Abstract
Melanin is made by several important pathogenic fungi and has been implicated in the pathogenesis of a number of fungal infections. This study investigated whether the thermally dimorphic fungal pathogen Histoplasma capsulatum var. capsulatum produced melanin or melanin-like compounds in vitro and during infection. Growth of H. capsulatum mycelia in chemically defined minimal medium produced pigmented conidia. Growth of H. capsulatum yeast in chemically defined minimal medium with l-3,4-dihydroxyphenylalanine (DOPA) or (-)-epinephrine produced pigmented cells. Treatment of the pigmented cells with proteolytic enzymes, denaturant, and hot concentrated acid yielded dark particles that were similar in size and shape to their respective propagules. Melanin-binding monoclonal antibodies (MAb) labeled pigmented conidia, yeast, and the isolated particles as determined by immunofluorescence microscopy. Electron spin resonance spectroscopy revealed that pigmented yeast cells and particles derived from pigmented cells were stable free radicals consistent with their identification as melanins. Tissues from mice infected with H. capsulatum and from biopsy specimens from a patient with histoplasmosis contained fungal cells that were labeled by melanin-binding MAb. Digestion of infected mouse tissues yielded dark particles that reacted with the melanin-binding MAb and were similar in appearance to H. capsulatum yeast cells. Additionally, sera from infected mice contained antibodies that bound melanin particles. Phenoloxidase activity capable of synthesizing melanin from L-DOPA was detected in cytoplasmic yeast cell extracts. These findings indicate that H. capsulatum conidia and yeast can produce melanin or melanin-like compounds in vitro and that yeast cells can synthesize pigment in vivo. Since melanin is an important virulence factor in other pathogenic fungi, this pigment may have a similar role to play in the pathogenesis of histoplasmosis.
The dimorphic fungus Histoplasma capsulatum var. capsulatum, which has a worldwide distribution, is the most prevalent cause of fungal respiratory infections (30). The fungus usually causes a mild, often asymptomatic respiratory illness, but it may progress to life-threatening systemic disease particularly in immunocompromised individuals (12, 47). H. capsulatum grows as a saprophytic mold in the environment but undergoes phase transition to a yeast at mammalian physiological temperatures. In addition to its thermally dimorphic nature, the fungus can modify its microenvironment over a broad pH range, survive in nutrient-deficient environments, resist reactive oxygen and nitrogen species, and survive exposure to degradative enzymes (30). However, the mechanisms by which the fungus accomplishes these tasks in the environment and during infection are not completely understood.
Melanins are negatively charged, hydrophobic pigments of high molecular weight that are formed by the oxidative polymerization of phenolic and/or indolic compounds (48). These multifunctional polymers are found in species from all biological kingdoms (14). Melanin synthesis is associated with virulence for the human pathogenic fungus Cryptococcus neoformans. Melanization in C. neoformans is catalyzed by a laccase when the fungus is grown in the presence of certain dihydroxyphenolic compounds (49). In C. neoformans, pigment production protects the fungus against oxidants, extremes in temperature, UV light, amphotericin B, microbicidal peptides, and macrophages in vitro (reviewed in reference 6). C. neoformans cells isolated from pigeon excrement (a major environmental reservoir) are melanized (26). In mice, melanized C. neoformans cells down regulate the immune response early in infection (15). Additionally, both classical genetic and gene disruption studies have demonstrated that wild-type melanin-producing C. neoformans cells are more virulent than their corresponding albino mutants (18, 31). These results suggest that melanins aid in protecting C. neoformans against environmental insults, antimicrobial therapies, and host defense mechanisms.
The pathogenic fungi Exophiala dermatitidis and Sporothrix schenckii also produce melanins that appear to provide protection against host effector cells and oxidative insults (32, 36). Melanin may also have a role in virulence for Aspergillus species (reviewed in reference 13). Additionally, melanin-like pigments have recently been described for the dimorphic fungus, Paracoccidioides brasiliensis (11). Melanins have also been implicated in the virulence of several fungal plant pathogens (5, 48). Since the available data suggest that melanins may play an important role in virulence in diverse fungal species, we investigated whether H. capsulatum could synthesize melanin or melanin-like compounds. Utilizing techniques developed to study and isolate melanin in vitro and in vivo for C. neoformans and P. brasiliensis, we demonstrated that the conidial and yeast forms of H. capsulatum produce melanin or melanin-like compounds.
MATERIALS AND METHODS
Fungal strains and media.
H. capsulatum var. capsulatum strain G217B, a class 2 isolate, was obtained from the American Type Culture Collection (Rockville, Md.). H. capsulatum strain CIB 1980 was obtained from the Corporación para Investigaciones Biológicas (Medellín, Colombia). Yeast cells were maintained by bimonthly subculture into Sabouraud dextrose or brain heart infusion (BHI) agar slants (Gibco BRL, Grand Island, N.Y.) at 37°C. Mycelial and yeast forms of H. capsulatum were grown at 30 and 37°C, respectively, either in chemically defined medium (minimal medium: 15.0 mM glucose, 10.0 mM MgSO4, 29.4 mM KH2PO4, 13.0 mM glycine, 3.0 μM thiamine [pH 5.5]) in a rotary shaker at 150 rpm or on solid agar (minimal medium with 2% agar) with or without 1 mM L-DOPA (Sigma Chemical Co.; St. Louis, Mo.). Yeast cultures were also grown in minimal medium with 1 mM (-)-epinephrine. The cultures were grown for a minimum of 15 days in the dark to prevent photopolymerization. Yeast cultures were also grown in BHI medium without supplements at 37°C with shaking. Wild-type (Mel+) C. neoformans strain JEC21 and its albino mutant, (Mel−) HMC6 (a gift from G. Cox, Durham, N.C.), were used as positive and negative controls, respectively. These C. neoformans strains have been described previously (34). Candida albicans strain ER2841, a clinical isolate, was also grown on medium with and without L-DOPA under the conditions described. For brightfield and immunofluorescent analysis of mycelial growth and conidial production, H. capsulatum strain 17262, a Brazilian clinical isolate, was grown on slide culture on potato dextrose agar (Sigma) without supplements for 15 days at 30°C.
Isolation and purification of conidia and yeast particles, scanning electron microscopy, transmission electron microscopy, and electron spin resonance spectroscopy (ESR).
Melanin particles were isolated from pigmented conidia and yeast grown for 15 days by a modification of a described methodology (35). Briefly, cells were collected by centrifugation, autoclaved, washed with phosphate-buffered saline (PBS) and suspended in 1.0 M sorbitol-0.1 M sodium citrate (pH 5.5). Cell wall lysing enzymes (from Trichoderma harzianum; Sigma) were added at 10 mg/ml, and the suspensions were incubated at 30°C overnight. The resulting protoplasts were collected by centrifugation, washed with PBS, and treated with 1.0 mg of proteinase K (Roche Laboratories; Indianapolis, Ind.)/ml in a reaction buffer (10.0 mM Tris, 1.0 mM CaCl2, 0.5% sodium dodecyl sulfate [pH 7.8]) at 37°C overnight. The debris was collected, washed with PBS, and boiled in 6.0 M HCl for 1 h. The remaining particles were collected, washed in PBS, and dialyzed extensively against distilled water. This procedure has been shown to solubilize nonmelanized C. neoformans, P. brasiliensis, Saccharomyces cerevisiae, and C. albicans yeast cells (11, 34). Chitin is also solubilized by this treatment (11). Scanning electron microscopy of conidia and yeast of H. capsulatum and the particles derived from them were performed as described previously (27). Additionally, transmission electron microscopy was performed on pigmented and nonpigmented yeast cells as described previously (46).
ESR spectroscopy has been used to study and define melanins based on the properties of unpaired electrons present in melanin (9), and it has been used to identify the pigments produced in C. neoformans (46) and P. brasiliensis (11) as melanins. ESR spectroscopy analyses were performed on H. capsulatum yeast cells grown in BHI medium without supplements and on particles isolated from yeast cells grown in minimal L-DOPA medium as described previously (44), except that a Gunn diode was used as the microwave source.
Experimental infection of mice with H. capsulatum yeast cells.
H. capsulatum strain G217B yeast cells were grown in BHI for 48 h at 37°C, collected, and washed three times with PBS. Isogenic BALB/c female mice (6 to 8 weeks old; National Cancer Institute, Rockville, Md.) were anesthetized with intraperitoneal injections of ketamine-xylazine and infected intranasally with 2.5 × 106 yeast cells or PBS. The mice were killed after 14 days. Lungs were removed and placed into formalin and then embedded in paraffin. Additional mice were bled prior to infection and then at 14 and 40 days after infection. Sera were stored at −20°C prior to analysis for melanin-binding antibodies.
Immunofluorescence analyses.
Yeast were grown with or without L-DOPA were embedded in paraffin, and 4-μm sections were cut. A similar preparation was done with melanin-like particles derived from pigmented conidia or yeast. Lung tissue from infected mice was also embedded in paraffin and sectioned. The sections were deparaffinized in xylene, rehydrated in an ethanol series, treated with 20 μg of proteinase K/ml for 1 h at room temperature, and then heated in 10 mM citric acid in a microwave oven for 5 min. Additionally, formalin-fixed, paraffin-embedded lung tissue from a patient with histoplasmosis was prepared for immunohistochemistry as described above except that proteinase K was inactivated by heating in PBS at 65°C for 1 h instead of by boiling. Slides were also prepared with 10-μl suspensions containing 106 particles derived from yeast cells grown with (-)-epinephrine that were dried on poly-l-lysine-coated slides (Sigma) (24). Slides were incubated in Superblock (Pierce, Rockford, Ill.) blocking buffer for 4 h followed by incubation with 10 μg of the melanin-binding monoclonal antibody (MAb) 6D2/ml for 2 h at 37°C. MAb 6D2 (μκ) was generated against melanin derived from C. neoformans; it also binds other types of melanins but does not bind C. albicans, S. cerevisiae, or a laccase-deficient mutant of C. neoformans (34). After a wash, the slides were incubated with a 1:100 dilution of fluorescein isothiocyanate (FITC)-conjugated goat anti-mouse (GAM) immunoglobulin M (IgM) (Southern Biotechnologies Associates, Inc., Birmingham, Ala.) for 1.5 h at 37°C. The slides were washed, mounted using a 50% glycerol-50% PBS-0.1 M N-propyl gallate solution, and viewed with an Olympus (Melville, N.Y.) AX70 microscope equipped with an FITC filter. Negative controls consisted of slides incubated with the MAb 5C11 (μκ), which binds mycobacterial lipoarabinomannan (10), as the primary antibody or FITC-labeled antibody alone. Additionally, mycelial growth of H. capsulatum strain 17262 grown on slide culture on potato agar without supplements was analyzed. Briefly, the agar block and coverslip were discarded, and the slide was fixed in cold acetone for 5 min. Slides were then processed for immunofluorescence analysis as described above, except that a 1:10 dilution of FITC-GAM IgM was used.
Melanin particles from H. capsulatum yeast cells were attached to poly-l-lysine-coated slides (Sigma), as described previously (24). The melanin was blocked with Superblock overnight at 4°C. Thawed sera were incubated at dilutions of 1:1, 1:10, 1:100, 1:1,000, and 1:10,000 in 2% bovine serum albumin for 1.5 h at 37°C. After a wash, the samples were incubated with a 1:100 dilution of FITC-conjugated GAM IgM-IgG for 1 h at 37°C. The slides were washed and then mounted and viewed as described above.
Polyacrylamide gel electrophoresis (PAGE) analysis for laccase-like activity.
The laccase-like activity of cytoplasmic yeast extract (CYE) was determined as described previously (44). Briefly, H. capsulatum yeast cells were collected and placed into a sonicator (Sonifier Ultrasonic Cell Disruptor 185; Branson, Sonic Power Company, division of Ultrasonic Corporation, Danbury, Conn.) at a 1:4 dilution with sterile PBS. The sonicator was fitted with an external jacket full of ice. Pellets were sonicated four times for 15 minutes with 5-min breaks between each cycle. The sonicated mixture was centrifuged at 5,000 × g for 30 min. The supernatant was collected, and its protein content was determined by the Coomassie blue method (29). Commercially prepared laccase (from Rhus vernificera; activity, 50 U per mg of solid) was obtained from Sigma. R. vernificera laccase (40 μg) and 200 μg of CYE from G217B were separated by 10% PAGE electrophoresis run at 18 mA overnight under nondenaturing conditions. As controls, each of the above samples was treated with 0.1 M KCN, an irreversible inhibitor of laccase activity, before being loaded onto the gel. Gels were incubated with 1 mM L-DOPA in 0.1 M citric acid-0.2 M Na2HPO4 (pH 6.0) buffer for 6 to 8 h.
Isolation of melanin particles from infected tissues. H. capsulatum strain G217B yeast cells were grown in BHI for 48 h at 37°C, collected, and washed three times with PBS. Isogenic BALB/c female mice (6 to 8 week old; National Cancer Institute, Rockville, Md.) were anesthetized with intraperitoneal injections of ketamine-xylazine and infected intranasally with 2.5 × 106 G217B yeast cells or PBS. The mice were killed after 14 days. The lungs were immediately removed, homogenized, and subjected to the melanin isolation protocol described above.
Additional lungs were obtained from uninfected mice. Yeast cells grown in BHI for 48 h (nonmelanized) or 15 days (melanized) were used to spike the lung tissue, which was then homogenized and treated with the melanin isolation procedure. Residual material was then processed for immunofluorescence analysis and scanning electron microscopy as described above.
RESULTS
Melanization of H. capsulatum conidia and yeast cells.
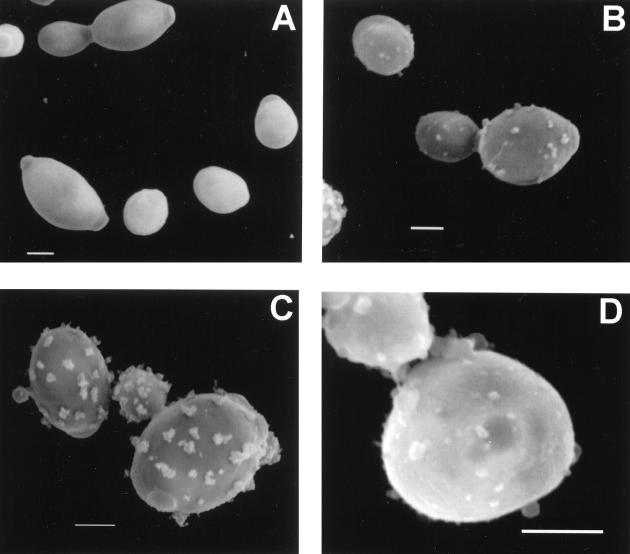
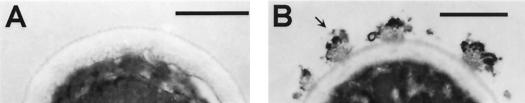
Pigmentation of conidia produced by mycelia grown in minimal medium without L-DOPA and yeast cells grown in the presence of L-DOPA occurred after 7 days of incubation on agar and 5 days in liquid media. Pigmentation also occurred in liquid cultures with (-)-epinephrine after 5 days. No pigmentation occurred in yeast cells grown in the absence of a substrate. Treatment of the pigmented cells with proteolytic and glycolytic enzymes, denaturant, and hot acid resulted in the isolation of black particles that were similar in size and shape to their respective propagules as determined by brightfield and scanning electron microscopy. Scanning electron microscopy demonstrated that pigmented conidia and yeast cells grown with L-DOPA and their corresponding isolated particles had tufts on their surfaces, whereas yeast cells grown in the absence of L-DOPA were smooth (Fig. 1). The wild-type C. neoformans JEC21 produced melanin when grown in the presence of L-DOPA, whereas the albino mutant MHC6 and C. albicans ER 2841 did not (data not shown). Nonpigmented H. capsulatum, C. neoformans, and C. albicans yeast cells treated with the melanin isolation protocol were completely solubilized. No hyphal structures were identified after H. capsulatum mycelia were subjected to the isolation protocol. An additional control tested calcofluor white labeling of pigmented H. capsulatum conidia and yeast cells and their respective isolated black particles. Calcofluor staining occurred on the walls of the conidia and yeast cells, but not on the particles (data not shown). Transmission electron microscopy of H. capsulatum yeast cells grown with or without L-DOPA demonstrates that the cell walls of yeast grown with L-DOPA were coated with electron-dense granules (Fig. 2). These granules were similar to those seen with melanized S. schenckii conidia (32) and correspond to the tufts seen on the H. capsulatum cells by scanning electron microscopy. H. capsulatum yeast cells grown in the absence of L-DOPA lacked these granules (Fig. 2). Additionally, the outer cell wall appeared more electron dense in the cells grown in L-DOPA compared to cells grown in minimal medium alone.
FIG. 1.
Scanning electron micrographs of H. capsulatum strain CIB 1980 yeast cells grown in minimal medium without (A) or with (B) L-DOPA. Scanning electron micrograph of particles isolated from yeast cells grown in the presence of L-DOPA (C) or conidia grown without L-DOPA (D) following treatment with enzymes, denaturant, and hot acid. Bars, 1 μm.
FIG. 2.
Transmission electron micrographs of H. capsulatum strain CIB 1980 yeast cells grown in minimal medium without (A) or with (B) L-DOPA. Arrow indicates a pigment granule. Bars, 0.5 μm.
ESR spectroscopy.
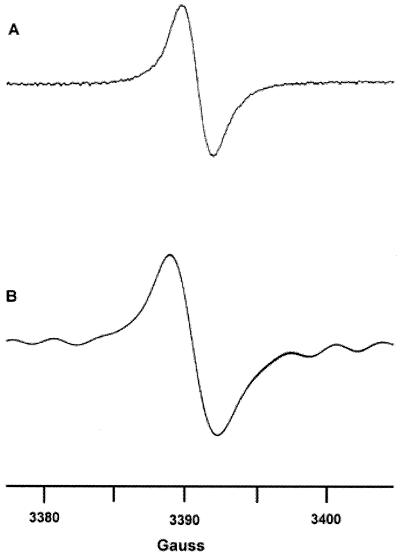
ESR spectroscopy of the black particles collected from H. capsulatum CIB 1980 yeast cells grown in the presence of L-DOPA produced a signal indicative of a stable free-radical population consistent with the pigment being identified as a melanin (Fig. 3) (9). The spectrum was nearly identical to the signals generated with C. neoformans (44, 46) and P. brasiliensis (11). H. capsulatum G217B yeast cells grown in BHI medium without L-DOPA or on BHI agar became a dark brownish color after more than 2 weeks of growth, and treatment of these cells by the melanin isolation protocol resulted in the collection of dark particles (data not shown). ESR spectroscopy was performed on H. capsulatum G217B yeast cells grown in BHI medium without L-DOPA for 21 days. Although of lower amplitude, the signal generated was similar to that seen with the particles purified from the pigmented CIB 1980 yeast cells (Fig. 3). The quantities of particles derived from H. capsulatum conidia or infected tissue were insufficient for study by ESR spectroscopy.
FIG. 3.
(A) ESR spectroscopy of melanin particles collected from H. capsulatum strain CIB 1980 grown for 15 days in minimal medium with L-DOPA. (B) ESR spectroscopy of H. capsulatum strain G217B grown in BHI medium for 21 days. ESR spectroscopy of yeast cells grown in minimal medium without a phenolic substrate for 21 days does not produce a free radical signal.
Immunofluorescence analyses.
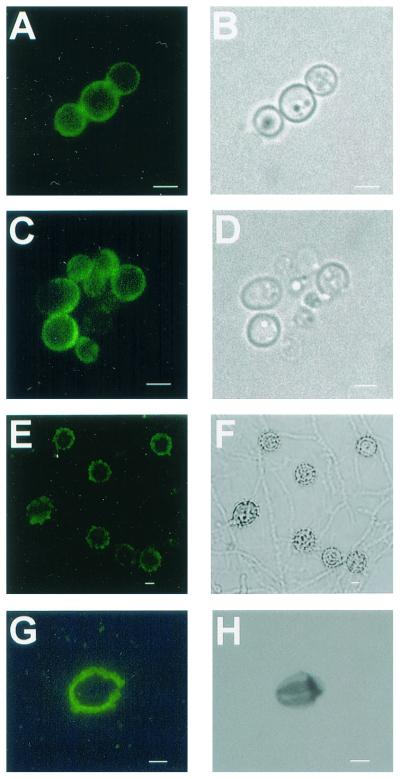
MAb 6D2 bound strongly to sections of pigmented H. capsulatum strain CIB 1980 conidia or yeast cells embedded in paraffin and conidia from H. capsulatum strain 17262 mycelial growth on slide culture (Fig. 4). MAb 6D2 reacted only with conidia and did not bind to hyphal structures from the mycelia of H. capsulatum strain 17262. Negative reactivity with hyphal cell structures provided a useful internal control for nonspecific binding in these experiments. Yeast cells grown in the presence of L-DOPA bound MAb 6D2, whereas those grown in the absence of L-DOPA were not reactive. Particles isolated from pigmented conidia or yeast cells grown with L-DOPA were labeled by MAb 6D2 (Fig. 4). Particles isolated from H. capsulatum strain CIB 1980 or G217B grown with (-)-epinephrine were also labeled by MAb 6D2 (data not shown). There was no reactivity with conidia, yeast, or particles with the negative control, MAb 5C11.
FIG. 4.
Corresponding immunofluorescence (A) and brightfield (B) images of pigmented H. capsulatum strain CIB 1980 yeast cells and the particles isolated from the cells following treatment with enzymes and chemicals (C and D). Corresponding images of mycelial growth of H. capsulatum strain 17262 (E and F) and particles isolated from pigmented conidia (G and H) are shown. Bars, 1 μm.
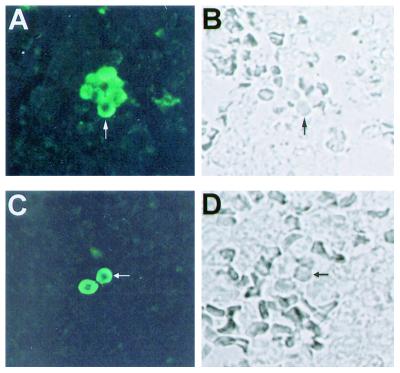
Tissue sections of lung from mice infected with H. capsulatum G217B for 14 days and from a patient with histoplasmosis were stained with Gomori's methenamine silver to confirm the presence of yeast cells (not shown). MAb 6D2 demonstrated reactivity to yeast cells in infected tissue (Fig. 5) but not for control tissue from uninfected mice. No reactivity was observed with the irrelevant isotype-matched negative control MAb 5C11 or when FITC-labeled GAM-IgM was used without MAb 6D2. Similarly, sections from a patient with histoplasmosis were shown to have yeast cells that were labeled by the melanin-binding MAb (Fig. 5).
FIG. 5.
Corresponding immunofluorescence and brightfield microscopy demonstrating the labeling of H. capsulatum strain G217B yeast cells by the melanin-binding MAb 6D2 in infected murine (A and B) and human (C and D) lung. Arrows indicate the yeast cells. Original magnification, ×250.
Murine H. capsulatum infection elicits antibodies to melanin.
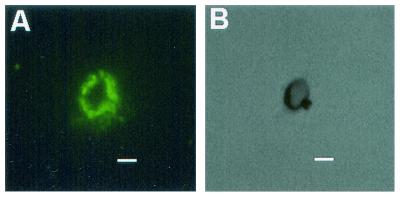
Sera from mice infected with H. capsulatum reacted with melanin particles (Fig. 6). The sera from mice 14 days postinfection reacted with the melanin in a ring appearance along the outer surface of the particles only when minimally diluted (1:10 dilution). Sera from mice 40 days after infection produced ring immunofluorescent patterns at dilutions of 1:1,000 (Fig. 6). At a 1:10,000 dilution, some particles had small areas of fluorescence. Preimmune sera did not react with the particles.
FIG. 6.
Corresponding immunofluorescence (A) and brightfield (B) microscopy demonstrating the labeling of melanin particles derived from H. capsulatum yeast cells by serum from mice 40 days following infection, diluted 1:1,000 in 2% bovine serum albumin. Bars, 1 μm.
Isolation of melanin particles from infected tissue.
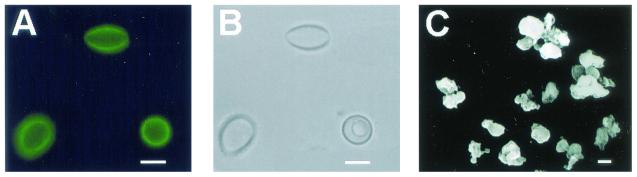
Treatment of infected lungs with enzymes, denaturant, and acid resulted in the isolation of dark particles. Similarly, uninfected lung tissue spiked with melanized cells subjected to the melanin isolation procedure resulted in the collection of black particles. No particles were isolated from lung tissue spiked with nonmelanized cells. The particles reacted with MAb 6D2 in a manner similar to that seen with particles isolated from in vitro-melanized cells (Fig. 7). The particles did not react with control MAb. Scanning electron microscopy revealed that the particles were similar in shape and size to that seen with in vitro-melanized cells (Fig. 7).
FIG. 7.
Corresponding immunofluorescence (A) and brightfield microscopy (B) depicting black particles recovered from the lungs of mice infected with H. capsulatum strain G217B following homogenization and treatment of tissue with enzymes, denaturant, and hot acid, similar in size and shape to H. capsulatum yeast cells. A scanning electron micrograph (C) of the black particles is shown. Bar, 1 μm.
PAGE analysis of laccase-like activity.
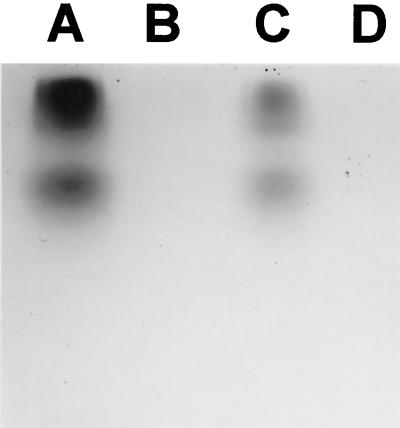
To determine whether H. capsulatum had laccase-like activity, cytoplasmic extracts from H. capsulatum were electrophoresed in a nondenaturing gel and then incubated with L-DOPA for 6 h. This resulted in the formation of dark bands consistent with polymerized DOPA-melanin (Fig. 8). The same results were observed with the commercially available R. vernificera laccase. Treatment of H. capsulatum CYE and R. vernificera laccase with KCN abrogated the enzymatic activity.
FIG. 8.
Nonreducing sodium dodecyl sulfate-PAGE gel of CYE of H. capsulatum strain G217B developed with L-DOPA. Synthesis of a black pigment consistent with melanin occurs in situ on the gel. Lane A, commercial laccase (40 U equivalent); lane B, as for lane A but treated with KCN; lane C, 200 μg of CYE of H. capsulatum isolate G217B; lane D, as for lane C but treated with KCN. Similar results were obtained using CYE of H. capsulatum strain CIB 1980.
DISCUSSION
In 1968, Berliner described brown and albino phenotypes of mycelial phase colonies isolated from primary subcultures grown on Emmons' modified Sabouraud agar (4), and brown phenotype filamentous H. capsulatum were more virulent in an animal model (7). Additionally, there are two reports of brown-red pigment formation by mycelial phase isolates of H. capsulatum on Sabourand (21) and bird seed agar (38), which is commonly used in the identification of C. neoformans by the brown color effect in clinical laboratories (17). However, melanin production with H. capsulatum has not previously been reported. Diverse fungal pathogens have been shown to produce melanin-like pigments that are associated with virulence (16). We applied techniques that were developed to study melanization in C. neoformans and P. brasiliensis to investigate whether H. capsulatum produces melanin. The evidence supporting the formation of melanin by H. capsulatum in vitro and in vivo is as follows: (i) treatment of pigmented H. capsulatum conidia and yeast with enzymes and chemicals resulted in the isolation of black particles similar in size and shape to their respective propagules, (ii) ESR spectroscopy analysis of pigmented yeast and yeast-derived particles indicated the presence of a stable free radical compound consistent with melanin, (iii) transmission electron micrographs of melanized yeast cells demonstrate the presence of pigment granules that resembled those observed with melanized S. schenckii conidia (32), (iv) reactivity of a melanin-binding MAb to the cell surface of pigmented H. capsulatum cells grown in vitro and to the pigmented particles derived from these cells, (v) reactivity of a melanin-binding MAb to the cell wall of H. capsulatum in infected murine and human tissues, (vi) recovery of melanin-like particles similar in size and form to H. capsulatum yeast cells from infected mouse tissue after chemical and enzymatic treatment that were also reactive with a melanin-binding MAb, (vii) detection of laccase-like activity in protein extracts of H. capsulatum, and (viii) occurrence of an antibody response to melanin in mice infected with H. capsulatum. In combination, these observations provide overwhelming evidence that H. capsulatum can make melanin in vitro and in vivo.
We observed that H. capsulatum conidia become progressively pigmented when grown on potato dextrose agar or in minimal chemical medium without exogenous phenolic compounds. Immunofluorescence labeling by melanin-binding MAb occurred only on the conidia of mycelial growth. The absence of binding on hyphal structures is consistent with the specificity of the reagent for melanin, since digestion of mycelia with enzymes and detergents resulted in the isolation of particles similar to conidia and completely solubilized hyphal structures. The ability to produce melanin when grown in minimal medium without L-DOPA indicates that H. capsulatum conidia possess the enzymatic processes necessary to synthesize precursors required for the formation of the polymer. Aspergillus fumigatus conidia produce dihydroxynaphthalene (DHN) melanin, and pigment synthesis has been associated with virulence (40-43). S. schenckii conidial melanization similarly appears to protect against various host defense mechanisms (32). Two conidial pigmentation genes have been identified in Aspergillus nidulans, but no information is currently available about the effect of melanin production on virulence (3, 19, 20). Conidial melanization has also been recently shown to occur in P. brasiliensis in the absence of exogenous phenolic substrates (11). As hypothesized for P. brasiliensis (11), the production of melanin by H. capsulatum conidia without the addition of phenolic precursors suggests that the pigment may be all or partly DHN melanin. Pigmented H. capsulatum conidia and yeast have tufts on their exterior surface which, although larger in size, are similar to the granules seen on S. schenckii conidia (32). C. neoformans yeast cells melanize in the environment (26), which serves to protect the cells from a variety of environmental stresses (reviewed in reference 6), including the ability to survive and replicate in phagocytic predators (39). Since H. capsulatum conidia synthesize melanin in the absence of exogenous phenolic substrate, it is probable that conidia are melanized in the environment. Thus, melanization may protect the conidia from environmental insults. This result is also important for pathogenesis because it indicates that initial infection occurs with a melanized propagule. Further investigation will be required to determine the actual type of melanin produced.
In contrast to H. capsulatum conidia, the yeast form requires exogenous phenolic compounds to melanize in vitro. This is similar to requirements for melanization in C. neoformans (28) and P. brasiliensis yeast cells (11). The utilization of a different enzymatic pathway for the synthesis of melanin by H. capsulatum yeast compared to conidia is consistent with prior reports of differential activation of genes in the H. capsulatum yeast phase compared to that of the mycelial phase (1, 37). The detection of laccase-like activity in nondenaturing protein gels is consistent with the presence of a laccase-like enzyme in H. capsulatum. Multiple bands are identified on these gels (Fig. 8). Further studies are required to determine whether the bands are products of a single gene or of multiple genes. However, the formation of tufts of pigment on the surface of H. capsulatum yeast cells is more similar to the granules seen on S. schenckii conidia in which DHN melanin is synthesized (32). Thus, identification and characterization of the putative laccase-like enzyme and the generation of mutants lacking the enzyme will be required to conclusively demonstrate the method by which the yeast form of H. capsulatum synthesizes melanin.
The finding that H. capsulatum yeast cells were pigmented when grown on BHI media or agar provides additional evidence for the formation of melanin or melanin-like compounds during infection. BHI is made from the brains and hearts of cows, and these organs are rich in phenolic compounds, which could serve as substrates for melanization by H. capsulatum. C. neoformans has been shown to be capable of synthesizing melanin when grown on minimal medium agar plates supplemented with homogenates of murine brains and lungs (34). Thus, since phenolic compounds are present in and constantly replenished in tissue (34), our findings suggest that these compounds are a sufficient substrates for the production of melanin by H. capsulatum under these circumstances.
In fact, our data indicate that H. capsulatum yeast cells synthesized melanin-like pigments during mammalian infection. We showed that a melanin-binding MAb labeled both yeast cells in infected tissues and particles resembling yeast cells isolated from infected tissues. These results are consistent with the recent reports that demonstrated that C. neoformans and P. brasiliensis yeast cells are melanized in vivo (11, 25, 34). Additionally, infection with nonmelanized H. capsulatum yeast cells resulted in the development of an antibody response against melanin, which implies that the cells synthesized melanin or melanin-like compounds during the infection. This is similar to the development of an antibody response to melanin during murine cryptococcosis (24).
In summary, our results indicate that H. capsulatum conidia and yeast cells synthesize melanin or melanin-like pigments in vitro and during infection. Since the production of melanin has been linked with virulence in several pathogenic fungi, the effects of pigment production in H. capsulatum should be explored. Immunity to H. capsulatum is complex and varies with early and late infection (2, 8). Melanized C. neoformans cells down regulate T-cell function (15), and melanization of H. capsulatum conidia and yeast may affect inflammation and immunity in histoplasmosis. Additionally, melanization of C. neoformans protects the fungus from phagocytosis by macrophages and from oxygen- and nitrogen-derived radicals (44). Macrophages play a critical role in histoplasmosis (22). It is possible that melanization protects H. capsulatum against immune effector cells, by either altering phagocytosis or protecting the fungus against nitric oxide and other macrophage-derived fungicidal chemicals. Current therapy for histoplasmosis in immunocompromised patients is inadequate, and patients with AIDS require lifelong maintenance therapy with antifungals to reduce the likelihood of recurrent disease. Melanization of C. neoformans decreases its susceptibility to amphotericin B (45), and agents that interfere with melanin polymerization are therapeutic in mice (23, 33). Thus, formation of melanin or melanin-like compounds could be an additional reason for the difficulties in treating histoplasmosis. Genetic manipulation strategies have been developed for H. capsulatum that will be instrumental in future studies investigating genes responsible for melanin formation in conidia and yeast (37). Development of melanin-deficient mutants of H. capsulatum conidia and yeast cells and investigating whether compounds, such as N-(phosphonomethyl)-glycine (23), can block the production of melanin by the fungus will provide a mechanism for improving our understanding of the function of this polymer in H. capsulatum.
Acknowledgments
A. Casadevall and A. J. Hamilton share senior authorship of this paper.
We thank the UNESCO/ASM travel award (1999) for sponsoring B. L. Gómez during her time in A. Casadevall's laboratory. B. L. Gómez was also supported by the Wellcome Trust (United Kingdom). A. Casadevall was supported by National Institutes of Health (NIH) grants RO1-AI33774, AI13342, and HL59842 and is a recipient of a Burroughs Wellcome Fund Scholar Award in Experimental Therapeutics. J. D. Nosanchuk was supported by NIH grant KO8-AI01489. P. Aisen was supported by grant 5 R01 DK15056. A. Restrepo is supported by the CIB and Colciencias in Colombia, and S. Diez is supported by an ORS (United Kingdom Government) award.
Editor: T. R. Kozel
REFERENCES
- 1.Abidi, F. E., H. Roh, and E. J. Keath. 1998. Identification and characterization of a phase-specific, nuclear DNA binding protein from the dimorphic pathogenic fungus Histoplasma capsulatum. Infect. Immun. 66:3867-3873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allendorfer, R., G. D. Brunner, and G. S. Deepe, Jr. 1999. Complex requirements for nascent and memory immunity in pulmonary histoplasmosis. J. Immunol. 162:7389-7396. [PubMed] [Google Scholar]
- 3.Aramayo, R., and W. E. Timberlake. 1990. Sequence and molecular structure of the Aspergillus nidulans yA (laccase I) gene. Nucleic Acids Res. 18:3415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berliner, M. D. 1968. Primary subcultures of Histoplasma capsulatum. I. Macro and micro-morphology of the mycelial phase. Sabouraudia 6:111-118. [PubMed] [Google Scholar]
- 5.Butler, M. J., and A. W. Day. 1998. Fungal melanins: a review. Can. J. Microbiol. 44:1115-1136. [Google Scholar]
- 6.Casadevall, A., A. L. Rosas, and J. D. Nosanchuk. 2000. Melanin and virulence in Cryptococcus neoformans. Curr. Opin. Microbiol. 3:354-358. [DOI] [PubMed] [Google Scholar]
- 7.Daniels, L. S., M. D. Berliner, and C. C. Campbell. 1968. Varying virulence in rabbits infected with different filamentous types of Histoplasma capsulatum. J. Bacteriol. 96:1535-1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Deepe, G. S., Jr. 2000. Immune response to early and late Histoplasma capsulatum infections. Curr. Opin. Microbiol. 3:359-362. [DOI] [PubMed] [Google Scholar]
- 9.Enochs, W. S., M. J. Nilges, and H. M. Swartz. 1993. A standardized test for the identification and characterization of melanins using electron paramagnetic (EPR) spectroscopy. Pigment Cell Res. 6:91-99. [DOI] [PubMed] [Google Scholar]
- 10.Glatman-Freedman, A., J. M. Martin, P. F. Riska, B. R. Bloom, and A. Casadevall. 1996. Monoclonal antibodies to surface antigens of Mycobacterium tuberculosis and their use in a modified enzyme-linked immunosorbent spot assay for detection of mycobacteria. J. Clin. Microbiol. 34:2795-2802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gómez, B. L., J. D. Nosanchuk, S. Diez, S. Youngchim, P. Aisen, L. E. Cano, A. Restrepo, A. Casadevall, and A. J. Hamilton. 2001. Detection of melanin-like pigments in the dimorphic fungal pathogen Paracoccidioides brasiliensis in vitro and during infection. Infect. Immun. 69:5760-5767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Graybill, J. R. 1988. Histoplasmosis and AIDS. J. Infect. Dis. 158:623-626. [DOI] [PubMed] [Google Scholar]
- 13.Hamilton, A. J., and M. D. Holdom. 1999. Antioxidant systems in the pathogenic fungi of man and their role in virulence. Med. Mycol. 37:375-389. [DOI] [PubMed] [Google Scholar]
- 14.Hill, Z. H. 1992. The function of melanin or six blind people examine an elephant. BioEssays 14:49-56. [DOI] [PubMed] [Google Scholar]
- 15.Huffnagle, G. B., G.-H. Chen, J. L. Curtis, R. A. McDonald, R. M. Strieter, and G. B. Toews. 1995. Down-regulation of the afferent phase of T cell-mediated pulmonary inflammation and immunity by a high melanin-producing strain of Cryptococcus neoformans. J. Immunol. 155:3507-3516. [PubMed] [Google Scholar]
- 16.Jacobson, E. S. 2000. Pathogenic roles for fungal melanins. Clin. Microbiol. Rev. 13:708-717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Korth, H., and G. Pulverer. 1971. Pigment formation for differentiating Cryptococcus neoformans from Candida albicans. Appl. Microbiol. 21:541-542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kwon-Chung, K. J., I. Polacheck, and T. J. Popkin. 1982. Melanin-lacking mutants of Cryptococcus neoformans and their virulence for mice. J. Bacteriol. 150:1414-1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mayorga, M. E., and W. E. Timberlake. 1992. The developmentally regulated Aspergillus nidulans wA gene encodes a polypeptide homologous to polyketide and fatty acid synthases. Mol. Gen. Genet. 235:205-212. [DOI] [PubMed] [Google Scholar]
- 20.Mayorga, M. E., and W. E. Timberlake. 1990. Isolation and molecular characterization of the Aspergillus nidulans wA gene. Genetics 126:73-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morris, P. R., A. A. Terreni, and A. F. DiSalvo. 1986. Red-pigmented Histoplasma capsulatum—an unusual variant. J. Med. Vet. Mycol. 24:231-233. [PubMed] [Google Scholar]
- 22.Newman, S. L. 1999. Macrophages in host defense against Histoplasma capsulatum. Trends Microbiol. 7:67-71. [DOI] [PubMed] [Google Scholar]
- 23.Nosanchuk, J. D., R. Ovalle, and A. Casadevall. 2001. Glyphosate inhibits melanization of Cryptococcus neoformans and prolongs survival of mice after systemic infection. J. Infect. Dis. 183:1093-1099. [DOI] [PubMed] [Google Scholar]
- 24.Nosanchuk, J. D., A. L. Rosas, and A. Casadevall. 1998. The antibody response to fungal melanin in mice. J. Immunol. 160:6026-6031. [PubMed] [Google Scholar]
- 25.Nosanchuk, J. D., A. L. Rosas, S. C. Lee, and A. Casadevall. 2000. Melanization of Cryptococcus neoformans in human brain tissue. Lancet 355:2049-2050. [DOI] [PubMed] [Google Scholar]
- 26.Nosanchuk, J. D., J. N. Rudolph, A. L. Rosas, and A. Casadevall. 1999. Evidence that Cryptococcus neoformans is melanized in pigeon excreta: implications for pathogenesis. Infect. Immun. 67:5477-5479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nosanchuk, J. D., P. Valadon, M. Feldmesser, and A. Casadevall. 1999. Melanization of Cryptococcus neoformans in murine infection. Mol. Cell. Biol. 19:745-750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Polacheck, I., and K. J. Kwon-Chung. 1988. Melanogenesis in Cryptococcus neoformans. J. Gen. Microbiol. 134:1034-1041. [DOI] [PubMed] [Google Scholar]
- 29.Read, S. M., and D. H. Northcote. 1981. Minimization of variation in the response to different proteins of the Coomassie blue G dye-binding assay for protein. Anal. Biochem. 116:53-64. [DOI] [PubMed] [Google Scholar]
- 30.Retallack, D. M., and J. P. Woods. 1999. Molecular epidemiology, pathogenesis, and genetics of the dimorphic fungus Histoplasma capsulatum. Microbes Infect. 1:817-825. [DOI] [PubMed] [Google Scholar]
- 31.Rhodes, J. C., I. Polacheck, and K. J. Kwon-Chung. 1982. Phenoloxidase activity and virulence in isogenic strains of Cryptococcus neoformans. Infect. Immun. 36:1175-1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Romero-Martinez, R., M. Wheeler, A. Guerrero-Plata, G. Rico, and H. Torres-Guerrero. 2000. Biosynthesis and functions of melanin in Sporothrix schenckii. Infect. Immun. 68:3696-703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rosas, A. L., J. D. Nosanchuk, and A. Casadevall. 2001. Passive immunization with melanin-binding monoclonal antibodies prolongs survival of mice with lethal Cryptococcus neoformans infection. Infect. Immun. 69:3410-3412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rosas, A. L., J. D. Nosanchuk, M. Feldmesser, G. M. Cox, H. C. McDade, and A. Casadevall. 2000. Synthesis of polymerized melanin by Cryptococcus neoformans in rodents. Infect. Immun. 68:2845-2853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rosas, A. L., J. D. Nosanchuk, B. L. Gomez, W. A. Edens, J. M. Henson, and A. Casadevall. 2000. Isolation and serological analyses of fungal melanins. J. Immunol. Methods 244:69-80. [DOI] [PubMed] [Google Scholar]
- 36.Schnitzler, N., H. Peltroche-Llacsahuanga, N. Bestier, J. Zundorf, R. Lutticken, and G. Haase. 1999. Effect of melanin and carotenoids of Exophiala (Wangiella) dermatitidis on phagocytosis, oxidative burst, and killing by human neutrophils. Infect. Immun. 67:94-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sebghati, T. S., J. T. Engle, and W. E. Goldman. 2000. Intracellular parasitism by Histoplasma capsulatum: fungal virulence and calcium dependence. Science 290:1368-1372. [DOI] [PubMed] [Google Scholar]
- 38.Staib, F., and G. Grosse. 1996. Brown-red pigment formation by the mycelial phase of a clinical isolate of Histoplasma capsulatum on Staib agar. A preliminary report. Zentbl. Bakteriol. 283:515-521. [DOI] [PubMed] [Google Scholar]
- 39.Steenbergen, J. N., H. A. Shuman, and A. Casadevall. 2001. Cryptococcus neoformans interactions with amoebae suggest an explanation for its virulence and intracellular pathogenic strategy in macrophages. Proc. Natl. Acad. Sci. USA 98:15245-15250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tsai, H. F., Y. C. Chang, R. G. Washburn, M. H. Wheeler, and K. J. Kwon-Chung. 1998. The developmentally regulated alb1 gene of Aspergillus fumigatus: its role in modulation of conidial morphology and virulence. J. Bacteriol. 180:3031-3038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tsai, H. F., I. Fujii, A. Watanabe, M. H. Wheeler, Y. C. Chang, Y. Yasuoka, Y. Ebizuka, and K. J. Kwon-Chung. 2001. Pentaketide melanin biosynthesis in Aspergillus fumigatus requires chain-length shortening of a heptaketide precursor. J. Biol. Chem. 276:29292-29298. [DOI] [PubMed] [Google Scholar]
- 42.Tsai, H. F., R. G. Washburn, Y. C. Chang, and K. J. Kwon-Chung. 1997. Aspergillus fumigatus arp1 modulates conidial pigmentation and complement deposition. Mol. Microbiol. 26:175-183. [DOI] [PubMed] [Google Scholar]
- 43.Tsai, H. F., M. H. Wheeler, Y. C. Chang, and K. J. Kwon-Chung. 1999. A developmentally regulated gene cluster involved in conidial pigment biosynthesis in Aspergillus fumigatus. J. Bacteriol. 181:6469-6477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang, Y., P. Aisen, and A. Casadevall. 1995. Cryptococcus neoformans melanin and virulence: mechanism of action. Infect. Immun. 63:3131-3136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang, Y., and A. Casadevall. 1994. Growth of Cryptococcus neoformans in presence of L-dopa decreases its susceptibility to amphotericin B. Antimicrob. Agents Chemother. 38:2648-2650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang, Y., and A. Casadevall. 1996. Melanin, melanin “ghosts,” and melanin composition in Cryptococcus neoformans. Infect. Immun. 64:2420-2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wheat, J. 1996. Histoplasmosis in the acquired immunodeficiency syndrome. Curr. Top. Med. Mycol. 7:7-18. [PubMed] [Google Scholar]
- 48.Wheeler, M. H., and A. A. Bell. 1988. Melanins and their importance in pathogenic fungi. Curr. Top. Med. Mycol. 2:338-387. [DOI] [PubMed] [Google Scholar]
- 49.Williamson, P. R. 1994. Biochemical and molecular characterization of the diphenol oxidase of Cryptococcus neoformans: identification as a laccase. J. Bacteriol. 176:656-664. [DOI] [PMC free article] [PubMed] [Google Scholar]