activity was determined throughout growth. Differences between various incubation conditions were found to be greatest in the 7- to 8-h batch cultures (Fig. 4).
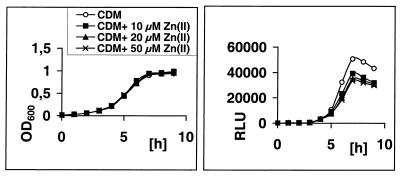
FIG. 4.
Temporal lsp operon expression levels in GAS corresponding to various divalent cation concentrations in the growth medium. The bacteria were grown as batch cultures in CDM supplemented with 10, 20, or 50 μM Zn2+ [Zn(II)]. The lsp operon expression was measured by firefly luciferase reporter activities of aliquots drawn hourly according to a protocol described in Materials and Methods and is shown as the data of one representative measurement.
The CDM formula does not include Zn2+, Cu2+, or Co2+ ions. Supplements of either Zn2+ or Cu2+ ions led to a decreased lsp operon expression, reaching a maximum of a 30% reduction at 20 μM concentrations. Co2+ ions supplemented to up to 100 μM did not influence the lsp operon transcription (data not shown). In all assays, the bacterial growth, as judged by measuring the optical density at 600 nm, was not affected by the presence of the cations.
Although complete CDM contains 50 μM Ca2+, leaving out the Ca2+ salt did not affect GAS growth nor lsp operon expression (data not shown). Upon addition of 500 μM EGTA, a specific Ca2+ chelator, to the theoretically Ca2+-depleted CDM, GAS growth entirely stopped. Growth was restored to normal levels by the addition of 300 or 400 μM Ca2+. The lsp operon transcription rate was not affected by these manipulations in growing GAS cells.
Complete CDM contains 33 μM MnSO4. Leaving out the Mn2+ salt led to growth arrest. Doubling the Mn2+ salt content to 66 μM did not affect the growth rate. The lsp operon expression was found to be independent of Mn2+ concentrations.
Complete CDM contains 36 μM FeSO4. Leaving out the Fe2+ salt increased the growth rate by ca. 20% and simultaneously, increased the lsp operon transcription to a comparable extent. Since the bacteria need iron for their growth, it is expected that the obviously low but crucial amounts were contained in traces in the other salts used for the preparation of CDM. Doubling the Fe2+ concentration in CDM to 72 μM decreased the growth rate and lsp operon expression by ca. 30%. Supplementation of trivalent Fe ions by the addition of Fe(NO3)3 to a 5 to 20 μM final concentration did not influence the growth rate or the lsp operon transcription (data not shown).
Interactions between whole GAS cells, Lsp, and human matrix proteins.
Several LraI-type proteins have been reported to affect adherence to human matrix proteins or other microorganisms. For example, the Lsp-equivalent Lmb was shown to influence the laminin binding of S. agalactiae (51).
To determine whether Lsp influences adhesion, insertional mutations in the lsp operon were engineered by introducing pGhost5 or pFW5 plasmids into lsp or ORF2, respectively. The transcriptional effects of the mutations were confirmed by Northern blotting, and the expression of Lsp protein was determined by Western blotting. The lsp mutant had a truncated lsp message and no detectable Lsp in the envelope fraction. However, in the lsp mutant a large transcript containing ORF2 could be detected (data not shown). This transcript probably results from the presence of one or several promoters in pGhost5 that read through into genes downstream of the plasmid insertion. Thus, the lsp mutant lacked Lsp but, due to readthrough from plasmid promoters, still expressed some ORF2. The ORF2 mutant had a truncated ORF2 message and expressed normal levels of Lsp (data not shown).
Both mutants had unaltered growth kinetics in liquid and on solid media. The mutants also had unchanged resistance to the antibiotics penicillin, vancomycin, chloramphenicol, kanamycin, tetracycline, cotrimoxazol, and ofloxacin. The mutants had an unchanged microscopic morphology with respect to chain length and interbacterial aggregation (data not shown). In addition, no significant differences in growth rate between the mutants and wild type could be seen in CDM modified for the content of divalent cations (see above).
The two mutants were tested for adherence to immobilized human matrix proteins. The fibronectin binding of the lsp mutant and the laminin binding of both mutants were markedly diminished to about half of the wild-type values (Table 2). The direct role of Lsp in fibronectin binding was examined. Preincubation of the immobilized fibronectin with dissolved recombinant Lsp did not influence the subsequent attachment of wild-type or mutant strains (data not shown). Therefore, to test whether Lsp directly interacted with fibronectin, binding assays were done. Fibronectin was immobilized and soluble labeled Lsp was added to the membranes. The converse experiments were also done in which Lsp was immobilized and soluble fibronectin was added. With both approaches, no interaction between Lsp and fibronectin could be detected (data not shown).
TABLE 2.
Quantitative assessment of interactions between serotype M49 GAS wild-type or lsp operon mutant bacteria and immobilized matrix proteins or A549 eukaryotic cells
| Parametera | Mean ± SEM
|
||
|---|---|---|---|
| Wild type | lsp mutant | ORF2 mutant | |
| % Binding to: | |||
| Fibronectin | 100 ± 18 | 50 ± 38 | 105 ± 30 |
| Laminin | 100 ± 29 | 59 ± 13* | 64 ± 22* |
| Collagen I | 100 ± 22 | 95 ± 27 | 103 ± 21 |
| % Eukaryotic cell attachment | 11.3 ± 5.7 | 5.8 ± 2.8* | 3.0 ± 1.2* |
| % Eukaryotic cell internalization | 5.5 ± 3.7 | 2.5 ± 0.9* | 1.4 ± 1.3* |
Binding to immobilized human matrix proteins during a 1-h incubation period was measured by utilizing FITC-labeled whole bacteria cultured to the stationary phase. Values obtained with wild-type bacteria were arbitrarily defined as 100%. Values of lsp and ORF2 mutants were calculated in relation to the corresponding wild-type values. Eukaryotic cell attachment and internalization were assessed by using stationary-phase bacteria and A549 epithelial cells according to a protocol for a classical antibiotic protection assay. The initial inoculum and the ratio of adherent to internalized bacteria were quantitated by viable counts. The initial inoculum was arbitrarily defined as 100%, and adherent/internalized bacteria were calculated in relation to the corresponding initial inoculum. Each assay was repeated on at least four independent occasions. Variation between assays is shown as a standard deviation of the mean. Values marked with asterisks indicate statistically significant differences (P < 0.05) according to the Mann-Whitney U test.
Eukaryotic cell attachment and internalization of lsp operon mutants.
Fibronectin binding has been described as a prerequisite for GAS to attach to and be internalized into eukaryotic cells (24, 40), although this mechanism could be less important for serotype M49 GAS bacteria (39).
To address the biological consequence of decreased fibronectin binding of the M49 lsp mutant, the interaction of both the lsp and ORF2 mutant with eukaryotic cells was inspected. Both the cell attachment and internalization rates of the two mutants were decreased to ca. 25 to 51% of the wild-type values (Table 2).
In order to discriminate between a direct or indirect involvement of Lsp in these phenomena, the serotype M49 GAS wild-type strain was preincubated in Lsp antiserum or the preimmune serum for 1 h prior to being added to the eukaryotic cells. Dilution steps of 1:2 and 1:4 of the anti-Lsp antiserum, but not of the preimmune serum, led to bacterial adherence reductions of 28 and 22%, respectively. Higher dilutions of the antiserum had no effect.
The decreased GAS internalization rates were determined by a antibiotic protection assay that quantitates the number of viable bacteria in eukaryotic cells where the bacteria are protected from antibiotics that are present in the medium but cannot penetrate eukaryotic cells. If GAS are lysing the eukaryotic cells, there would be fewer eukaryotic cells available to protect the GAS from the antibiotic. This could also lead to a decrease in the number of viable GAS recovered from the assay.
Therefore, the host cell viability was examined at the end of the internalization period utilizing the WST-1 reagent assay and electron microscopy. With both approaches, no differences in host cell viability upon contact with wild-type or mutant bacteria could be detected.
Virulence gene expression in lsp locus mutants.
It appeared that Lsp did not directly interact with fibronectin but still affected GAS interaction with matrix proteins and host cells. Therefore, the transcription of known virulence genes in the lsp operon mutants was assessed (Fig. 5; Table 3).
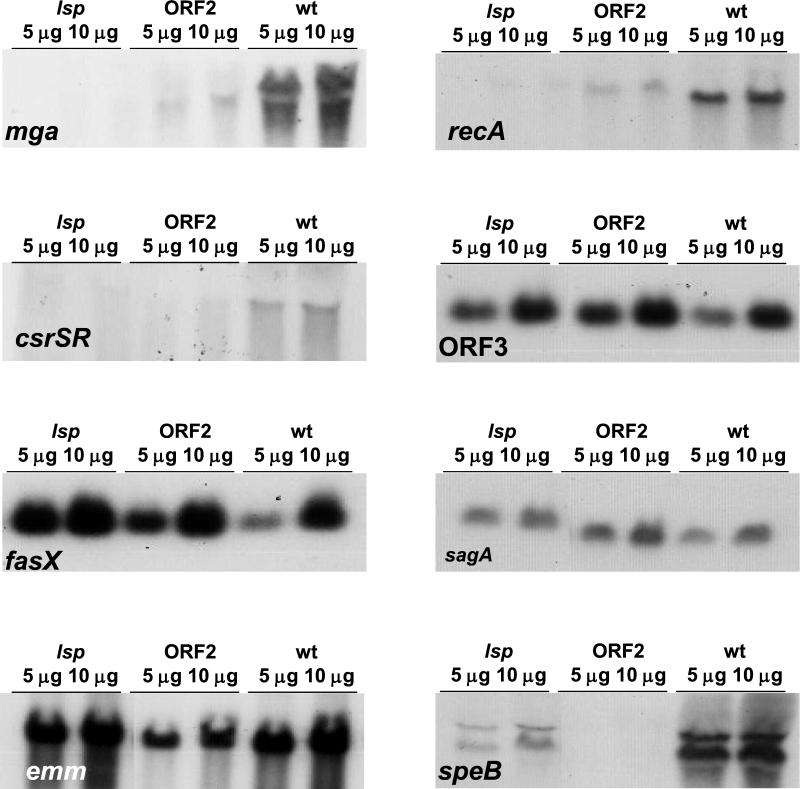
FIG. 5.
Selected semiquantitative Northern blot hybridizations of specific mRNAs (italics) in serotype M49 wild-type (wt) and lsp operon mutant bacteria (lsp and ORF2). Total RNA (5 and 10 μg) was analyzed by denaturing agarose gel electrophoresis and Northern blot hybridizations. For further details, see the legend of Table 3 and the corresponding section in Materials and Methods.
TABLE 3.
Semiquantitative transcription analysis of selected virulence-associated genes in serotype M49 GAS wild-type or lsp operon mutant bacteria
| Group and probe target | Resultsa with:
|
||
|---|---|---|---|
| Wild type | lsp mutant | ORF2 mutant | |
| Housekeeping gene | |||
| recA | S | −− | −− |
| Surface-expressed virulence factors | |||
| emm49 | S | O | O |
| fbp54 | ND | ND | ND |
| has | S | O | O |
| prtF2 | W | −− | O |
| sof/sfbII | W | − | − |
| Secreted virulence factors | |||
| sagA/pel | S | O | O |
| ska | W | + | + |
| slo | W | − | − |
| speB | S | − | −− |
| Virulence regulators | |||
| csrRS | W | − | − |
| fasX | S | + | + |
| mga | S | −− | −− |
| nra | W | − | − |
| ropB | S | O | O |
| Genes of the lsp locus | |||
| lsp | W | + | + |
| ORF2 | W | + | + |
| ORF3 | S | + | + |
Semiquantitative Northern blot hybridizations were performed with total RNA prepared from a mixture of stationary- and early-log-phase bacteria. The total RNA was serially diluted in twofold steps before being subjected to denaturing gel electrophoresis. By visual inspection, hybridization signals from wild-type cells were judged as strong (S), weak (W), or not detectable (ND). The strength of these signals was compared to those from lsp or ORF2 mutant bacteria. The differences were recorded as unchanged (O), one or two dilution steps stronger (+) or weaker (−), or three or more dilution steps weaker (−−) compared to the corresponding wild-type signals. The results represent at least three independent RNA preparations and Northern blot hybridizations for each gene.
The lsp mutant was the only mutant that exhibited decreased fibronectin binding. Of the genes encoding fibronectin-binding surface factors, the protein F2 (prtF2) and serum opacity factor (sof) genes were decreased in the lsp mutant but were not decreased or were less pronounced in the ORF2 mutant. This finding could explain the reduced fibronectin binding of the lsp mutant. However, this does not explain the altered host cell interactions found in both the lsp and ORF2 mutants. The expression of the secreted cysteine protease (speB) gene was strongly diminished in both lsp operon mutants. The speB gene has previously been shown to be involved in serotype M49 GAS eukaryotic cell adherence (39). A significant decrease in the transcription rates of three global regulatory genes or operons—csrRS, mga, and nra—was also seen in both lsp operon mutants. All three regulators have been associated with eukaryotic cell interaction (9, 25, 39).
The truncated lsp operon messages in both lsp operon mutants were present at higher levels than the full-length transcripts in the wild type, indicating the possible existence of a positive autoregulatory loop for lsp operon transcription.
Of note, the recA messages were reduced in both mutants. Since several other genes (e.g., the emm gene) were expressed at unchanged levels, the lower expression level for recA did not indicate a global reduction in mRNA transcription rate or mRNA stability in the mutants.
DISCUSSION
The ability of GAS to bind a variety human matrix proteins and the highly complex regulation of the binding properties could explain why GAS can infect almost every tissue or organ in humans, but at specific stages of the infection process bacteria are found only in limited tissue types. Laminin binding could contribute to the spread of GAS to additional sites in the human body during late infection stages. Thus, the identification of Lsp, exhibiting 98.7% identity to the potential laminin-adhesin Lmb from S. agalactiae (51), stimulated the present investigation.
Lsp's high degree of homology to Lmb immediately indicated that Lsp could be another member of the constantly growing LraI protein family in streptococci. It contained the requisites of the signature sequence and the structural organization of a LraI protein. Lsp's association with the cell envelope and display on the cell surface added strong arguments for the classification of Lsp as an LraI protein. S. pyogenes is regarded as an exclusive human pathogen, and Lsp was found to be present in all 27 strains examined. S. agalactiae cause both human and animal infections and, interestingly, Lmb is found predominantly in human pathogenic strains.
In both S. pyogenes and S. agalactiae, the LraI-like gene is associated with ORF2, a gene whose exact function is still unknown. In S. pyogenes the two genes are located between general virulence genes (mga regulon) and housekeeping genes (dipeptide permease) (42, 46). In S. agalactiae, these genes are located on a composite transposon (14). This finding led to the hypothesis that lsp antedated lmb and was transferred from S. pyogenes to S. agalactiae by mobile genetic elements.
Since lmb and ORF2 are polycistronically transcribed in S. agalactiae, it was likely they also formed an operon in S. pyogenes. Although only a weak potential transcription terminator could be identified in the intergenic region between ORF2 and ORF3, Northern blotting clearly demonstrated that lsp and ORF2 were expressed as a two-gene operon, whereas ORF3 was monocistronically transcribed. Thus, the lsp operon exhibited an organization that was different from the majority of LraI operons where the lraI gene and two other ORFs form an operon. This unique pattern of transcription is consistent with homology data that suggests that lsp and lmb form a unique subfamily of LraI proteins (Lmb subfamily). The strong sequence divergence of ORF2 and ORF3 from the other genes of three-gene LraI operons (e.g., the FimA subfamily) further supports grouping of the lsp and lmb operons into a distinct subfamily.
Sequence divergence of ORF2 and ORF3 suggested that the lsp and lmb operons may have different biologic functions from their counterparts in the FimA subfamily. Unlike one of the ORFs from the FimA subfamily, ORF2 from the lsp and lmb operons did not have signal motifs for association with the membrane or ATPase domains (2). Instead, lsp and lmb operon ORF2 proteins had EF hand motifs and the GAS ORF3 a double-glycine leader. Thus, the ORF2 protein could be involved in trafficking, homeostasis, or storage of divalent cations or act as a carrier to provide other proteins with divalent cations needed for their stability (38).
However, differences in the function between Lsp and the FimA subfamily LraI proteins were identified when the role of the lsp operon in the transport of divalent cations was examined. lsp operon mutants were not affected in their growth kinetics when exposed to varied concentrations of six different divalent cations. These results were in clear contrast to the results for FimA subfamily LraI proteins (10, 33). These LraI operon mutants had growth defects unless the growth media was supplemented with unusually high concentrations of divalent cations.
Consistent with the observation that Lsp does not participate in divalent cation transport, the lsp operon does not have a DtxR-type regulator gene close to the lsp operon and lsp transcription is relatively insensitive to divalent cation concentrations. The only exception is modest effects of high concentrations of Zn2+ and Cu2+ ions on the lsp operon expression. Because these cations affect lsp transcription, it is formally possible that Lsp could participate in the transport of these two ions at high extracellular cation concentrations by providing an alternative cation-binding lipoprotein. Lipoprotein-binding protein cross talk has been demonstrated for other streptococcal lipoprotein transport systems and may be due to the ability of lipoproteins to diffuse laterally across the cell membrane (11, 42).
Two FimA family LraI lipoproteins (PsaA and AdcA) have been identified in the complete genome sequence of a serotype M1 GAS strain (13), and an equivalent of MtsA (28) has been identified in the M1 sequence. These sequences could also be found in the serotype M49 genome (A. Podbielski, unpublished results). Therefore, while the primary function of Lsp does not appear to be ion transport, there are a number of LraI family transport systems and Lsp could serve as an alternative binding protein for one of these systems.
Like other LraI systems, Lsp is important for bacterium-host interactions. The reduction in fibronectin binding of Lsp mutants and the reduction of laminin binding of both lsp and ORF2 mutants suggest a role for the lsp operon in matrix glycoprotein interaction. Both of the lsp operon mutants also showed reduced levels of eukaryotic cell adhesion and internalization. These defects in matrix glycoprotein binding and eukaryotic cell interactions could be because Lsp acts as an adhesin or because the lsp operon is involved in the regulation of other genes important for these interactions.
When characterizing the role of Lsp in fibronectin binding, we found it is unlikely Lsp functions as a direct fibronectin adhesin. The lsp mutant, but not the ORF2 mutant, displayed a marked decrease in binding to fibronectin. However, no indication of the direct binding of Lsp and fibronectin was found either by adding recombinant Lsp to the adherence assay with whole bacteria or by testing the interaction of isolated Lsp and human matrix proteins.
The lsp mutant, but not the ORF2 mutant, showed a decreased transcription of the genes for the fibronectin-binding proteins prtF2 and sof. This decrease in fibronectin-binding protein transcription probably explains the reduced fibronectin binding in the lsp mutant. It can be concluded that Lsp affects interaction with fibronectin through the regulation of fibronectin-binding proteins. Another member of the Lmb subfamily of LraI proteins, Lmb, does not play a role in fibronectin binding. Among the FimA subfamily, only interactions with fibrin as an extracellular matrix component have been investigated with scbA or fimA mutants. The inactivation of the S. cristae scbA gene (8) did not affect fibrin binding, whereas a S. parasanguinis fimA mutation led to this effect (5). The present results are complemented by the documentation of a direct laminin binding of the Lsp-equivalent in a serotype M1 GAS strain (58).
Since both the lsp and ORF2 mutants showed decreased cell invasion but only the lsp mutant had decreased fibronectin binding, it is likely that fibronectin binding is not as important for cell adherence and internalization in serotype M49 GAS strains as it is for other strains. Molinari et al. have recently published similar observations about a potentially marginal role of fibronectin binding for M49 GAS invasion (39).
In the present study, there are at least four possible explanations for the decreased eukaryotic cell invasion of the lsp and ORF2 mutants. (i) The decreased laminin binding of both mutants could be the molecular basis for their behavior toward eukaryotic cells. This human matrix protein was previously demonstrated to stimulate the internalization of GAS strains into epithelial cells (9). (ii) The decreased eukaryotic cell invasion of the lsp and ORF2 mutants could be due to the participation of Lsp in a complex oligo- or multifactor adhesive structure. Lsp could be important for assembly, stability, or turnover of this structure. This Lsp-stabilized structure could contain different proteins in different streptococcal species. This would explain the phenotypic differences observed in LraI mutants of diverse streptococcal species or GAS strains (58). (iii) Another, although less-probable explanation, for changes in adhesiveness is that Lsp could have a general effect on the GAS surface charge. (iv) Finally, the lsp operon could be involved in the regulation of genes important for internalization into eukaryotic cells.
Transcription analysis of several virulence-associated genes revealed multiple effects of the lsp operon mutations on GAS gene expression. Transcription of the cysteine protease SpeB was decreased in both the lsp and ORF2 mutants. SpeB was previously shown to influence both GAS cell attachment and internalization (6, 39, 60). Superimposed and with presumably much broader effects, the transcription rates of three global positive (Mga) and negative (CsrR and Nra) regulator genes were diminished in both mutants. For each of these regulators, significant effects on eukaryotic cell interactions were demonstrated in specific mutants (9, 23, 25, 39). Simultaneous defects in two or more of these regulatory genes have not been examined. Since transcription of all three of these regulators was affected in lsp operon mutants, there may be additive affects of these regulators that remain to be elucidated.
Acknowledgments
We thank A. Flosdorff for expert technical assistance and T. Auerbach for patient typing.
A.P. is supported by DFG grant Po391/3-4; A.P., B.K., and A.B.-K. are supported by DFG grant Po391/8-1; B.S. is supported by DFG grant Sp511/2-2, and B.A.B. is supported by AHA grant 016430U.
Editor: E. I. Tuomanen
REFERENCES
- 1.Bartsevich, V. V., and H. B. Pakrasi. 1995. Molecular identification of an ABC transporter complex for manganese: analysis of a cyanobacterial mutant strain impaired in the photosynthetic oxygen evolution process. EMBO J. 14:1845-1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bartsevich, V. V., and H. B. Pakrasi. 1999. Membrane topology of MntB, the transmembrane protein component of an ABC transporter system for manganese in the cyanobacterium Synechocystis sp. strain PCC 6803. J. Bacteriol. 181:3591-3593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berry, A. M., and J. C. Paton. 1996. Sequence heterogeneity of PsaA, a 37-kilodalton putative adhesin essential for virulence of Streptococcus pneumoniae. Infect. Immun. 64:5255-5262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Briles, D. E., E. Ades, J. C. Paton, J. S. Sampson, G. M. Carlone, R. C. Huebner, E. Swiatlo, and S. Hollingshead. 2000. Intranasal immunization of mice with a mixture of the pneumococcal proteins PsaA and PspA is highly protective against nasopharyngeal carriage of Streptococcus pneumoniae. Infect. Immun. 68:796-800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burnette-Curley, D., V. Wells, H. Viscount, C. L. Munro, J. C. Fenno, P. Fives-Taylor, and F. L. Macrina. 1995. FimA, a major virulence factor associated with Streptococcus parasanguis endocarditis. Infect. Immun. 63:4669-4674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burns, E. H., S. Lukomski, J. Rurangirwa, A. Podbielski, and J. M. Musser. 1998. Genetic inactivation of the extracellular cysteine protease enhances in vitro internalization of group A streptococci by human epithelial and endothelial cells. Microb. Pathog. 24:333-339. [DOI] [PubMed] [Google Scholar]
- 7.Cockayne, A., P. J. Hill, N. B. Powell, K. Bishop, C. Sims, and P. Williams. 1998. Molecular cloning of a 32-kilodalton lipoprotein component of a novel iron-regulated Staphylococcus epidermidis ABC transporter. Infect. Immun. 66:3767-3774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Correia, F. F., J. M. DiRienzo, T. L. McKay, and B. Rosan. 1996. scbA from Streptococcus crista CC5A: an atypical member of the lraI gene family. Infect. Immun. 64:2114-2121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cue, D., P. E. Dombek, H. Lam, and P. P. Cleary. 1998. Streptococcus pyogenes serotype M1 encodes multiple pathways for entry into human epithelial cells. Infect. Immun. 66:4593-4601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dintilhac, A., G. Alloing, C. Granadel, and J. P. Claverys. 1997. Competence and virulence of Streptococcus pneumoniae: Adc and PsaA mutants exhibit a requirement for Zn and Mn resulting from inactivation of putative ABC metal permeases. Mol. Microbiol. 25:727-739. [DOI] [PubMed] [Google Scholar]
- 11.Dintilhac, A., and J. P. Claverys. 1997. The adc locus, which affects competence for genetic transformation in Streptococcus pneumoniae, encodes an ABC transporter with a putative lipoprotein homologous to a family of streptococcal adhesins. Res. Microbiol. 149:119-131. [DOI] [PubMed] [Google Scholar]
- 12.Fenno, J. C., D. J. LeBlanc, and P. Fives-Taylor. 1989. Nucleotide sequence analysis of a type 1 fimbrial gene of Streptococcus sanguis FW213. Infect. Immun. 57:3527-3533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ferretti, J. J., W. M. McShan, D. Ajdic, D. J. Savic, G. Savic, K. Lyon, C. Primeaux, S. Sezate, A. N. Suvorov, S. Kenton, H. S. Lai, S. P. Lin, Y. Qian, F. Z. Najar, Q. Ren, H. Zhu, L. Song, J. White, X. Yuan, S. W. Clifton, B. A. Roe, and R. McLaughlin. 2001. Complete genome sequence of an M1 strain of Streptococcus pyogenes. Proc. Natl. Acad. Sci. USA 98:4658-4663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Franken, C., G. Haase, C. Brandt, J. Weber-Heynemann, S. Martin, C. Lämmler, A. Podbielski, R. Lütticken, and B. Spellerberg. 2001. Horizontal gene transfer and host specificity of beta-haemolytic streptococci: the role of a putative composite transposon containing scpB and lmb. Mol. Microbiol. 41:925-935. [DOI] [PubMed] [Google Scholar]
- 15.Ganeshkumar, N., N. Arora, and P. E. Kolenbrander. 1993. Saliva-binding protein (SsaB) from Streptococcus sanguis 12 is a lipoprotein. J. Bacteriol. 175:572-574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ganeshkumar, N., P. M. Hannam, P. E. Kolenbrander, and B. C. McBride. 1991. Nucleotide sequence of a gene coding for a saliva-binding protein (SsaB) from Streptococcus sanguis 12 and possible role of the protein in coaggregation with Actinomyces. Infect. Immun. 59:1093-1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hanski, E., and M. Caparon. 1992. Protein F, a fibronectin-binding protein, is an adhesin of the group A streptococcus (Streptococcus pyogenes). Proc. Natl. Acad. Sci. USA 89:6172-6176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harlow, E., and D. Lane. 1999. Using antibodies: a laboratory manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 19.Havarstein, L. S., R. Hakenbeck, and P. Gaustad. 1997. Natural competence in the genus Streptococcus: evidence that streptococci can change pherotype by interspecies recombinational exchanges. J. Bacteriol. 179:6589-6594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hayashi, S., and H. C. Wu. 1990. Lipoproteins in bacteria. J. Bioenerg. Biomembr. 22:451-471. [DOI] [PubMed] [Google Scholar]
- 21.Hill, P. J., A. Cockayne, P. Landers, J. A. Morrissey, C. M. Sims, and P. Williams. 1998. SirR, a novel iron-dependent repressor in Staphylococcus epidermidis. Infect. Immun. 66:4123-4129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Inukai, M., M. Nakajima, M. Osawa, T. Haneishi, and N. M. Arai. 1978. Globomycin, a new peptide antibiotic with spheroplast froming activitiy. II. Isolation, physico-chemical and biological characterization. J. Antibiot. 31:421-425. [DOI] [PubMed] [Google Scholar]
- 23.Jadoun, J., O. Eyal, and S. Sela. 2002. Role of CsrR, hyaluronic acid, and SpeB in the internalization of Streptococcus pyogenes M type 3 strain by epithelial cells. Infect. Immun. 70:462-469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jadoun, J., V. Ozeri, E. Burstein, E. Skutelsky, E. Hanski, and S. Sela. 1998. Protein F1 is required for efficient entry of Streptococcus pyogenes into epithelial cells. J. Infect. Dis. 178:147-158. [DOI] [PubMed] [Google Scholar]
- 25.Jadoun, J., and S. Sela. 2000. Mutation in csrR global regulator reduces Streptococcus pyogenes internalization. Microb. Pathog. 29:311-317. [DOI] [PubMed] [Google Scholar]
- 26.Jakubovics, N. S., and H. F. Jenkinson. 2001. Out of the iron age: new insights into the critical role of manganese homeostasis in bacteria. Microbiology 147:1709-1718. [DOI] [PubMed] [Google Scholar]
- 27.Jakubovics, N. S., A. W. Smith, and H. F. Jenkinson. 2000. Expression of the virulence-related Sca (Mn2+) permease in Streptococcus gordonii is regulated by a diphtheria toxin metallorepressor-like protein ScaR. Mol. Microbiol. 38:140-153. [DOI] [PubMed] [Google Scholar]
- 28.Janulczyk, R., J. Pallon, and L. Björck. 1999. Identification and characterization of a Streptococcus pyogenes ABC transporter with multiple specificities for metal cations. Mol. Microbiol. 34:596-606. [DOI] [PubMed] [Google Scholar]
- 29.Jedrzejas, M. J. 2001. Pneumococcal virulence factors: structure and function. Microb. Mol. Biol. Rev. 65:187-207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jenkinson, H. F. 1994. Cell surface protein receptors in oral streptococci. FEMS Microbiol. Lett. 121:133-140. [DOI] [PubMed] [Google Scholar]
- 31.Jenkinson, H. F., S. D. Terry, R. McNab, and G. W. Tannock. 1993. Inactivation of the gene encoding surface protein SspA in Streptococcus gordonii DL1 affects cell interactions with human salivary agglutinin and oral actinomyces. Infect. Immun. 61:3199-3208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kitten, T., C. L. Munro, S. M. Michalek, and F. L. Macrina. 2000. Genetic characterization of a Streptococcus mutans LraI family operon and role in virulence. Infect. Immun. 68:4441-4451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kolenbrander, P. E., R. N. Andersen, R. A. Baker, and H. F. Jenkinson. 1998. The adhesion-associated sca operon in Streptococcus gordonii encodes an inducible high-affinity ABC transporter for Mn2+ uptake. J. Bacteriol. 180:290-295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kolenbrander, P. E., R. N. Andersen, and N. Ganeshkumar. 1994. Nucleotide sequence of the Streptococcus gordonii PK488 coaggregation adhesin gene, scaA, and ATP-binding cassette. Infect. Immun. 62:4469-4480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kreikemeyer, B., M. D. P. Boyle, B. A. L. Buttaro, M. Heinemann, and A. Podbielski. 2001. Group A streptococcal growth phase-associated virulence factor regulation by a novel operon (Fas) with homologies to two-component-type regulators requires a small RNA molecule. Mol. Microbiol. 39:392-406. [DOI] [PubMed] [Google Scholar]
- 36.Law, J., G. Buist, A. Haandrikman, J. Kok, G. Venema, and K. Leenhouts. 1995. A system to generate chromosomal mutations in Lactococcus lactis which allows fast analysis of targeted genes. J. Bacteriol. 177:7011-7018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lowe, A. M., P. A. Lambert, and A. W. Smith. 1995. Cloning of an Enterococcus faecalis endocarditis antigen: homology with adhesins from some oral streptococci. Infect. Immun. 63:703-706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Michiels, J., C. Xi, J. Verhaert, and J. Vanderleyen. 2002. The function of Ca2+ in bacteria: a role for EF-hand proteins? Trend. Microbiol. 10:87-93. [DOI] [PubMed] [Google Scholar]
- 39.Molinari, G., M. Rohde, S. R. Talay, G. S. Chhatwal, S. Beckert, and A. Podbielski. 2001. Role played by the group A streptococcal negative regulator Nra on bacterial interactions with epithelial cells. Mol. Microbiol. 40:99-114. [DOI] [PubMed] [Google Scholar]
- 40.Molinari, G., S. R. Talay, P. Valentin-Weigand, M. Rohde, and G. S. Chhatwal. 1997. The fibronectin-binding protein of Streptococcus pyogenes, SfbI, is involved in the internalization of group A streptococci by epithelial cells. Infect. Immun. 65:1357-1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Podbielski, A., A. Kaufhold, and P. P. Cleary. 1993. PCR-mediated amplification of group A streptococcal genes encoding immunoglobulin-binding proteins. ImmunoMethods 2:55-64. [Google Scholar]
- 42.Podbielski, A., and B. A. B. Leonard. 1998. The group A streptococcal dipeptide permease (Dpp) is involved in uptake of essential amino acids and affects expression of cysteine protease. Mol. Microbiol. 28:1323-1334. [DOI] [PubMed] [Google Scholar]
- 43.Podbielski, A., B. Pohl, M. Woischnik, C. Körner, K. H. Schmidt, E. Rozdzinski, and B. A. B. Leonard. 1996. Molecular characterization of group A streptococcal (GAS) oligopeptide permease (Opp) and its effect on cysteine protease production. Mol. Microbiol. 21:1087-1099. [DOI] [PubMed] [Google Scholar]
- 44.Podbielski, A., B. Spellerberg, M. Woischnik, B. Pohl, and R. Lütticken. 1996. Novel series of plasmid vectors for gene inactivation and expression analysis in group A streptococci (GAS). Gene 177:137-147. [DOI] [PubMed] [Google Scholar]
- 45.Podbielski, A., M. Woischnik, B. A. B. Leonard, and K. H. Schmidt. 1999. Characterization of nra, a global negative regulator gene in group A streptococci. Mol. Microbiol. 31:1051-1064. [DOI] [PubMed] [Google Scholar]
- 46.Podbielski, A., M. Woischnik, B. Pohl, and K. H. Schmidt. 1996. What is the size of the group A streptococcal vir regulon? The Mga regulator affects expression of secreted and surface virulence factors. Med. Microbiol. Immunol. 185:171-181. [DOI] [PubMed] [Google Scholar]
- 47.Que, Q., and J. D. Helmann. 2000. Manganese homeostasis in Bacillus subtilis is regulated by MntR, a bifunctional regulator related to the diphtheria toxin repressor family of proteins. Mol. Microbiol. 35:1454-1468. [DOI] [PubMed] [Google Scholar]
- 48.Sampson, J. S., S. P. O'Connor, A. R. Stinson, J. E. Tharpe, and H. Russell. 1994. Cloning and nucleotide sequence analysis of psaA, the Streptococcus pneumoniae gene encoding a 37-kilodalton protein homologous to previously reported Streptococcus sp. adhesins. Infect. Immun. 62:319-324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Singh, K. V., T. M. Coque, G. M. Weinstock, and B. E. Murray. 1998. In vivo testing of an Enterococcus faecalis efaA mutant and use of efaA homologs for species identification. FEMS Immunol. Med. Microbiol. 21:323-331. [DOI] [PubMed] [Google Scholar]
- 50.Spatafora, G., M. Moore, S. Landgren, E. Stonehouse, and S. Michalek. 2001. Expression of Streptococcus mutans fimA is iron-responsive and regulated by a DtxR homologue. Microbiology 147:1599-1610. [DOI] [PubMed] [Google Scholar]
- 51.Spellerberg, B., E. Rozdzinski, S. Martin, J. Weber-Heynemann, R. Lütticken, and A. Podbielski. 1999. Lmb, a protein with similarities to the LraI adhesin family, mediates attachment of Streptococcus agalactiae to human laminin. Infect. Immun. 67:871-878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sutcliffe, I. C., and R. R. B. Russell. 1995. Lipoproteins of gram-positive bacteria. J. Bacteriol. 177:1123-1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sutcliffe, I. C., L. Tao, J. J. Ferretti, and R. R. B. Russell. 1993. MsmE, a lipoprotein involved in sugar transport in Streptococcus mutans. J. Bacteriol. 175:1853-1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Switalski, L. M., P. Speziale, M. Höök, T. Wadström, and R. Timpl. 1984. Binding of Streptococcus pyogenes to laminin. J. Biol. Chem. 259:3734-3738. [PubMed] [Google Scholar]
- 55.Talay, S. R., E. Ehrenfeld, G. S. Chhatwal, and K. N. Timmis. 1991. Expression of the fibronectin-binding components of Streptococcus pyogenes in Escherichia coli demonstrates that they are proteins. Mol. Microbiol. 5:1727-1734. [DOI] [PubMed] [Google Scholar]
- 56.Talkington, D. F., B. G. Brown, J. A. Tharpe, A. Koenig, and H. Russell. 1996. Protection of mice against fatal pneumococcal challenge by immunization with pneumococcal surface adhesin A (PsaA). Microb. Pathog. 21:17-22. [DOI] [PubMed] [Google Scholar]
- 57.Terao, Y., S. Kawabata, E. Kunitomo, J. Murakami, I. Nakagawa, and S. Hamada. 2001. Fba, a novel fibronectin-binding protein from Streptococcus pyogenes, promotes bacterial entry into epithelial cells, and the fba gene is positively transcribed under the Mga regulator. Mol. Microbiol. 42:75-86. [DOI] [PubMed] [Google Scholar]
- 58.Terao, Y., S. Kawabata, E. Kunitomo, I. Nakagawa, and S. Hamada. 2002. Novel laminin-binding protein of Streptococcus pyogenes, Lbp, is involved in adhesion to epithelial cells. Infect. Immun. 70:993-997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tettelin, H., K. E. Nelson, I. T. Paulsen, J. A. Eisen, T. D. Read, S. Peterson, J. Heidelberg, R. T. DeBoy, D. H. Haft, R. J. Dodson, A. S. Durkin, M. Gwinn, J. F. Kolonay, W. C. Nelson, J. D. Peterson, L. A. Umayam, O. White, S. L. Salzberg, M. R. Lewis, D. Radune, E. Holtzapple, H. Khouri, A. M. Wolf, T. R. Utterback, C. L. Hansen, L. A. McDonald, T. V. Feldblyum, S. Angiuoli, T. Dickinson, E. K. Hickey, I. E. Holt, B. J. Loftus, F. Yang, H. O. Smith, J. C. Venter, B. A. Dougherty, D. A. Morrison, S. K. Hollingshead, and C. M. Fraser. 2001. Complete genome sequence of a virulent isolate of Streptococcus pneumoniae. Science 293:498-506. [DOI] [PubMed] [Google Scholar]
- 60.Tsai, P. J., K. Y. Kuo, Y. S. Lin, H. Y. Lei, F. F. Chen, J. R. Wang, and J. J. Wu. 1998. Effect of group A streptococcal cysteine protease on invasion of epithelial cells. Infect. Immun. 66:1460-1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.van de Rijn, I., and R. E. Kessler. 1980. Growth characteristics of group A streptococci in a new chemically defined medium. Infect. Immun. 27:444-448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Vercelloti, G. M., J. B. McCarthy, P. Lindholm, P. K. Peterson, H. S. Jacob, and L. T. Furcht. 1985. Extracellular matrix proteins (fibronectin, laminin, and type IV collagen) bind and aggregate bacteria. Am. J. Pathol. 120:13-21. [PMC free article] [PubMed] [Google Scholar]
- 63.Viscount, H. B., C. L. Munro, D. Burnette-Curley, D. L. Peterson, and F. L. Macrina. 1997. Immunization with FimA protects against Streptococcus parasanguis endocarditis in rats. Infect. Immun. 65:994-1002. [DOI] [PMC free article] [PubMed] [Google Scholar]