Abstract
Oligosaccharides (OSs) related to the pneumococcal type 14 capsular polysaccharide (Pn14PS) were studied for their ability to inhibit the binding between anti-PS14 antisera and native PS14. A synthetic tetrasaccharide corresponding to the repeating unit of the Pn14PS, a hexasaccharide mimic, and an octasaccharide fragment obtained by Pn14PS depolymerization were good inhibitors. CRM197 conjugates of the tetrasaccharide and an octasaccharide mimic were prepared by using either adipic acid diester or diethyl squarate linkers. The conjugate with the tetrasaccharide chains induced anti-Pn14PS antibodies when injected subcutaneously into mice, as determined by an enzyme-linked immunosorbent assay, and antibody titers increased with oligosaccharide loading. The adipic acid-linked tetrasaccharide conjugates elicited higher antibody titers than those prepared with a squarate spacer. The lower anti-Pn14PS antibody response of the octasaccharide mimic conjugate indicates the importance of the backbone galactose residue for an appropriate antibody response. The OS-CRM197 conjugate prepared from a single repeat unit of the Pn14PS is a potential vaccine candidate.
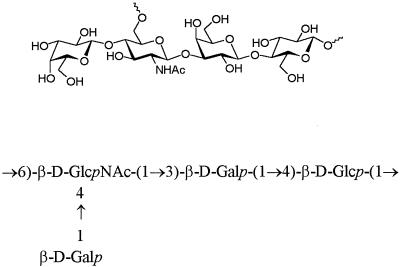
Streptococcus pneumoniae is a major cause of morbidity and mortality in children and adults in both industrialized and developing countries (10). Although the licensed 14- and 23-valent pneumococcal capsular polysaccharide (PnPS) vaccines are safe and effective in reducing the incidence of invasive disease in healthy adults (5, 24), they are weakly immunogenic in children less than 2 years old and in the elderly, the two groups at highest risk (8, 22, 25, 31). Whereas PSs are T-cell-independent immunogens, conjugates in which the PS is covalently attached to a protein carrier elicit a T-cell-dependent antisaccharide response even in infants, as evidenced by a booster effect upon subsequent immunizations. A number of PnPS serotypes have been conjugated to various carrier proteins and have been shown to be immunogenic and protective in various animal models (13, 20, 30) and in humans (1, 32). Intact PS, small oligosaccharides (OSs), or OSs of undefined length have been used to prepare those conjugates (3, 13, 36). The capsular polysaccharide of S. pneumoniae type 14 (Fig. 1) consists of a branched tetrasaccharide repeating unit (21) which is identical to the asialo core antigen of the type III group B Streptococcus PS (38). The poor immunogenicity of the Pn14PS compared to other PnPSs (18) may be due to structural similarities between antigenic determinants of the Pn14PS and human OS structures (e.g., human milk OSs and blood group carbohydrate structures). To avoid cross-reactivity with human tissue and the induction of autoreactive antibodies (6), we have been investigating the synthesis of well-defined OS fragments (26, 27) corresponding to the Pn14PS to enable specific protective epitopes to be defined. Inhibition studies were performed both with synthetic OS structures and fragments from PS degradation to determine optimal OSs for conjugation. The protein carrier used in the OS conjugates was CRM197, a nontoxic, immunologically cross-reacting mutant protein of diphtheria toxin (9, 23) containing 39 lysine residues with a free α amino terminus for attachment of multiple OS chains. For the development of effective synthetic glycoconjugate vaccines, the effects of different OSs and different conjugation chemistries, as well as the effect of the ratio of protein to carbohydrate (OS loading), on the immunogenicity of the glycoconjugates were studied.
FIG. 1.
Tetrasaccharide repeating unit of the Pn14PS.
MATERIALS AND METHODS
Materials.
Pn14PS, N-hydroxysuccinimide-activated adipic acid diester, and CRM197 were provided by N. Ravenscroft, Chiron Vaccines SpA, Siena, Italy.
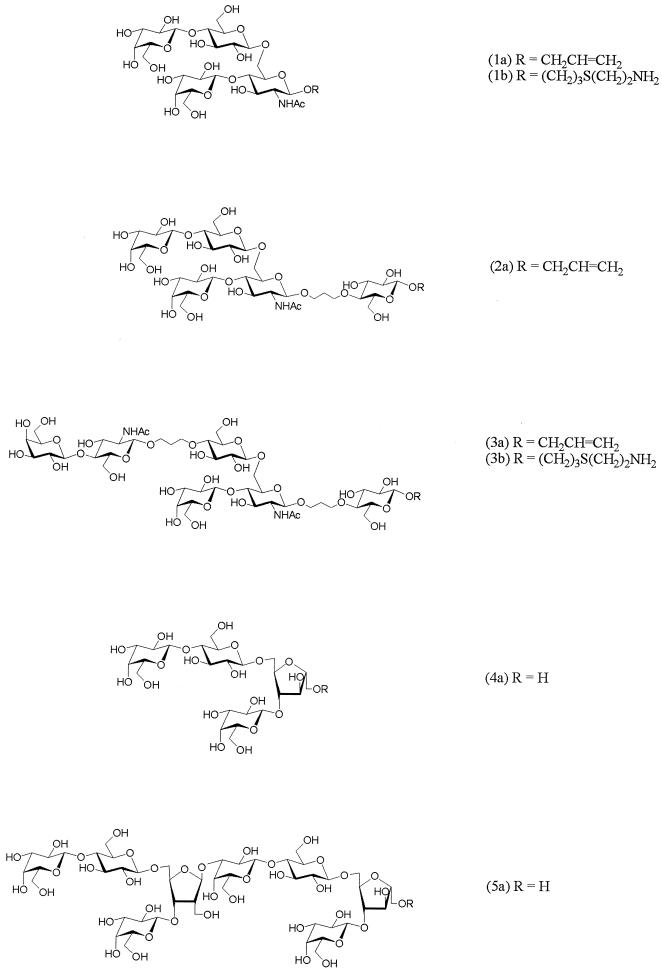
Preparation of OSs (Fig. 2).
FIG. 2.
OSs for inhibition studies and for coupling to CRM197. OS compound designations are given in the parentheses (designations with the letter a were used for inhibition, and designations with the letter b were used for coupling to CRM197).
Synthetic OSs 1a to 3a were prepared by a chemoenzymatic approach, as previously described (26, 27). Linear OS acceptors and alkyl-bridged OS mimics of fragments of the Pn14PS were synthesized chemically and subsequently galactosylated to the branched OS structures using β-1,4-galactosyltransferase. Subsequent conversion of the anomeric allyl groups of OSs 1a and 3a into amino-derivatized spacers by the light-induced free-radical addition of cysteamine (19) gave the β-aminoethylthio-extended glycosides 1b and 3b suitable for coupling to the protein carrier.
The 2,5-anhydro-d-mannose-containing tetrasaccharide 4a and octasaccharide 5a were obtained by depolymerization of the completely de-N-acetylated Pn14PS by deaminative cleavage (12, 21) and subsequent borohydride reduction. PS degradation was monitored by thin-layer chromatography on Silica Gel 60 F254 (Merck) with detection by charring with 0.2% orcinol in 20% methanolic sulfuric acid using 2:1:1 n-butanol-acetic acid-water as the solvent.
(i) De-N-acetylation.
A solution of Pn14PS (32 mg) in anhydrous hydrazine (3 ml) containing hydrazine sulfate (150 mg) was stirred under argon for 20 h at 100°C. The solution was concentrated in vacuo and coconcentrated repeatedly with toluene. The residue was dissolved in water and desalted by gel filtration on HiTrap (Sephadex G-25 Superfine; Pharmacia Biotech), eluted with 5 mM ammonium bicarbonate.
(ii) Deamination and in situ reduction.
The de-N-acetylated PS (16 mg) was dissolved in 33% acetic acid (1 ml). At 0°C, a 5% solution of sodium nitrite in water (1 ml) was added, and the solution was stirred for 2 h at room temperature. The solution was adjusted to pH 8 with 4 M ammonia, and a solution of sodium borohydride (30 mg) in water (1 ml) was added. After the solution was stirred for 2 h at room temperature, the mixture was neutralized with 4 M acetic acid and coevaporated repeatedly with 10% acetic acid in methanol and then with methanol. The residue was desalted on an AG 50WX12 (H+) column and eluted with water, and the eluate was concentrated. The residue was fractionated on a Toyopearl HW-40S column and eluted with 5 mM ammonium bicarbonate. Product-containing fractions were lyophilized to give OSs 4a (11.6 mg, 88%) and 5a (1.4 mg, 12%). Rf values were 0.17 (OS 4a) and 0.05 (OS 5a). Mass spectrometry values were as follows: m/z 649.2 [M − H]− (OS 4a); m/z 1282.3 [M − H]− (OS 5a).
Partial acid hydrolysis of octasaccharide 5a and monosaccharide analysis.
Octasaccharide 5a was hydrolyzed with 0.3 M trifluoroacetic acid (2 ml) for 15 min at 100°C. Trifluoroacetic acid was then removed by lyophilization, and the residue was fractionated on a Toyopearl HW-40S column and eluted with 5 mM ammonium bicarbonate.
OSs 4a and 5a were subjected to methanolysis (methanolic 1 M hydrogen chloride, 24 h, 85°C), and the resulting mixtures of methyl glycosides were trimethylsilylated with 1:1:5 (vol/vol/vol) hexamethyldisilazane-trimethylchlorosilane-pyridine and quantitatively analyzed by gas-liquid chromatography.
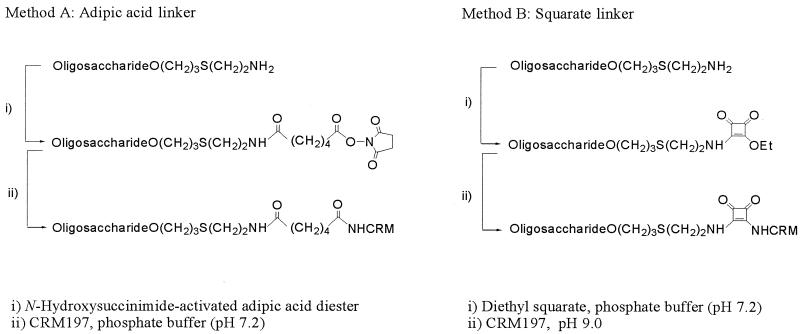
Activation of OSs and coupling to CRM197 (Fig. 3). (i) Method A (activation with N-hydroxysuccinimide-activated adipic acid diester).
FIG. 3.
Methods for coupling to lysine residues of the carrier protein.
OS 1b (4.0 μmol) was dissolved in water (100 μl) and then in triethylamine (20 μmol), a solution of the N-hydroxysuccinimide diester of adipic acid (48 μmol, 16 mg) in dimethyl sulfoxide (900 μl) was added, and the mixture was stirred for 2 h at room temperature. The activated OS was precipitated by the addition of acetone (10 ml) and recovered by centrifugation at 4°C. The precipitate was washed with acetone (5 times) to give the activated OS in 50% yield, which was reacted with CRM197 (10 mg) in sodium phosphate buffer (10 mM, pH 7.2, 200 μl) containing 10% sucrose for 1 day at room temperature (conjugate 1).
OS 3b (3.7 μmol) was conjugated by the same procedure to 5 mg of CRM197 (conjugate 6).
(ii) Method B (activation with diethyl squarate).
OS 1b (3.7 μmol) was dissolved in sodium phosphate buffer (0.1 M, pH 7.2, 150 μl), then a solution of diethyl squarate (3.5 μmol) in ethanol (150 μl) was added, and the solution was stirred for 2 h at room temperature. The mixture was evaporated to dryness, and the residue was dissolved in water (5 ml) and applied to a C18 Sep-Pak cartridge. The activated OS was eluted after the column was washed first with water and then with heptane and methanol. The eluate was concentrated and dissolved in water (200 μl), and CRM197 (10 mg) in sodium phosphate buffer (10 mM, pH 7.2, 200 μl) containing 10% sucrose was added; the solution was adjusted to pH 9 by the addition of 0.1 M potassium carbonate and left for 3 days at room temperature (conjugate 3).
OS 3b (1.85 μmol) was conjugated by the same procedure to 5 mg of CRM197 (conjugate 5).
Different OS incorporations for OS 1b were obtained by dividing the activated OS of a second reaction mixture into portions of 40 μl (conjugate 2) and 160 μl (conjugate 4), which were each reacted with 5 mg of CRM197 in sodium phosphate buffer (10 mM, pH 7.2, 100 μl) at pH 9.
Isolation of OS-CRM197 conjugates.
The OS-CRM197 conjugates were purified either by HiTrap gel filtration with 10 mM sodium phosphate buffer (pH 7.2) and subsequent concentration with Centriprep-30 or by HiTrap gel filtration with water and subsequent reconstitution with 10 mM sodium phosphate buffer (pH 7.2), using Centriprep-30.
Characterization of neoglycoproteins.
The level of incorporation of OS (average loading) onto CRM197 was determined by matrix-assisted laser desorption ionization-time of flight (MALDI-TOF) mass spectrometry. MALDI-TOF spectra were recorded on a Voyager-DE (PerSeptive Biosystems) instrument operated at an accelerating voltage of 24 kV. Samples were desalted and dissolved in 50% acetonitrile containing 0.1% trifluoroacetic acid and applied in a sinapinic acid matrix. Protein concentration was determined using the Sigma Diagnostics micro protein determination kit.
Animals and immunization.
Inbred 8- to 10-week-old female BALB/c mice were obtained from Charles Rivers Ltd., Mairgate, Kent, United Kingdom. The mice (five mice per group) were immunized (day 0) subcutaneously with 0.2 ml of OS-CRM197 conjugate in phosphate-buffered saline (PBS) without adjuvant (2.5 μg of OS/mouse). On day 28, each mouse was given a booster dose of the same preparation as that used for primary immunization. Mice were bled on day 28 for primary response and on day 42 for secondary response.
Determination of the antigenicity of OS by inhibition ELISA.
To investigate the antigenicity of the synthetic OSs, a competition enzyme-linked immunosorbent assay (ELISA) was performed, using polyclonal mouse antisera raised with a commercial Pn14PS-CRM197 conjugate vaccine (Wyeth/Lederle), as follows: ELISA plates were coated with native Pn14PS as for the conventional ELISA. Subsaturating dilutions of the mouse immune anti-PS14 sera were incubated overnight at 4°C with different concentrations of the different OSs. The next day, the sera were applied to the plates in duplicate, and ELISA was performed to measure anti-PS14 antibody levels as described below. The results were expressed as the changes in optical density (OD) using immune sera preincubated with the OS and compared to immune sera alone.
ELISA for measuring anti-Pn14PS and anti-CRM197 IgG.
Pn14PS-specific and CRM197-specific immunoglobulin G (IgG) antibodies were measured by indirect ELISA as follows. Maxisorp microtiter plates (Nunc) were coated with Pn14PS in PBS or with CRM197 in 0.1 M carbonate buffer (pH 9.6) by overnight incubation. The next day, plates were blocked with PBS containing 1% bovine serum albumin. Samples of diluted (11 serial twofold dilutions) immune mouse sera, preadsorbed with the cell wall C polysaccharide, were distributed into the wells (100 μl/well). The plates were incubated at room temperature for 2 h; biotin-conjugated goat anti-mouse IgG (Serotec, Kidlington, Oxford, United Kingdom), followed by streptavidin-horseradish peroxidase, was added; and the plates were incubated for 1 h. The enzyme substrate o-phenylenediamine was added, and the reaction was terminated after 30 min by the addition of 50 μl of 3 M hydrogen chloride per well. Between each incubation stage, plates were washed with PBS containing 0.05% Tween 20. The OD at 492 nm was measured using an automated reader (Labsystems Multiscan mass spectrometer) and Genesis software. Results are presented as titers (reciprocal of the serum dilution giving OD of 0.5). A titer of <25 was considered negative. The specificity of the antibodies against the PS was demonstrated by an inhibition ELISA with PS14.
RESULTS
Preparation of OSs for inhibition studies.
OSs used for inhibition studies were obtained by either chemoenzymic synthesis as allyl glycosides (OSs 1a to 3a) as previously described (26, 27) or by chemical Pn14PS degradation (OSs 4a and 5a) (Fig. 2). In the latter case, fragmentation of the Pn14PS was achieved by hydrazinolysis and subsequent nitrous acid deamination of the completely de-N-acetylated PS. Borohydride reduction then gave, in addition to the 2,5-anhydro-d-mannitol-containing tetrasaccharide 4a, the octasaccharide fragment 5a, which contains a 2-deoxy-2-C-hydroxymethyl-pentofuranose unit. In a competing reaction to the deaminative cleavage reaction of the glycosidic bond of the β-d-glucosamine residues, the 2-deoxy-2-C-formyl-pentofuranoside is formed by deaminative ring contraction with simultaneous elimination of the substituent at O-3 without glycosidic bond cleavage (33, 35). OS 5a was analyzed by partial acid hydrolysis and monosaccharide analysis.
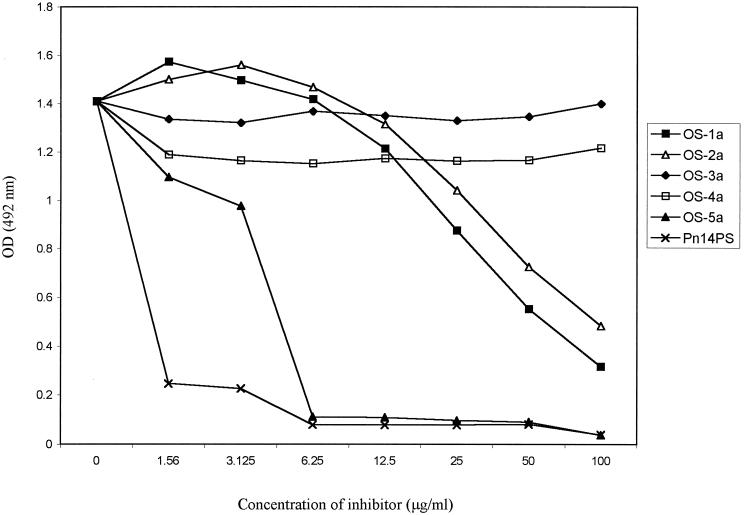
Inhibition of binding of anti-Pn14PS polyclonal antisera to Pn14PS with Pn14 synthetic OS.
To investigate the ability of the various type 14 OS to bind to anti-Pn14PS antibodies, a competition ELISA was performed using polyclonal mouse antisera raised with a commercial Pn14PS-CRM197 conjugate (Wyeth/Lederle). Native Pn14PS was used as the coating antigen, and synthetic OSs were used as the inhibitors. Results presented in Fig. 4 indicate that synthetic OSs 1a (tetrasaccharide) and 2a (hexasaccharide mimic), but not OSs 3a (octasaccharide mimic) and 4a (modified tetrasaccharide), were able to inhibit the binding of anti-Pn14PS antibodies to PS in the ELISA in a dose-dependent fashion, with the OSs 1a and 2a being able to cause 82 and 69% inhibition, respectively, at 100 μg/ml. A much greater level (∼100%) of inhibition was seen with only 6 μg of OS 5a per ml and native Pn14PS. Subsequently, OSs 1a and 3a were conjugated, after activation, to CRM197 for evaluation in immunogenicity studies.
FIG. 4.
Specificity of the anti-PS IgG response. Sera from mice immunized with PS14-CRM197 conjugate vaccine were absorbed overnight at 4°C with increasing concentrations of different synthetic OSs or native PS prior to measurement of anti-PS14 level by a conventional ELISA. Results are expressed as OD and compared to that of unadsorbed immune serum.
Coupling of activated OSs to CRM197.
Synthetic OSs 1b and 3b (15, 35) equipped with an amino spacer were attached to CRM197 in order to evaluate the structural requirements for a glycoconjugate vaccine. The saccharides used here represent the branched tetrasaccharide repeating unit and an octasaccharide mimic fragment of Pn14PS. The saccharides were linked to the ɛ amino groups of lysine residues of the carrier protein by activation with N-hydroxysuccinimide-activated adipic acid diester (method A) or with diethyl squarate (method B) (15, 35) (Fig. 3). The average degree of incorporation of OS chains onto CRM197 (Table 1) was calculated from molecular mass differences between OS-CRM197 conjugates and CRM197 (measured as 58,560 Da) as determined by MALDI-TOF mass spectrometry. The influence of different saccharide/protein molar ratios on the immunogenicity of the glycoconjugates was investigated with the tetrasaccharide-squarate adducts (conjugates 2 to 4).
TABLE 1.
OS incorporation and coupling method used for CRM197 conjugates
| Conjugate | OS | Coupling method | OS incorporation |
|---|---|---|---|
| 1 | 1b | Adipic acid | 4.0 |
| 2 | 1b | Squarate | 4.8 |
| 3 | 1b | Squarate | 11.5 |
| 4 | 1b | Squarate | 23.8 |
| 5 | 3b | Squarate | 11.5 |
| 6 | 3b | Adipic acid | 8.5 |
Immunogenicity of OS 14-CRM197 conjugates in mice and effect of different spacers.
The immunogenicity of CRM197 conjugates prepared from two synthetic OSs (1b and 3b) attached with two different spacers (adipic acid or squarate) in a mouse model was evaluated. In the present study, conjugates made of the synthetic tetrasaccharide (conjugates 1 and 2), but not the octasaccharide mimic (conjugates 5 and 6) using adipic acid or squarate as spacers, elicited a small Pn14PS-specific IgG response after a single immunization (Table 2). Booster injections of OS 1b-CRM197 conjugates 1 and 2 induced massive increases in anti-Pn14PS IgG levels (378- and 225-fold for adipic diester- and squarate-linked conjugates, respectively), with the adipic acid-containing conjugates being significantly more immunogenic than the squarate-containing conjugates. Surprisingly, a small but significant (P < 0.05) response was detected in mice given booster doses of OS 3b conjugate 6 containing adipic acid, while the squarate-containing OS 3b conjugate 5 remained nonimmunogenic after the booster dose.
TABLE 2.
IgG antibody response to Pn14PS and to CRM197 in mice immunized with OS-CRM197 conjugates prepared with different linkersa
| Immunogen | Linker | Anti-Pn14PS IgG (geometric mean titer) after the following no. of vaccinations:
|
|
|---|---|---|---|
| One | Two | ||
| Conjugate 1 | Adipic acid | 1,176 | 442,218 |
| Conjugate 2 | Squarate | 498 | 111,813 |
| Conjugate 5 | Squarate | 429 | 787 |
| Conjugate 6 | Adipic acid | 366 | 9,323 |
| Saline | NDb | 347 | |
Mice (five mice per group) were immunized subcutaneously with 2.5 μg of OS conjugated to CRM197.
ND, not determined.
Effect of OS loading on the immunogenicity of tetrasaccharide conjugates.
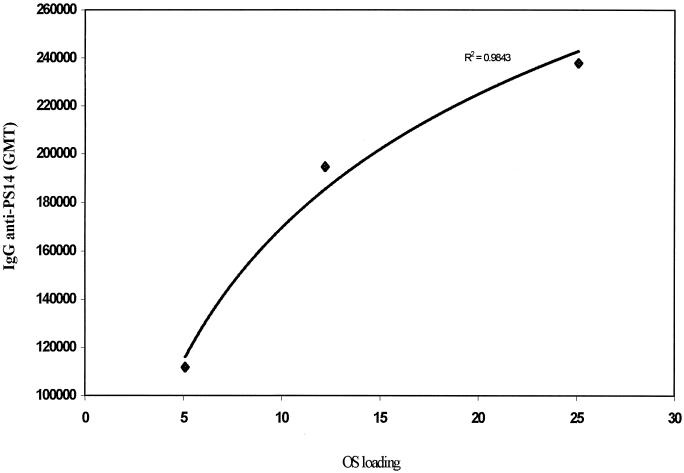
To investigate the relationship between OS loading and immunogenicity, OS 1b-CRM197 conjugates 2 to 4 (containing a squarate spacer) with different OS loading values (Table 1) were used to immunize mice on days 0 and 28, and sera were tested for anti-Pn14PS IgG 2 weeks after the booster dose. Figure 5 showed, within the range of OS loading values tested, a significant positive correlation (r2 = 0.98) between OS loading and the level of anti-Pn14PS IgG levels, with the highest OS loading inducing the most immunogenic conjugate.
FIG. 5.
Relationship between anti-PS 14 antibody level and OS loading. Mice were immunized with conjugates 2 to 4 containing different OS loading values. Immune sera were tested for anti-PS14 antibody levels using ELISA, and results are expressed as geometric mean titer (GMT) plotted against OS loading.
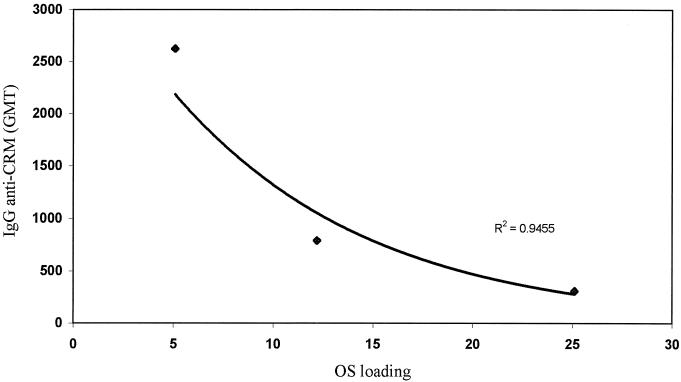
In the same experiments, the antibody response to the carrier protein was somewhat different. Within the squarate-containing conjugates, the level of anti-CRM197 IgG was, within the OS loading range tested, inversely proportional to the level of OS loading (Fig. 6).
FIG. 6.
Relationship between anti-CRM197 antibody level and OS loading. Mice were immunized with conjugates 2 to 4 containing different OS loading values. Immune sera were tested for anti-CRM197 antibody levels using ELISA, and results are expressed as geometric mean titer (GMT) plotted against OS loading.
DISCUSSION
In an attempt to determine the OS size and structural requirements for the synthesis of an effective type 14 pneumococcal conjugate vaccine, different type 14 OSs were synthesized and assessed for their abilities to bind to anti-Pn14PS antibodies and to induce a specific anti-Pn14PS immune response when conjugated to CRM197. In addition, the influence of coupling chemistry and OS loading on the immunogenicity of OS-protein conjugates was evaluated.
Binding studies of anti-Pn14PS polyclonal antisera to Pn14PS using synthetic pneumococcal OSs and OSs from PS depolymerization as inhibitors showed that the synthetic tetrasaccharide 1a, the hexasaccharide mimic 2a, and the octasaccharide fragment 5a, but not the synthetic octasaccharide mimic 3a or the modified tetrasaccharide 4a from Pn14PS degradation, were able to inhibit the binding of PS14 to anti-PS14 antisera in a dose-dependent fashion. Inhibition of the synthetically accessible tetrasaccharide 1a turned out to be slightly better than by the hexasaccharide mimic 2a, suggesting that an OS as small as a single repeating unit of the PS retains a key epitope. In contrast to the tetrasaccharide O-glycoside 1a, which represents a tetrasaccharide repeating unit of the Pn14PS, the C-glycoside 4a obtained by PS depolymerization was not able to inhibit the binding of anti-Pn14PS antibodies. This is probably because PS degradation generates a tetrasaccharide containing a 2,5-anhydro-d-mannitol residue instead of N-acetyl-β-d-glucosamine in the natural repeating tetrasaccharide.
The octasaccharide 5a containing a 2-deoxy-2-C-hydroxymethyl-pentofuranose residue was, in contrast to the C-glycoside 4a, also able to inhibit the binding.
Although the octasaccharide 5a gave the greatest level of inhibition (similar to the native PS), it was not used in coupling experiments due to low quantities of OS material and to the need to introduce an amino group for activation with a spacer before coupling to the protein carrier. The octasaccharide mimic 3a, which represents two repeating units of the Pn14 PS in which the galactose moieties of the repeating trisaccharide backbone were substituted by a flexible spacer, showed no inhibition of antibody-PS binding, indicating that the backbone galactose moiety is essential for the antigenicity of the OS. However, the octasaccharide mimic 3a was, in addition to the tetrasaccharide 1a, chosen as a second OS structure for coupling to the carrier protein in order to investigate the correlation between inhibition of anti-Pn14PS polyclonal antisera binding and conjugate immunogenicity. Overall, the results of the inhibition studies suggest that as structural elements both the backbone galactose residue and an O-glycosidic branching point seem to be essential for the antigenicity of the OS.
Tetrasaccharide 1a and the octasaccharide mimic 3a were used in diverse coupling reactions and subsequent attachment to the carrier protein for immunogenicity evaluation.
In accordance with the inhibition studies, the tetrasaccharide-CRM197 conjugates 1 to 4, with either adipic acid or squarate as a linker, elicited a PS-specific IgG response after a booster injection, whereas the octasaccharide mimic conjugates 5 and 6 gave only small responses or even remained nonimmunogenic. Conjugate 1 with the adipic acid linker was more immunogenic than the squarate-coupled conjugate 2 with a comparable amount of average OS loading. This may be an effect of differences in the distribution of glycan chains on CRM197 due to the different coupling conditions with conjugation reactions at pH 7.2 for adipic acid and pH 9 for squarate and/or differences in the distance or linkage between the OS and protein carrier.
The correlation between OS loading and immunogenicity of the squarate-linked conjugates 2 to 4 is probably a direct result of better cross-linking and activation of the PS-specific B cells with increased OS loading. The inverse correlation between the anticarrier antibody level and OS loading suggests that as OS loading is increased, more lysine residues are occupied and hence less B-cell epitopes are exposed for cross-linking and activation of carrier-specific B cells.
In contrast to previously published results with Pn14PS conjugates obtained by depolymerization of partial de-N-acetylated PS conjugated to tetanus toxoid (17), where it was shown that a minimum of two repeating units of the PS is required to generate an antigenic epitope, a synthetic single repeating unit of the natural PS which is different from the modified structure of the single repeat obtained by PS degradation was sufficient for eliciting a massive anti-PS14 IgG response.
Present glycoconjugate vaccines against bacterial infections use either high-molecular-mass PSs or mixtures of large OSs obtained by structure-specific chemical degradations (2, 7, 16). The PSs have, to date, been randomly activated, and the final products are often difficult to characterize and control. Conjugates prepared from OSs obtained by size fractionation of the bacterial PS depend on suitable specific degradation methods.
The results reported here show that synthetically accessible OSs can be used to prepare immunogenic conjugates, in accordance with the findings with synthetic S. pneumoniae type 6B di-, tri-, and tetrasaccharide-protein conjugates (14), synthetic S. pneumoniae type 3 di-, tri-, and tetrasaccharide-CRM197 conjugates (4), or antitumor therapeutics (11, 28, 29, 34, 37). This approach will eventually allow the preparation of well-defined conjugates using OSs related to bacterial PSs for which no simple degradative scheme can be devised and the stimulation of immune responses against the most important protective epitopes while avoiding potentially damaging autoimmune responses. Moreover, conjugates prepared from synthetic OSs are likely to become vital research tools for the investigation of structural features which are important in stimulating immune responses to carbohydrates.
Acknowledgments
This work was supported in part by the European Union program VACNET (grant ERB BIO 4CT960158).
We thank N. Ravenscroft (Chiron Vaccines SpA) for providing the activated adipic acid linker and information on the coupling method and R. Gutiérrez Gallego (Utrecht University) for recording the MALDI-TOF spectra.
Editor: E. I. Tuomanen
REFERENCES
- 1.Ahman, H., H. Kayhty, A. Vuorela, O. Leroy, and J. Eskola. 1999. Dose dependency of antibody response in infants and children to pneumococcal polysaccharides conjugated to tetanus toxoid. Vaccine 17:2726-2732. [DOI] [PubMed] [Google Scholar]
- 2.Anderson, P., and R. Betts. 1989. Human adult immunogenicity of protein coupled pneumococcal capsular antigens of serotypes prevalent in otitis media. Pediatr. Infect. Dis. J. 8:S50-S53. [PubMed] [Google Scholar]
- 3.Anderson, P. W., M. E. Pichichero, R. A. Insel, R. Betts, R. Eby, and D. H. Smith. 1986. Vaccines consisting of periodate-cleaved oligosaccharides from the capsule of Haemophilus influenzae type b coupled to a protein carrier: structural and temporal requirements for priming in the human infant. J. Immunol. 137:1181-1186. [PubMed] [Google Scholar]
- 4.Benaissa-Trouw, B., D. J. Lefeber, J. P. Kamerling, J. F. G. Vliegenthart, H. Snippe, and K. Kraaijeveld.2001. Synthetic di-, tri-, and tetrasaccharide-CRM197 conjugates induce protection against Streptococcus pneumoniae type 3 in mice. Infect. Immun. 69:4698-4701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bolan, G., C. V. Broome, R. R. Facklam, B. D. Plikaytis, D. W. Fraser, and W. F. Schlech. 1986. Pneumococcal vaccine efficacy in selected populations in the United States. Ann. Intern. Med. 104:1-6. [DOI] [PubMed] [Google Scholar]
- 6.Colling, R. G., T. C. Pearson, and J. C. Brown. 1983. Association of carbohydrate-specific cold agglutinin antibody production with immunization by group C, group B type III, and Streptococcus type XIV streptococcal vaccines. Infect. Immun. 41:205-213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Costantino, P., F. Norelli, A. Giannozzi, S. D'Ascenzi, A. Bartoloni, S. Kaur, D. Tang, R. Seid, S. Viti, R. Paffetti, M. Bigio, C. Pennatini, G. Averani, V. Guarnieri, E. Gallo, N. Ravenscroft, C. Lazzeroni, R. Rappuoli, and C. Ceccarini. 1999. Size fractionation of bacterial capsular polysaccharides for their use in conjugate vaccines. Vaccine 17:1251-1263. [DOI] [PubMed] [Google Scholar]
- 8.Cowan, M. J., A. J. Ammann, D. W. Wara, V. M. Howie, L. Schultz, N. Doyle, and M. Kaplan. 1978. Pneumococcal polysaccharide immunization in infants and children. Pediatrics 62:721-727. [PubMed] [Google Scholar]
- 9.Crane, D. T., B. Bolgiano, and C. Jones. 1997. Comparison of the diphtheria mutant toxin, CRM197, with a Haemophilus influenzae type-b polysaccharide-CRM197 conjugate by optical spectroscopy. Eur. J. Biochem. 246:320-327. [DOI] [PubMed] [Google Scholar]
- 10.De Velasco, E. A., A. F. M. Verheul, J. Verhoef, and H. Snippe. 1995. Streptococcus pneumoniae: virulence factors, pathogenesis and vaccines. Microbiol. Rev. 59:591-603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dickler, M. N., G. Ragupathi, N. X. Liu, C. Musselli, D. J. Martino, V. A. Miller, M. G. Kris, F. T. Brezicka, P. O. Livingston, and S. C. Grant. 1999. Immunogenicity of a fucosyl-GM1-keyhole limpet hemocyanin conjugate vaccine in patients with small cell lung cancer. Clin. Cancer Res. 5:2773-2779. [PubMed] [Google Scholar]
- 12.Erbing, C., B. Lindberg, and S. Svensson. 1973. Deamination of methyl 2-amino-2-deoxy-α- and β-d-glucopyranosides. Acta Chem. Scand. 27:3699-3704. [Google Scholar]
- 13.Giebink, G. S., M. Koskela, P. P. Vella, M. Harris, and C. T. Le. 1993. Pneumococcal capsular polysaccharide-meningococcal outer membrane protein complex conjugate vaccines: immunogenicity and efficacy in experimental pneumococcal otitis media. J. Infect. Dis. 167:347-355. [DOI] [PubMed] [Google Scholar]
- 14.Jansen, W. T. M., S. Hogenboom, M. J. L. Thijssen, J. P. Kamerling, J. F. G. Vliegenthart, J. Verhoef, H. Snippe, and A. F. M. Verheul. 2001. Synthetic 6B di-, tri-, and tetrasaccharide-protein conjugates contain pneumococcal type 6A and 6B common and 6B-specific epitopes that elicit protective antibodies in mice. Infect. Immun. 69:787-793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kamath, V. P., P. Diedrich, and O. Hindsgaul. 1996. Use of diethyl squarate for the coupling of oligosaccharide amines to carrier proteins and characterization of the resulting neoglycoproteins by MALDI-TOF mass spectrometry. Glycoconjugate J. 13:315-319. [DOI] [PubMed] [Google Scholar]
- 16.Klein, D. L., and R. W. Ellis. 1997. Conjugate vaccines against Streptococcus pneumoniae, p. 503-525. In M. M. Levine, G. C. Woodrow, J. B. Kaper, and G. S. Cobon (ed.), New generation vaccines. Marcel Dekker, New York, N.Y.
- 17.Laferriere, C. A., R. K. Sood, J. M. de Muys, F. Michon, and H. J. Jennings. 1998. Streptococcus pneumoniae type 14 polysaccharide-conjugate vaccines: length stabilization of opsonophagocytic conformational polysaccharide epitopes. Infect. Immun. 66:2441-2446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Landesman, S. H., and G. Schiffman. 1981. Assessment of the antibody response to pneumococcal vaccine in high-risk populations. Rev. Infect. Dis. 3:S184-S196. [DOI] [PubMed] [Google Scholar]
- 19.Lee, C. J., R. A. Lock, P. W. Andrew, T. J. Mitchell, D. Hansman, and J. C. Paton. 1994. Protection of infant mice from challenge with Streptococcus pneumoniae type 19F by immunization with a type 19F polysaccharide-pneumolysoid conjugate. Vaccine 12:875-878. [DOI] [PubMed] [Google Scholar]
- 20.Lee, R. T., and Y. C. Lee. 1974. Synthesis of 3-(2-aminoethylthio)propyl glycosides. Carbohydr. Res. 37:193-201. [DOI] [PubMed] [Google Scholar]
- 21.Lindberg, B., J. Lönngren, and D. A. Powell. 1977. Structural studies on the specific type-14 pneumococcal polysaccharide. Carbohydr. Res. 58:177-186. [DOI] [PubMed] [Google Scholar]
- 22.Mäkela, P. H., M. Sibakov, E. Herva, J. Henrichsen, J. Luotonen, M. Timonen, M. Leinonen, M. Koskela, J. Pukander, S. Pontynen, P. Gronroos, and P. Karma. 1980. Pneumococcal vaccine and otitis media. Lancet 2:547-551. [DOI] [PubMed] [Google Scholar]
- 23.Mekada, E., and T. Uchida. 1985. Binding properties of diphtheria toxin to cells are altered by mutation in the fragment A domain. J. Biol. Chem. 260:12148-12153. [PubMed] [Google Scholar]
- 24.Mufson, M. A., H. E. Krause, and G. Schiffman. 1983. Long-term persistence of antibody following immunization with pneumococcal polysaccharide vaccine. Proc. Soc. Exp. Biol. Med. 173:270-275. [DOI] [PubMed] [Google Scholar]
- 25.Musher, D. M., A. J. Chapman, A. Goree, S. Jonsson, D. Briles, and R. E. Baughn. 1986. Natural and vaccine-related immunity to Streptococcus pneumoniae. J. Infect. Dis. 154:245-256. [DOI] [PubMed] [Google Scholar]
- 26.Niggemann, J., J. P. Kamerling, and J. F. G. Vliegenthart. 1998. Application of β-1,4-galactosyltransferase in the synthesis of complex branched-chain oligosaccharide mimics of fragments of the capsular polysaccharide of Streptococcus pneumoniae type 14. J. Chem. Soc. Perkin Trans. 1:3011-3020. [Google Scholar]
- 27.Niggemann, J., J. P. Kamerling, and J. F. G. Vliegenthart. 1998. β-1,4-Galactosyltransferase-catalyzed synthesis of the branched tetrasaccharide repeating unit of Streptococcus pneumoniae type 14. Bioorg. Med. Chem. 6:1605-1612. [DOI] [PubMed] [Google Scholar]
- 28.Ragupathi, G., L. Howard, S. Capello, et al. 1999. Vaccines prepared with sialyl-Tn and sialyl-Tn trimers using the 4-(4-maleimidomethyl)cyclohexane-1-carboxyl hydrazide linker group result in optimal antibody titers against ovine submaxillary mucin and sialyl-Tn-positive tumor cells. Cancer Immunol. Immun. 48:1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ragupathi, G., S. F. Slovin, S. Adluri, et al. 1999. A fully synthetic globo H carbohydrate vaccine induces a focused humoral response in prostate cancer patients: a proof of principle. Angew. Chem. Int. 38:563-566. [DOI] [PubMed] [Google Scholar]
- 30.Rodriguez, M. E., G. P. van den Dobbelsteen, L. A. Oomen, O. de Weers, L. van Buren, M. Beurret, J. T. Poolman, and P. Hoogerhout. 1998. Immunogenicity of Streptococcus pneumoniae type 6B and 14 polysaccharide-tetanus toxoid conjugates and the effect of uncoupled polysaccharide on the antigen-specific immune response. Vaccine 16:1941-1949. [DOI] [PubMed] [Google Scholar]
- 31.Sell, S. H., P. F. Wright, W. K. Vaughn, J. Thompson, and G. Schiffman. 1981. Clinical studies of pneumococcal vaccines in infants. I. Reactogenicity and immunogenicity of two polyvalent polysaccharide vaccines. Rev. Infect. Dis. 3(Suppl.):S97-S107. [DOI] [PubMed] [Google Scholar]
- 32.Shinefield, H. R., S. Black, P. Ray, I. Chang, N. Lewis, B. Fireman, J. Hackell, P. R. Paradiso, G. Siber, R. Kohberger, D. V. Madore, F. J. Malinowski, A. Kimura, C. Le, I. Landaw, J. Aguilar, and J. Hansen. 1999. Safety and immunogenicity of heptavalent pneumococcal CRM197 conjugate vaccine in infants and toddlers. Pediatr. Infect. Dis. J. 18:757-763. [DOI] [PubMed] [Google Scholar]
- 33.Shively, J. E., and H. E. Conrad. 1976. Formation of anhydrosugars in the depolymerization of heparin. Biochemistry 15:3932-3942. [DOI] [PubMed] [Google Scholar]
- 34.Slovin, S. F., G. Ragupathi, S. Adluri, G. Ungers, K. Terry, S. Kim, M. Spassova, W. G. Bornmann, M. Fazzari, L. Dantis, K. Olkiewicz, K. O. Lloyd, P. O. Livingston, S. J. Danishefsky, and H. I. Scher. 1999. Carbohydrate vaccines in cancer: immunogenicity of a fully synthetic globo H hexasaccharide conjugate in man. Proc. Natl. Acad. Sci. USA 96:5710-5715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tietze, L. F., M. Arlt, M. Beller, K. H. Glüsenkamp, E. Jähde, and M. F. Rajewsky. 1991. Squaric acid diethyl ester: a new coupling reagent for the formation of drug biopolymer conjugates. Synthesis of squaric acid ester amides and diamides. Chem. Ber. 124:1215-1221. [Google Scholar]
- 36.Vella, P. P., S. Marburg, J. M. Staub, P. J. Kniskern, W. Miller, A. Hagopian, C. Ip, R. L. Tolman, C. M. Rusk, L. S. Chupak, and R. W. Ellis. 1992. Immunogenicity of conjugate vaccines consisting of pneumococcal capsular polysaccharide types 6B, 14, 19F, and 23F and a meningococcal outer membrane protein complex. Infect. Immun. 60:4977-4983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang, Z. G., L. J. Williams, X. F. Zhang, A. Zatorski, V. Kudryashov, G. Ragupathi, M. Spassova, W. Bornmann, S. F. Slovin, H. I. Scher, P. O. Livingston, K. O. Lloyd, and S. J. Danishefsky. 2000. Polyclonal antibodies from patients immunized with a globo H-keyhole limpet hemocyanin vaccine: isolation, quantification, and characteristics of immune responses by using totally synthetic immobilized tumor antigens. Proc. Natl. Acad. Sci. USA 97:2719-2724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wessels, M. R., V. Pozsgay, D. L. Kasper, and H. J. Jennings. 1987. Structure and immunochemistry of an oligosaccharide repeating unit of the capsular polysaccharide of type III group B streptococcus: a revised structure for the type III group B streptococcal polysaccharide antigen. J. Biol. Chem. 262:8262-8267. [PubMed] [Google Scholar]