Abstract
Dysphagia occurs in only a small percentage of patients with lung cancer, but the frequency of this cancer means that large numbers are affected. Non-quantitative analysis of a large Scottish series of lung cancer cases indicates the following eight broad categories of dysphagia according to underlying mechanisms: mediastinal disease; cervical lymphadenopathy; brainstem lesions; gastrointestinal tract metastases; associated systemic disorders; second primaries; oropharyngeal and oesophageal infections; and radiation-induced oesophageal toxicity.
INTRODUCTION
Carcinoma of the lung affects 125 men per 100 000 and 33 women per 100 000 of the UK population1. About 80% of cases are non-small-cell and 20% small-cell carcinomas2. Although respiratory symptoms predominate, substantial numbers of patients experience difficulty in swallowing. LeRoux3 reported that 1-2% of lung cancer patients had dysphagia at presentation—about the same proportion as those with wheeze or stridor. When the full clinical course of the disease is considered the percentage experiencing dysphagia rises to 6-7%4,5.
According to Stankey et al.6 in 1969, the dysphagia associated with lung cancer could be accounted for in all cases by three possible mechanisms—first and most commonly, extrinsic compression of the oesophagus within the mediastinum; second, compression of the pharynx and upper oesophagus by lymph-node deposits within the neck; and third and most infrequently, oesophageal stenosis secondary to antecedent mediastinal radiotherapy. To these three causes Makker et al.7, in 1995, added secondary achalasia. The present communication aims to expand this list further towards completeness.
In a prospective study from September 1999 to April 2001 in the Edinburgh Cancer Centre, cases of lung cancer associated with different causes of dysphagia were collected. In addition, both a literature search and a survey of the department's consultants (covering a cumulative oncological experience of 153 years) were undertaken to identify rarer causes. Six separate condition-related causes and two treatment-related causes of dysphagia were identified. The eight causes, with illustrative case histories, are discussed in detail below.
MEDIASTINAL DISEASE
Case history
A man of 53 had experienced dizziness and increasing dysphagia to solids for the previous month. CT scans revealed multiple cerebral metastases in addition to massive mediastinal lymphadenopathy almost completely obliterating the lumen of his oesophagus (Figure 1). Small-cell carcinoma was diagnosed on transoesophageal biopsy. A nasogastric tube was inserted endoscopically to allow enteral nutrition. After treatment with dexamethasone his dizziness improved and he received two cycles of cisplatin and etoposide chemotherapy. Within a few days of the first cycle his swallowing improved subjectively and the nasogastric tube was removed, but soon after the second cycle he became confused. Palliative whole-brain radiotherapy was given without apparent benefit and he died 12 days later.
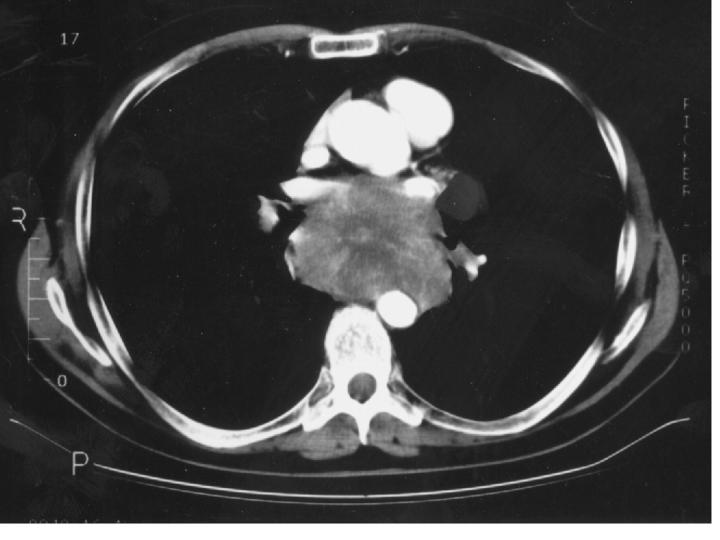
Figure 1.
CT scan of chest showing massive mediastinal lymphadenopathy (secondary to small-cell carcinoma) obliterating the oesophageal lumen
Comment
In both the prospective data collection and the consultant survey the most commonly reported cause of lung-cancer-associated dysphagia was mediastinal disease (92% of all cases).
Direct invasion may occur with lesions in the left main bronchus but perioesophageal or subcarinal lymphnode deposits are more often responsible6. Anatomically, the subcarinal lymph nodes, limited in their potential for lateral growth and therefore tending to expand posteriorly, are the group most likely to intrude on the adjacent oesophagus6. In addition to a direct physical effect on the passage of food, both small-cell and non-small-cell carcinomas have been reported to produce secondary achalasia by interfering with oesophageal motility7,8. Whether this phenomenon reflects direct invasion of the nerve supply of the oesophagus is unclear. Changes in oesophageal motility have been observed after pneumonectomy9, suggesting that nerve damage within the mediastinum might sometimes be the mechanism in lung cancer, but another possibility is a paraneoplastic effect on gastrointestinal motility10,11.
CERVICAL LYMPHADENOPATHY
Case history
A woman aged 49 reported a dragging feeling in her chest and right-sided cervical lymphadenopathy. Lymph-node biopsy revealed adenocarcinoma. A 15 mm right midzone peripheral nodule with right hilar, right pretracheal, subcarinal and left para-aortic lymphadenopathy was apparent on a CT scan of the chest. In a clinical trial she received chemotherapy with gemcitabine and cisplatin. After two cycles of treatment the cervical lymphadenopathy had progressed. Over the next 6 weeks she reported worsening dysphagia, as the lymph-nodes on both sides of her neck enlarged into masses approximately 7 cm across (Figure 2). She became unable to swallow solids or liquids and was admitted for intravenous hydration and nasogastric feeding. Barium swallow showed extrinsic compression of the oesophagus at the level of the thoracic inlet but good flow throughout the rest of the oesophagus. Palliative radiotherapy to the neck and mediastinum produced little improvement in her dysphagia. She continued to be fed nasogastrically at home until her death 10 weeks later.
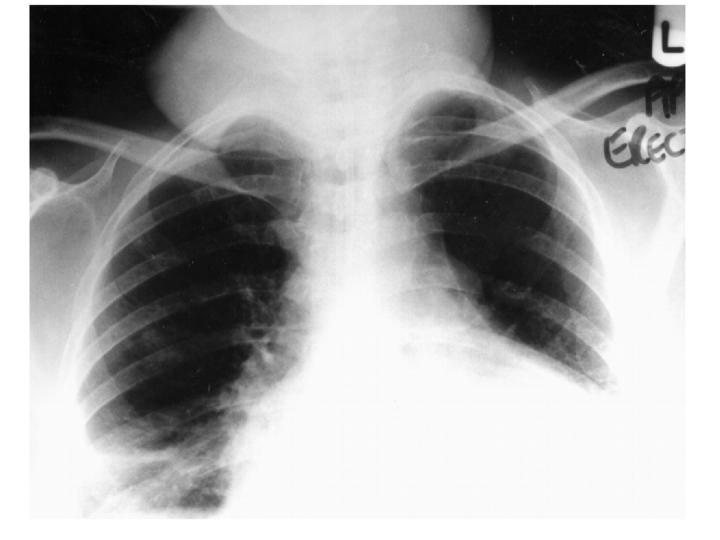
Figure 2.
Chest X-ray of patient with cervical lymphadenopathy showing extent of bilateral soft-tissue masses in cervical region
Comment
Cervical lymph nodes are involved in 15-20% of patients with lung cancer4.
BRAINSTEM LESIONS
Case history
A woman of 64 reported cough and intermittent hoarseness, and a CT scan showed a large left hilar mass encasing the left pulmonary artery with multiple enlarged mediastinal lymph-nodes. Small-cell carcinoma was found on bronchoscopic biopsy, and she had a complete radiological response to four cycles of cisplatin and etoposide. The chemotherapy was followed by consolidation radiotherapy (40 Gray in fifteen fractions over 22 days) to the mediastinum with prophylactic cranial irradiation (30 Gray in ten fractions over 12 days). 22 months later she developed progressive dysphagia over 2 weeks. A chest X-ray revealed complete collapse of the left lung. She received palliative radiotherapy to the mediastinum (20 Gray in five fractions over 5 days). Two weeks from the end of radiotherapy she was admitted because of the sensation of food sticking in her throat, reduced oral intake and dehydration. The presumptive diagnosis was radiation oesophagitis, and she improved over 7 days with parenteral fluid support. 12 days later, although tolerating semisolid food, she became unable to swallow liquids. A barium swallow showed no obstructive lesion but there was considerable aspiration into the lungs. On neurological assessment her speech was nasal and movement was reduced on the left side of the soft palate. The patient was intolerant of MRI scanning but a contrast CT scan of the brain revealed a 1 cm metastasis in the right side of the brainstem (Figure 3). Despite intravenous dexamethasone her level of consciousness deteriorated and she died 6 days later.
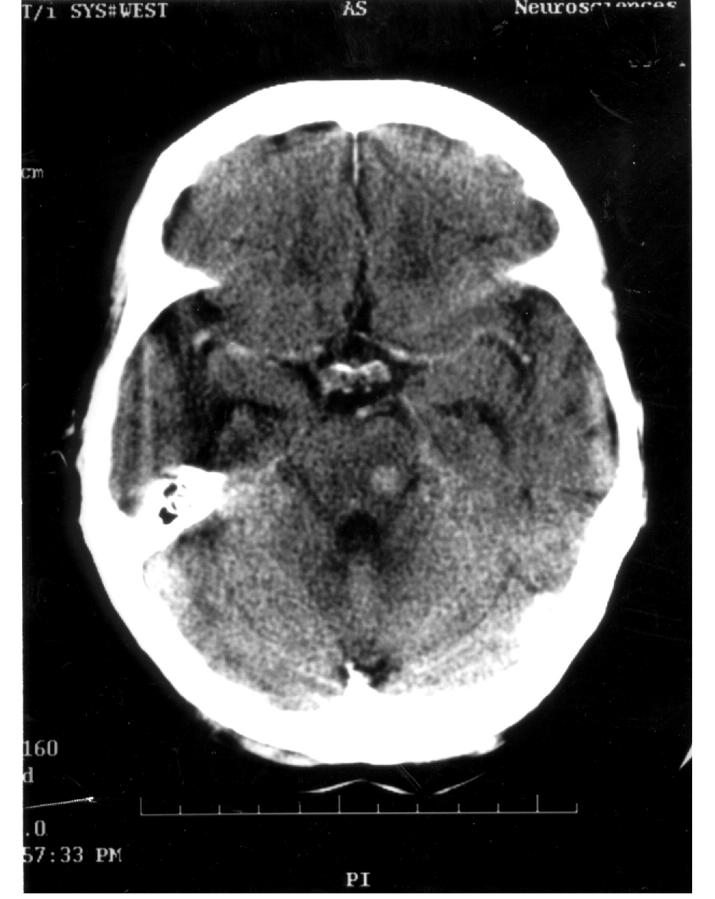
Figure 3.
Contrast enhanced CT scan of brain showing solitary right brainstem metastasis from small-cell carcinoma
Comment
Damage to the nucleus ambiguus in the ventral medulla, comprising the motor nuclei of the IXth, Xth and XIth cranial nerves, may affect both phonation and swallowing (bulbar palsy)12. A similar clinical appearance (but with more prominent upper-motor-neuron signs including an exaggerated jaw jerk) may be seen with lesions within the internal capsule affecting the supranuclear control of this region (pseudobulbar palsy). Either syndrome may be caused by metastatic disease. From a large study based in South East Scotland, 50% of intracranial tumours are secondaries and by far the most common source of these (53%) is lung cancer13. In necropsy series cerebral metastases can be documented in about 20% of lung cancer cases4. In LeRoux's series 3.3% of lung cancer patients presented with intracranial lesions3: the commonest manifestation is hemiplegia, but about 5% have isolated cranial nerve palsies.
Occasionally, brainstem dysfunction can also be treatment-related. In a phase I study of diethylnorspermine the dose-limiting toxicity of the drug was a central nervous system syndrome characterized by ataxia, dysarthria and dysphagia14.
GASTROINTESTINAL TRACT METASTASES
Case history
A woman aged 62 reported pain in the left side of her tongue. On examination the tongue was fixed: no ulceration was present but a mass was palpable at the base which on biopsy contained metastatic adenocarcinoma. A CT scan of the chest and abdomen revealed a left lower lobe mass and multiple liver metastases. Histology of the lung mass was the same as that of the tongue lesion. She received combination chemotherapy with mitomycin C, ifosfamide and cisplatin. After two cycles her tongue was less uncomfortable but after four it was worse again and her oral intake declined. She was admitted for pain control and died 6 weeks later.
Comment
Gastrointestinal metastases are found at necropsy in 2-10% of patients with lung cancer15, 16. Lung cancer is the commonest source of oropharyngeal metastases17, and in the rest of the upper gastrointestinal tract only malignant melanoma and breast cancer are more frequent sources of metastasis18. The most common gastrointestinal site for lung-cancer metastasis is the oesophagus, particularly the middle third16. Often the metastatic spread is clinically unsuspected. In one necropsy series only 15% of patients with lung cancer directly involving the oesophagus had a history of dysphagia16.
ASSOCIATED SYSTEMIC DISORDERS
Case history
A man aged 76 reported weight loss and waterbrash but nothing abnormal was seen on oesophagogastroscopy. Within 2 months he developed progressive dysphagia, periorbital oedema and weakness and discomfort in his shoulders and thighs. His creatine kinase was 1205 U/L (normal range 24-161) and muscle biopsies and electromyography indicated dermatomyositis. A screening CT scan of his chest revealed a right apical mass which on biopsy proved to be squamous cell carcinoma. The muscle tenderness and swallowing improved with prednisolone. He was given palliative radiotherapy to the mediastinum and right apex and died 4 months later.
Comment
Dermatomyositis is associated with an underlying visceral malignancy in 15-35% of cases19. The most common underlying malignancies are lung, breast, female genital tract, stomach, rectum, kidney and testis20. Symptoms and signs characteristically include a heliotrope rash, Gottron's papules in the hands and proximal limb myalgia and weakness. If the musculature of the upper gastrointestinal tract is involved the consequences can include nasal speech, regurgitation and dysphagia20. Clinical dysphagia is seen in 40-60% of patients with dermatomyositis or polymyositis, although manometric abnormalities are found in 69-80%21. The management consists of steroids with or without steroid-sparing agents such as azathioprine; upper oesophageal dysfunction responds better than lower21. Effective antitumour therapy sometimes lessens the inflammation.
What other paraneoplastic conditions cause dysphagia? Systemic sclerosis, in which the oesophagus is commonly involved, has been associated with lung cancer though the association is not as strong as for dermatomyositis22. Tomkin suggests that haemoptysis in the context of systemic sclerosis should always stimulate a search for underlying lung malignancy22. The Eaton—Lambert syndrome develops in 3% of patients with small-cell lung carcinoma23. Although this autoimmune condition is principally characterized by proximal muscle fatiguability, dysphonia and dysphagia can occur23.
SECOND PRIMARY
Case history
A woman of 56 had a right upper lobectomy for poorly differentiated adenocarcinoma of the lung. 7 years later she began to have dysphagia and odynophagia. Endoscopic biopsies of an obstructing mucosal lesion revealed a squamous carcinoma of the upper oesophagus 20-26 cm from the incisors. Dilatation was followed by palliative radiotherapy to the upper oesophagus. She remained well until her death 18 months later.
Comment
The ‘association’ of cancers may reflect nothing more than the chance occurrence of two common conditions within an individual. Indeed, the fact that several different histological types of lung cancer, including both adenocarcinoma and small-cell carcinoma, have been reported in association with squamous carcinoma of the oesophagus does support such a ‘coincidence theory’24,25. However, the fact that lung and oesophageal cancers have been reported synchronously26 as well as metachronously (as in this case) does raise the possibility that lifestyle or genetic factors could converge within individuals to increase the risk of malignant transformations at several sites at the same time. The most notable example concerns patients with squamous cell carcinomas of the head and neck, 16% of whom go on to develop second neoplasms (nearly one-third of which will be primary lung cancers)27. Therefore a malignancy distinct from the original lung primary should always be borne in mind as the possible cause of a patient's dysphagia.
OROPHARYNGEAL AND OESOPHAGEAL INFECTIONS
Case history
A man aged 56 who had smoked 30 cigarettes a day for 40 years was referred to the ear, nose and throat clinic with increasing hoarseness of voice. A left vocal cord palsy was noted in addition to poor short-term memory and multiple skin nodules. An excision biopsy of one of the skin nodules revealed neoplastic cells with scanty mucin production consistent with metastatic spread from a lung primary. On CT scanning a large peripheral lung mass was seen abutting the pleural surface in the left upper lobe with hilar, aortopulmonary and subcarinal lymphadenopathy. A CT scan of his head showed multiple cerebral metastases. He was started on oral dexamethasone 16 mg daily, with some improvement in his short-term memory, and palliative radiotherapy to the whole brain was initiated; after 7 days the daily dose of dexamethasone was reduced to 8 mg. 5 days later he began to feel nauseated, and after another 48 hours he developed dysphagia and started to regurgitate food soon after eating. A barium swallow showed free flow of contrast but with widespread mucosal ulceration throughout the length of the oesophagus highly suggestive of oesophageal candidiasis. His swallowing improved after 7 days of oral fluconazole 100 mg. He was transferred to a hospice three weeks after admission, where he died 10 days later.
Comment
Oropharyngeal candidal infections are common in cancer patients28. They are usually obvious clinically but seldom cause enough oropharyngeal discomfort to limit intake29. Candidal infections of the oesophagus are, in contrast, commonly associated with both severe dysphagia and odynophagia. Although a well recognized complication of leukaemias and lymphomas (or their treatment), oesophageal candidiasis is less often associated with lung cancer28. Abnormal oesophageal motility (due to obstruction or achalasia) may be a predisposing factor28, as may be use of corticosteroids, antibiotics or radiotherapy29. The definitive diagnosis of oesophageal candida rests on oesophagoscopy and biopsy30, but in practice antifungal agents such as fluconazole are often used empirically. The presence or absence of oropharyngeal candida is not predictive of oesophageal disease30.
Viral and bacterial stomatitis and oesophagitis can occur in the lung cancer population but they are sufficiently rare that additional risk factors, such as underlying HIV infection, should probably be sought28.
RADIATION-INDUCED OESOPHAGEAL TOXICITY
Case history
A man aged 55 sought advice for hoarseness and left anterior chest pain. A CT scan revealed a 4.7 × 4 cm left hilar mass extending anterior to the left main bronchus with right paratracheal lymphadenopathy. A biopsy specimen obtained at video-assisted thoracoscopy showed adenocarcinoma of the lung. In a clinical trial he received weekly gemcitabine and concomitant radical radiotherapy (60 Gray in thirty fractions over 46 days) to the left lung mass and mediastinum. Odynophagia gradually developed two weeks into his radiotherapy. The discomfort progressed, limiting his intake of solid food and hot liquids. Soluble aspirin and Mucaine (aluminium and magnesium hydroxide and oxetacaine) were ineffective but some symptomatic relief was achieved with dihydrocodeine. There was no response to a trial of fluconazole. His swallowing difficulties disappeared 6 weeks after the end of his radiotherapy. He remained well, without dysphagia or evidence of progression, 8 months later.
Comment
Symptomatic acute radiation oesophagitis usually develops 2 or 3 weeks after the beginning of treatment and may last for several months. Kaasa et al.31 followed the subjective reporting of dysphagia in 51 patients with inoperable non-small-cell lung cancer treated with 42 Gray in fifteen fractions over 3 weeks. After 2 weeks of treatment 75% reported dysphagia. Three weeks after the end of treatment this had fallen to 64% and 8 weeks later to 22%. Maguire et al.5 similarly found that 75% of their patients receiving high-dose conformal radiotherapy for lung cancer (64.2-85.6 Gray) experienced dysphagia. However, the degree of dysphagia was very variable. On a well-recognized morbidity scale (RTOG), only 11% had grade 3 acute oesophageal toxicity (requiring parenteral fluid support). Factors that seemed to predispose to severe acute toxicity were the existence of dysphagia before treatment and the use of hyperfractionated treatment regimens. Chronic oesophageal toxicity was much rarer than acute toxicity in the series of Maguire et al. (18%), with grade 3 toxicity (requiring dilatation) developing in only 3% of patients. Again pretreatment dysphagia was a risk factor. The incidence of severe late toxicity seems to be increased when chemotherapy and radiotherapy32 are used together.
CONCLUSION
In lung cancer life expectancy is often short, and rapid palliation of symptoms is of paramount importance. Many different mechanisms can underlie dysphagia in lung cancer. By considering the eight broad categories discussed above the clinician should aim to reach the correct diagnosis and start appropriate treatment promptly.
References
- 1.Jensen OM, Esteve J, Moller H, Renard H. Cancer in the European Community and its member states. Eur J Cancer 1990;26: 1167-256 [DOI] [PubMed] [Google Scholar]
- 2.Clark R, Ihde DC. Small-cell lung cancer: treatment, progress and prospects. Oncology (Huntingt) 1998;12: 647-58 [PubMed] [Google Scholar]
- 3.LeRoux BT. The presentation of bronchial carcinoma. Scott Med J 1968;13: 31-7 [DOI] [PubMed] [Google Scholar]
- 4.Hyde L, Hyde C. Clinical manifestations of lung cancer. Chest 1974;65: 299-306 [DOI] [PubMed] [Google Scholar]
- 5.Maguire PD, Sibley GS, Zhou S-M, et al. Clinical and dosimetric predictors of radiation-induced oesophageal toxicity. Int J Rad Onc Biol Phys 1999;45: 97-103 [DOI] [PubMed] [Google Scholar]
- 6.Stankey RM, Roshe J, Sogocio RM. Carcinoma of the lung and dysphagia. Dis Chest 1969;55: 13-17 [DOI] [PubMed] [Google Scholar]
- 7.Makker HK, Chisholm R, Rate AJ, Bancewicz J, Bernstein A. Dysphagia due to secondary achalasia as an early manifestation of squamous cell carcinoma. Postgrad Med J 1995;71: 505-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goldin NR, Burns TW, Ferrante WA. Secondary achalasia: association with adenocarcinoma of the lung and reversal with radiation therapy. Am J Gastroenterol 1983;78: 203-5 [PubMed] [Google Scholar]
- 9.Dougenis D, Morrit GN, Vagianos C, Farr S, Hedley-Brown A. Motility disorders of the esophagus before and after pneumonectomy for lung carcinoma. Eur Surg Res 1996;28: 461-5 [DOI] [PubMed] [Google Scholar]
- 10.Fielding JC, Badenoch J, Millward-Sadler GH. Dysphagia, vomiting and obdurate constipation as a metabolic manifestation of malignancy. J Ir Med Assoc 1973;66: 384-5 [PubMed] [Google Scholar]
- 11.Chu G, Wilson PC, Carter CB, Lennon VA, Roberts-Thompson IC. Intestinal pseudoobstruction, type I antineuronal nuclear antibodies and small cell carcinoma of the lung. J Gastroenterol Hepatol 1993;8: 604-6 [DOI] [PubMed] [Google Scholar]
- 12.Patten J. The brain stem. In: Patten J, ed. Neurological Differential Diagnosis, 2nd edn. London: Springer-Verlag, 1996: 166
- 13.Counsell CE, Collie DA, Grant R. Incidence of intracranial tumours in the Lothian region of Scotland, 1989-90. J Neurol Neurosurg Psychiatry 1996;61: 143-50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Creaven PJ, Perez R, Pendyala L, et al. Unusual central nervous system toxicity in a phase I study of N-1, N-11-diethylnorspermine in patients with advanced malignancy. Invest New Drugs 1997;15: 227-34 [DOI] [PubMed] [Google Scholar]
- 15.Joffe N. Symptomatic gastrointestinal metastases secondary to bronchogenic carcinoma. Clin Radiol 1978;29: 217-25 [DOI] [PubMed] [Google Scholar]
- 16.Antler AS, Ongh Y, Pitchumoni CS, Davidian M, Thelmo W. Gastrointestinal metastases from malignant tumours of the lung. Cancer 1982;49: 170-2 [DOI] [PubMed] [Google Scholar]
- 17.Kim RY, Perry SR, Levy DS. Metastatic carcinoma to the tongue. Cancer 1979;43: 386-9 [DOI] [PubMed] [Google Scholar]
- 18.Higgins PM. Pyloric obstruction due to a metastatic deposit from carcinoma of the bronchus. Can J Surg 1962;5: 438-41 [PubMed] [Google Scholar]
- 19.Barnes BE. Dermatomyositis and malignancy. Ann Intern Med 1976;84: 68-76 [DOI] [PubMed] [Google Scholar]
- 20.Rowell NR, Goodfield MJD. Dermatomyositis. In: Champion RH, Burton JL, Burns DA, Breathnach SM, eds. Textbook of Dermatology, Vol. 3, 6th edn. Oxford: Blackwell Science, 1998: 2555-65 [Google Scholar]
- 21.Romans B, Cohen S. A rheumatologist's view of polymyositis/dermatomyositis: extracutaneous and extramuscular involvement and overlap syndromes. Clin Dermatol 1988;6: 15-22 [DOI] [PubMed] [Google Scholar]
- 22.Tomkin GH. Systemic sclerosis associated with carcinoma of the lung. Br J Dermatol 1969;81: 213-16 [DOI] [PubMed] [Google Scholar]
- 23.Deron P. Dysphagia with systemic diseases. Acta Oto-Rhino-Laryngol Belg 1994;48: 191-200 [PubMed] [Google Scholar]
- 24.Shuangshoti S, Shuangshoti S. Fatal haemorrhage from additional primary esophageal squamous cell carcinoma in a patient previously having primary bronchogenic adenocarcinoma. J Med Assoc Thai 1995;78: 443-8 [PubMed] [Google Scholar]
- 25.Fekete F, Gayet B, Kaisserian G, Zouari Z. Associated cancers of the esophagus and the lung. Chirurgie 1993;119: 59-60 [PubMed] [Google Scholar]
- 26.Shuangshoti S. Primary carcinomas of esophagus and bronchus with presentation simulating primary carcinoma of thyroid gland. J Med Assoc Thai 1982;65: 68-74 [PubMed] [Google Scholar]
- 27.Leon X, Quer M, Diez S, Orus C, Lopez-Pousa A, Burgues J. Second neoplasm in patients with head and neck cancer. Head Neck 1999;21: 204-10 [DOI] [PubMed] [Google Scholar]
- 28.Wilcox CM, Karowe MW. Esophageal infections: etiology, diagnosis and management. Gastroenterologist 1994;2: 188-206 [PubMed] [Google Scholar]
- 29.Redding SW, Zellars RC, Kirkpatrick WR, et al. Epidemiology of oropharyngeal candida colonization and infection in patients receiving radiation for head and neck cancer. J Clin Microbiol 1999;37: 3896-900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Isaac DW, Parham DM, Patrick CC. The role of esophagoscopy in diagnosis and management of esophagitis in children with cancer. Med Pediatr Oncol 1997;28: 299-303 [DOI] [PubMed] [Google Scholar]
- 31.Kaasa S, Mastkaasa A, Thorud E. Toxicity, physical function and everyday activity reported by patients with inoperable non-small cell lung cancer in a randomized trial (chemotherapy versus radiotherapy). Acta Oncol 1988;27: 343-9 [DOI] [PubMed] [Google Scholar]
- 32.Horwich A, Lokich JJ, Bloomer WD. Doxorubicin, radiotherapy and oesophageal stricture. Lancet 1975;ii: 80-3 [DOI] [PubMed] [Google Scholar]