Abstract
A T helper type 1 (Th1) response is essential for resolving genital infections with the mouse pneumonitis biovar of Chlamydia trachomatis (MoPn). However, T-cell-dependent anti-chlamydial antibody is produced and may also contribute to protective immunity. We produced a MoPn-specific CD4 Th2 clone (Th2-MoPn) to study the role of a Th2 response during infection. We found that Th2-MoPn was unable to eradicate chlamydiae from the genital tract (GT) when it was transferred into MoPn-infected nude mice. Mice that received Th2-MoPn produced greater titers of MoPn-specific serum immunoglobulin G (IgG) antibody than mice that received a MoPn-specific Th1 clone (Th1-MoPn) (log10 titers, 1.89 ± 0.84 and 0.58 ± 0.76 [mean ± standard deviation], respectively [P < 0.01]). Also, the IgG isotypes were different for the two groups; whereas IgG1 was associated with Th2-MoPn, IgG2a was associated with Th1-MoPn. Also, infected nude mice that received Th2-MoPn produced higher levels of IgA in vaginal secretions. Although clone Th2-MoPn was detected in the GT, it was less efficient at migrating (112 ± 35.6 labeled Th2 clone cells/105 GT cells) than Th1-MoPn (505 ± 51.6 Th1 clone cells/105 GT cells) (P < 0.001, as determined by a t test). This may have been due to reduced expression of α4β7 and P-selectin ligand 1 on Th2-MoPn. However, Th2-MoPn cells were retained in the GT during chronic infection and comprised 10 to 15% of the total GT cells 80 days after transfer. The data show that the MoPn-specific Th2 cells are important for serum and vaginal antibody production and may accumulate in the GT during chronic infection.
Genital infection caused by the obligate intracellular pathogen Chlamydia trachomatis is the most common bacterial sexually transmitted disease in the United States (8). The majority of genital infections in women are asymptomatic (41) and are often not treated. Sequelae resulting from untreated chlamydial genital infections include pelvic inflammatory disease, fallopian tube damage, ectopic pregnancy, and infertility (41, 46). Thus, development of a vaccine would have the potential to alleviate complications related to prolonged or repeated infections. Previous studies have indicated that cellular immune responses are essential for resolution of and immunity to murine chlamydial infection (40), and CD4+ cells are the primary effector cells (15, 25, 37, 42). Additionally, recent data obtained with the murine model suggest that the development of an ineffective, anti-chlamydial T-cell response may enhance the development of tissue pathology (49). Thus, understanding the factors that regulate immune responses within the genital tract (GT) is necessary for the design of future vaccines.
A T helper cell type 1 (Th1) response predominates in the murine GT during chlamydial infections (7) and is necessary to eradicate the bacteria (15, 31, 50). Anti-chlamydial Th1 cells appear to mediate protection through the production of cytokines, such as gamma interferon (IFN-γ) (13, 35, 39), which has been shown to directly inhibit chlamydial growth (6). In addition, the neutralization of interleukin-12 (IL-12) has also been shown to impede the clearance of chlamydiae (31) since IL-12 is necessary for the production of Th1 cells. Similarly, mice producing lower levels of IFN-γ exhibited a prolonged course of infection (14, 19, 50).
It has been more difficult to define the role of B cells in chlamydial genital infection. B-cell-deficient mice have been shown to resolve genital infections over a time span similar to that observed for immunologically intact control mice (17, 37, 43). However, studies performed with chlamydial immune (recovered from a previous infection with the mouse pneumonitis biovar of C. trachomatis [MoPn]) μ-chain knockout mice suggest that B cells or specific antibody may enhance protective T-cell responses (43, 47, 48). Furthermore, mice that were depleted of CD4 cells following resolution of infection were able to mount a protective response as long as B cells were present (26, 27). While anti-chlamydial immunoglobulin G (IgG) and IgA antibodies have been shown to reduce the amount of infection and inflammation in oviducts (9, 29, 38), the beneficial effect may depend on the antibody isotype. Peterson et al. (32) showed that passive administration of the IgG2b subclass, which is associated with a Th1 response, enhanced the shedding of chlamydiae in vivo, while administration of IgG1 antibodies, which are associated with a Th2 response, did not. Based on the importance of Th2 cells in B-cell maturation and differentiation, further investigation of the role of Th2 cells in B-cell help during chlamydial infection is needed.
The representation of distinct lymphocyte subsets within a local tissue site of inflammation is controlled by mechanisms that regulate lymphocyte recruitment (5). While a number of factors participate in regulating lymphocyte recruitment, particular adhesion molecule-lymphocyte receptor ligand pairs have been shown to influence the recruitment of Th1 and Th2 cell subsets. For instance, Austrup et al. (2) have shown that Th2 cells are virtually prevented from entering tissue sites of inflammation, such as arthritic joints and skin during a delayed-type hypersensitivity response. These authors also demonstrated that the absence of CD62E (E-selectin) and CD62P (P-selectin) binding domains on Th2 cells was responsible for the differential homing. Therefore, anti-chlamydial Th2 cell migration to the GT may be reduced. Since Th2 cells are important for IgA production and IgA is produced locally within the genital mucosa (21), reduced Th2 cell migration could result in lower IgA antibody levels. It is important to note that IgG levels are greater than or similar to the IgA levels found in vaginal secretions (24), which differ from the increased levels of IgA antibody found in other mucosal secretions. In this paper we describe production of a MoPn-specific Th2 clone (Th2-MoPn), which was used to examine the influence of a Th2 immune response in chlamydial immunity.
MATERIALS AND METHODS
Antibodies.
The following monoclonal antibodies were utilized in a flow cytometry analysis. Hybridomas secreting rat anti-CD4 (TIB207, IgG2b) and anti-CD54 (CRL1878, IgG2b) were purchased from the American Type Culture Collection (Rockville, Md.), and staining was performed by using undiluted supernatants. Rat anti-CD3 (29B, IgG2b; Gibco BRL, Gaithersburg, Md.) was used at a concentration of 10 μg/ml. The following rat monoclonal antibodies were purchased from PharMingen (San Diego, Calif.): anti-CD49d (9C10, IgG2a), anti-CD45RB (16A, IgG2a), anti-CD62L (Mel-14, IgG2a), anti-CD44 (IM7, IgG2b), anti-β7 (M293, IgG2a), anti-LPAM-1 (DATK-32, IgG2a), and anti-CD11a (M17/4, IgG2a). The concentrations used for staining ranged from 10 to 25 μg/ml. A rat IgG2b(κ) myeloma protein (IR863) and the rat monoclonal antibody IgG2a (R35-95) (PharMingen) were used as negative controls. Purified P-selectin-IgG1 fusion protein (PharMingen) was used at a concentration of 10 μg/ml. The phycoerythrin-conjugated affinity-purified F(ab)′2 fragment of goat anti-human IgG was purchased from Jackson Laboratories (West Grove, Pa.). The following anti-mouse monoclonal antibodies were utilized in T-cell purification by negative selection with magnetic beads. A hybridoma secreting rat anti-B220 (TIB-146, IgM) was purchased from the American Type Culture Collection. Rat anti-CD8 (53-6.7, IgG2a) and rat anti-MAC-1 (M1/70, IgG2b) were purchased from PharMingen.
Experimental animals.
Immunocompetent BALB/c mice and BALB/c nude (nu/nu) mice were purchased from Harlan-Sprague Dawley (Indianapolis, Ind.) and were housed under laminar flow by using specific-pathogen-free conditions according to the American Association of Accreditation of Laboratory Animal Care guidelines. The UAMS Institutional Animal Care and Use Committee approved experimental procedures. Sterilized food and water were supplied ad libitum, and the room was maintained with a cycle consisting of 12 h of light and 12 h of darkness.
Infection of mice.
Seven days prior to infection, mice received 2.5 mg of progesterone (Depo-Provera; Upjohn, Kalamazoo, Mich.) to maintain them in a state of anestrus (30, 37). Each mouse was infected by intravaginal inoculation of 1 × 107 inclusion-forming units (IFU) (2.4 × 103 50% infective doses) of MoPn in 30 μl of sucrose-phosphate-glutamate buffer. Infection was monitored every 10 days by isolation of MoPn from cervical-vaginal swabs as previously described (37).
Isolation and maintenance of T-cell lines and clones.
A MoPn-specific Th2 clone was isolated from a MoPn-specific Th2 line. The line was established by boosting BALB/c mice subcutaneously with 108 IFU of MoPn, followed by infection 5 weeks later. CD4+ lymphocytes were purified by negative selection from cervical lymph nodes (CLN) 1 week later. Briefly, lymph node cells were labeled with rat monoclonal antibodies against CD8, B220, and MAC-1 (see above). The cells were then incubated with goat anti-rat antibody conjugated to 10-nm magnetic beads as specified by the manufacturer (Miltenyl Biotechnological Equipment, Bergisch-Gladbach, Germany) and passed over a ferromagnetic column. The recovered cells were more than 95% CD4+ and less than 1% CD8+, 1% B220+, and 1% MAC-1+ (data not shown). The isolated CD4 cells were generated by restimulation in vitro in complete medium (CM), which consisted of RPMI 1640 (Gibco Laboratories, Grand Island, N.Y.) supplemented with 15 mM HEPES (Gibco), 1.0 mM sodium pyruvate (Gibco), 0.1 mM nonessential amino acids (Gibco), 2 mM glutamine (Gibco), 100 U of penicillin (Gibco) per ml, 100 μg of streptomycin (Gibco) per ml, 10% heat-inactivated fetal calf serum (Atlanta Biologicals, Norcross, Ga.), and 2 × 10−5 M 2-mercaptoethanol (Sigma), with 5 μg of UV-inactivated MoPn elementary bodies harvested from HeLa monolayers per ml and γ-irradiated syngeneic splenocytes as a source of antigen-presenting cells (APCs). IL-4 (200 U/ml) (Sigma) and anti-IFN-γ (1 μg/ml; PharMingen) were added to enhance Th2 differentiation. Following 4 days of culture, wells containing proliferating cells were expanded with MoPn in CM with IL-4. The wells that contained proliferating cells were further expanded as isolated clones. One clone, designated Th2-MoPn, was characterized and used for further studies.
A keyhole limpet hemocyanin (KLH)-specific Th1 line was derived from BALB/c mice that received KLH (100 μg) in complete Freund's adjuvant via intradermal injection at the base of the tail. Two weeks later the mice received a second injection of KLH in incomplete Freund's adjuvant. Ten days later, CD4 cells were isolated by negative selection from inguinal lymph nodes. The CD4 cells were stimulated in vitro in CM containing 12.5% (vol/vol) concanavalin A (ConA)-conditioned medium, 5 μg of KLH per ml, and 5 × 107 gamma-irradiated syngeneic APCs. The T cells were expanded and maintained in vitro as a primary line with biweekly treatment with antigen, APCs, and 12.5% (vol/vol) ConA-conditioned medium.
Lymphocyte proliferation assay.
T cells (5 × 104 cells) were cultured in the presence of 106 gamma-irradiated (2,000 rads) syngeneic splenocytes as APCs and antigen (5 μg/ml) as previously described (7). Proliferation was measured after 48 h by [3H]thymidine uptake after cells were harvested onto a self-aligning RG glass fiber filter (Packard, Meriden, Conn.) by using a Packard FilterMate cell harvester. The radioactive counts were determined with a Packard Matrix 96 direct beta counter. Control wells contained T cells and APCs alone without antigen.
ELISPOT assay.
We utilized the enzyme-linked immunospot (ELISPOT) assay to determine the frequency of IFN-γ- and IL-4-secreting cells in both MoPn-specific T-cell clones and the KLH line (7). Each 96-well plate with a nitrocellulose base (Millititer HA; Millipore Corp., Bedford, Mass.) was coated overnight at 4°C with 2 μg of antibodies (anti-murine IFN-γ or anti-IL-4; PharMingen) per ml. The plates were blocked for 1 h at 37°C with medium containing 5% fetal bovine serum. Various numbers of cells (1 × 105 to 1 × 103 cells) were added to individual wells and incubated overnight at 37°C in the presence of 5% CO2. The plates were then washed with phosphate-buffered saline containing 0.1% Tween 20, and biotinylated anti-cytokine antibodies (4 μg/well) were then added to individual wells. Following overnight incubation at 4°C, the plates were washed and incubated for 1 h with 100 μl of a 2.5-μg/ml avidin-peroxidase solution (Vector, Burlingame, Calif.). The plates were then developed with 3-amino-9-ethylcarbazole (Vector). The reddish brown spots were enumerated with a dissecting microscope. Control wells that were not coated with anti-cytokine antibodies were negative. The number of spot-forming cells was calculated by determining the mean number of spots counted in triplicate samples.
Adoptive transfer of T cells.
Clones Th1-MoPn and Th2-MoPn and cell line KLH-1 were harvested separately 3 to 5 days following stimulation with ConA-conditioned media. Following centrifugation over lympholyte-M (Cedarlane Laboratories), 1 × 107 cells per mouse were transferred intravenously via the retroorbital plexus of nude mice that had been infected 10 days previously (15). The infection was monitored by isolation of chlamydiae from cervical-vaginal swabs as described above. Animals were swabbed every 10 days for 80 days following transfer. To monitor lymphocyte migration in vivo, T-cell populations were labeled with a fluorescent dye, PKH-26 (Sigma), as previously described (11). The labeled samples were checked for viability and fluorescence intensity prior to adoptive transfer. Eighteen hours after transfer into recipient mice, the tissues were harvested and processed as described previously (11). The number of fluorescent cells in each tissue was determined by acquiring 10,000 fluorescently labeled cells by flow cytometry while the total number of cells analyzed was tabulated. The frequency of labeled cells in each tissue was expressed as the number of labeled cells per 100,000 total cells analyzed with the flow cytometer.
Flow cytometry.
Cell populations of interest were stained for flow cytometric analysis as previously described (18). The flow cytometry analysis was performed with a FACScan (Becton Dickinson, San Jose, Calif.) following calibration with CaliBRITE beads and AutoCOMP software beads (Becton Dickinson). Dead cells were excluded by forward and 90o angle light scatter, and at least 10,000 gated cells were analyzed per sample.
Determination of antibody levels by enzyme-linked immunosorbent assay.
Serum and vaginal secretions were collected 4 weeks after adoptive transfer of T cells, and antibody titers were determined as previously described (37). The antibody titer was calculated by determining the reciprocal of the last dilution with an optical density that was 3 standard deviations above the optical density of background wells (wells that did not contain sample).
Western blot analysis.
Western blot assays were used to identify serum antibodies produced against various chlamydial proteins, as previously described (36). Nitrocellulose strips containing electrophoretically separated MoPn were incubated with serum samples for 2 h at room temperature with constant agitation. The nitrocellulose strips were then washed three times in 155 mM NaCl. Bound antibody was identified by incubating the strips with peroxidase-conjugated anti-mouse IgG (diluted 1:10,000, vol/vol) for 1 h. After washing, the bound conjugated antibody was visualized by development in a 4-chloro-naphthol solution.
Immunohistochemistry.
Tissues were harvested and staining was carried out as previously described (20), with the following exceptions. After a tissue-blocking step with goat serum, the primary antibody (rat anti-mouse CD62P) and the rat monoclonal antibody IgG2a (R35-95; PharMingen) were incubated with tissue sections for 45 min at room temperature in a humidified chamber, and then the preparations were washed. A goat anti-rat IgG F(ab′)2 antibody conjugated to biotin at a concentration of 14 μg/ml (BioSource International) and then streptavidin conjugated to horseradish peroxidase (Zymed, San Francisco, Calif.) were added next, and the tissue sections were incubated for 45 min. Slides were developed as previously described (20). Photographs were generated by scanning the microscope slides with an Olympus DP10 color digital video camera.
Statistics.
Statistical differences between control and experimental groups were assessed with a t test, and significance was indicated by a P value of <0.05.
RESULTS
Characterization of a MoPn-specific Th2 clone.
Subcutaneous immunization of mice with MoPn was shown to produce an immune response containing both IL-4- and IFN-γ-producing cells during genital infection (19). We used this form of immunization to isolate a MoPn-specific Th2 clone (Th2-MoPn). This clone was found to express CD3, CD4, α/β T-cell receptor, and low levels of CD45RB and CD62L (data not shown). Also, Th2-MoPn was specific for MoPn and did not markedly proliferate to other chlamydial agents or nonchlamydial antigens (Table 1). In addition, the proliferative responses of a control CD4 Th1 cell line specific for KLH, designated KLH-1, and a previously described MoPn-specific CD4 Th1 clone (15) (Th1-MoPn) are also shown in Table 1. We determined the cytokine profile of the new clone and cell line by using an ELISPOT assay. As shown in Table 1, clone Th2-MoPn exhibited a Th2 cytokine profile in response to MoPn characterized by a high frequency of IL-4-producing cells in the absence of detectable IFN-γ. The KLH-1 cell line was predominately Th1 in character but also contained some IL-4-producing cells. The cytokine profile of Th1-MoPn has been previously shown to include IFN-γ and tumor necrosis factor alpha but not IL-2 or IL-4 (15).
TABLE 1.
Characterization of clone Th2-MoPn
| Antigen or cytokine |
3H incorporation (103 counts)a
|
No. of cytokine-producing cells/106 cellsb
|
||||
|---|---|---|---|---|---|---|
| Th2-MoPn | KLH-1 | Th1-MoPn | Th2-MoPn | KLH-1 | Th1-MoPn | |
| Antigens | ||||||
| MoPn | 8.0 ± 3.4 | 0.9 ± 0.3 | 12.3 ± 6.7 | |||
| C. trachomatis serovar E | 0.7 ± 0.2 | 1.1 ± 0.8 | 1.0 ± 1.6 | |||
| GPICc | 0.5 ± 0.3 | 1.1 ± 1.2 | 0.6 ± 0.2 | |||
| KLH | 0.6 ± 0.4 | 4.6 ± 2.3 | 0.4 ± 0.6 | |||
| Pigeon cytochrome c | 0.4 ± 0.3 | 0.4 ± 0.5 | 0.6 ± 0.1 | |||
| None | 0.5 ± 0.2 | 0.4 ± 0.2 | 0.5 ± 0.2 | |||
| Cytokines | ||||||
| IFN-γ | 0 | 6,139 ± 680 | NDd | |||
| IL-4 | 308,000 ± 21,000 | 2,039 ± 467 | ND | |||
Single-cell suspensions of 5 × 104 cells were stimulated in triplicate with 106 irradiated APCs and 5 μg of antigen per ml. After 2 days of culture [3H]thymidine was added, and incorporation was measured 24 h later. The values are means ± standard deviations for two separate experiments.
The values are means ± standard deviations for triplicate wells following stimulation with 5 μg of MoPn per ml (Th2-MoPn and Th1-MoPn) or 5 μg of KLH per ml (KLH-1).
GPIC, guinea pig agent of inclusion conjunctivitis.
ND, assay not performed.
Protective capacity of clone Th2-MoPn in vivo.
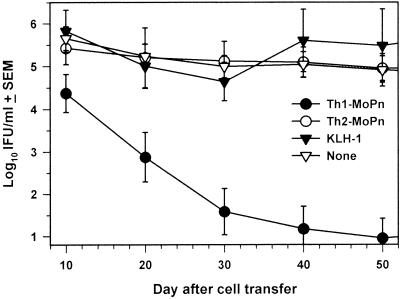
To explore the role of MoPn-specific Th2 cells in immunity during genital infection with Chlamydia, we adoptively transferred Th2-MoPn into recently infected BALB/c nude mice which were unable to clear a chlamydial infection. Briefly, 107 cells were adoptively transferred intravenously into nude mice that had been infected 10 days earlier. For comparison, equal numbers of Th1-MoPn and KLH-1 cells were also transferred into additional groups of mice. As shown in Fig. 1, the mice that received Th1-MoPn rapidly cleared the infection, as reported previously (15). By day 50 after cell transfer, 11 of 15 (73.3%) of the mice that received Th1-MoPn were culture negative. In contrast, none of the nude mice that received Th2-MoPn or KLH-1 or control mice had resolved the infection. These mice continued to shed high amounts of MoPn (>4 log10 IFU) for up to 80 days after cell transfer (data not shown). These results indicate that an anti-chlamydial Th2 response is not able to eradicate chlamydiae from the infected GT.
FIG. 1.
Inability of Th2-MoPn to resolve a chlamydial genital infection in nude mice. Clones Th2-MoPn and Th1-MoPn and cell line KLH-1 (1 × 107 cells) were transferred intravenously via the retroorbital plexus into nude mice that had been infected 10 days previously. The infection was monitored by determining the number of IFU of MoPn obtained with cervical-vaginal swabs. Animals were swabbed every 10th day for 80 days following transfer. The values are means ± standard errors of the means for 5 (KLH-1), 10 (Th2-MoPn), or 15 (Th1-MoPn and no cells) mice in two separate experiments.
MoPn-specific antibody production induced by Th2-MoPn in vivo.
The ability of Th2-MoPn to support antibody production in infected nude mice was assessed by using an enzyme-linked immunosorbent assay with serum collected 28 days after the adoptive transfer. Although Th2-MoPn was unable to eradicate MoPn from genital tissues in infected recipients, mice that received Th2-MoPn developed significantly greater MoPn-specific IgG serum antibody titers (log10 titers, 1.9 ± 0.8 [mean ± standard deviation]) than mice that received Th1-MoPn (log10 titers, 0.6 ± 0.8; P < 0.01). The antibody titers for mice that received Th2-MoPn were similar to the levels found after vaginal infection of immunologically competent BALB/c mice (15, 19). In addition, the IgG isotype differed depending on the clone transferred. Mice that received Th2-MoPn produced IgG1 (log10 titers, 2.1 ± 0.8) in response to MoPn infection, while mice that received Th1-MoPn developed anti-MoPn IgG2a serum antibody (log10 titers, 0.4 ± 0.7). Furthermore, significantly greater levels of MoPn-specific IgA antibody were detected in vaginal washes of mice that received Th2-MoPn (log10 titers, 1.8 ± 0.4) than in vaginal washes of mice that received Th1-MoPn (log10 titers, 0.5 ± 1.0) (P < 0.005). Anti-MoPn antibodies were not detected in the serum following the transfer of KLH-1 or in control mice that did not receive T cells. Despite these differences, we noted similarities in the antibody responses to MoPn of the groups that received clones Th2-MoPn and Th1-MoPn. Antibodies against the 44-kDa MoPn major outer membrane protein, as well as a 62-kDa outer membrane protein 2 (Omp2) and other bands, were found in the sera from both groups of mice by Western blot analysis (data not shown). Taken together, these data show that in vivo Th2-MoPn supported the production of primarily IgG1 and IgA antibodies that bind to multiple chlamydial antigens.
In vivo homing of a Th2 clone during MoPn genital infection.
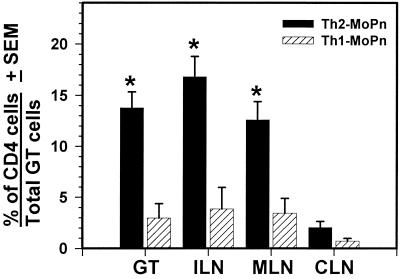
To confirm that clone Th2-MoPn survived in vivo, we sacrificed the mice 80 days following chlamydial genital infection and examined single-cell suspensions from various tissue sites. Immunofluorescent staining revealed that CD4+ cells were indeed present in the GT, iliac lymph nodes (ILN), and mesenteric lymph nodes (MLN) of mice that had received clone Th2-MoPn (Fig. 2). However, there was a significantly lower percentage of CD4+ cells in the GT, ILN, and MLN (P < 0.001) but not in the cervical lymph nodes in nude mice that had received clone Th1-MoPn (Fig. 2). This is consistent with our previous findings showing that clone Th1-MoPn does not readily migrate to the GT and associated lymph nodes in the absence of an active infection (11). However, some Th1-MoPn cells continue to migrate to the GT following resolution of a infection. These cells may represent a population of memory cells that retain tissue-specific migratory properties (23). In addition, we were unable to detect CD4+ cells in any tissues examined from control mice that did not receive T cells (data not shown). These data show that Th2-MoPn can migrate to infected GT tissues.
FIG. 2.
Immunofluorescent staining of T cells in the GT of nude mice 80 days following transfer of Th1-MoPn or Th2-MoPn. Single-cell suspensions were prepared from tissues of nude mice, processed, and stained with an anti-CD4 monoclonal antibody. The values are means ± standard deviations for 10 mice per group. An asterisk indicates that the number of CD4+ cells was significantly greater than the number of CD4+ cells for mice that received clone Th1-MoPn (P < 0.001). CLN, cervical lymph nodes.
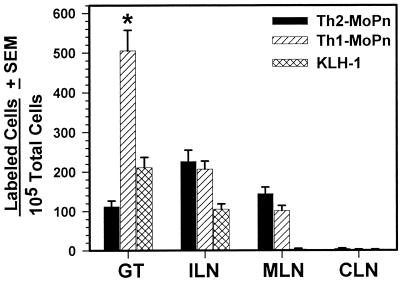
Due to the persistence of chlamydiae in the GT, we expected to find clone Th2-MoPn in lymphoid organs of mice following adoptive transfer. However, we were surprised to detect Th2-MoPn in the GT because it was previously shown that only Th1 cells were able to efficiently migrate to sites of tissue inflammation (2). To determine if the Th1 and Th2 clones differed in the ability to migrate to the MoPn-infected GT, we compared the rates of migration of Th1-MoPn and Th2-MoPn in vivo. Previous studies have shown that the greatest number of Th1-MoPn cells migrate to the GT 7 days after infection (11). Therefore, T cells were labeled with the fluorescent dye PKH-26 and transferred (5 × 106 cells/mouse) to BALB/c mice 7 days after vaginal inoculation. Control mice received 5 × 106 unlabeled clone cells. Eighteen hours later, the mice were sacrificed, and the numbers of fluorescently labeled cells in various tissues were determined by flow cytometry. As shown in Fig. 3, we found that significantly fewer Th2-MoPn cells than Th1-MoPn cells migrated to the GT (P < 0.0001). However, similar numbers of Th2-MoPn and Th1-MoPn cells migrated to the ILN and MLN. Compared to Th1-MoPn, few KLH-1 cells also appeared in the GT and ILN but did not migrate to the MLN, consistent with our previous report (11). In addition, none of the transferred T cells appeared in the cervical lymph nodes. Also, when each of the clones was adoptively transferred into uninfected mice, all tissues were negative for fluorescent cells (data not shown). Thus, Th2-MoPn does not appear to be actively recruited to the GT during infection since its migration rate is lower than the background migration rate displayed by a non-antigen-specific T-cell population, KLH-1. However, Th2-MoPn can enter GT tissues and appears to be retained in the GT during persistent infection (Fig. 2).
FIG. 3.
Abilities of Th1-MoPn and Th2-MoPn to migrate to the GT 7 days after MoPn infection. Each clone was labeled with PKH-26, and 5 × 106 cells were intravenously transferred into mice infected for 7 days with MoPn. Separate mice received unlabeled cells as a control. Single-cell suspensions were isolated from the tissues 18 h after transfer and analyzed by flow cytometry. Negligible numbers of labeled positive cells (0 to 4 cells) were detected in control mice, and these values were subtracted from those obtained for mice which received labeled cells. The values are means ± standard deviations for two separate experiments in which five mice per group were used for each experiment. An asterisk indicates that a value is significantly greater than the value obtained for clone Th2-MoPn (P < 0.0001). CLN, cervical lymph nodes.
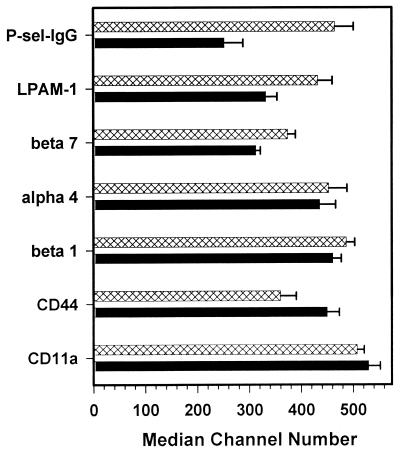
The recruitment of lymphocytes to sites of inflammation is facilitated by interactions between adhesion molecules expressed by local endothelial cells and homing receptors expressed on lymphocytes (5). Recently, it has been shown that differences in the abilities of Th1 and Th2 cells to bind to CD62P and CD62E resulted in migration differences in vivo (2). Although P-selectin ligand 1 (PSGL-1) is expressed on both subsets of T cells (4), binding to CD62P requires posttranslational glycoslylation of PSGL-1, which occurs in Th1 cells (45). T cells also bind to CD62E via PSGL-1, and CD62E-binding T cells are a subset of T cells that also bind CD62P (10, 12). In order to determine if differences in CD62P or CD62E binding could account for the observed differences in GT migration, we compared the abilities of Th2-MoPn and Th1-MoPn to bind CD62P conjugated to human IgG1 (P-sel-IgG), as well as the expression of other T-cell homing molecules. As shown in Fig. 4, Th2-MoPn bound P-sel-IgG with less intensity than Th1-MoPn bound P-sel-IgG (P = 0.055). The mucosal homing receptors β7 and lymphocyte Peyer's patch adhesion molecule 1 (LPAM-1) were also expressed at lower levels on Th2-MoPn. However, not all homing receptors were expressed at lower levels on Th2-MoPn. CD44 was expressed at higher levels on Th2-MoPn than on Th1-MoPn. Also, the two clones expressed similar levels of adhesion molecules α4, β1, and CD11a. Thus, differences in the abilities of Th1-MoPn and Th2-MoPn to migrate to the GT may, in part, be due to reduced expression of PSGL-1 and LPAM-1 (2, 11, 44, 45).
FIG. 4.
Homing receptor expression on clones Th1-MoPn and Th2-MoPn. Single-cell suspensions of clones Th2-MoPn (solid bars) and Th1-MoPn (cross-hatched bars) were stained for the stated surface markers. The values are averages ± standard deviations for two separate experiments.
CD62P expression in the GT during MoPn infection.
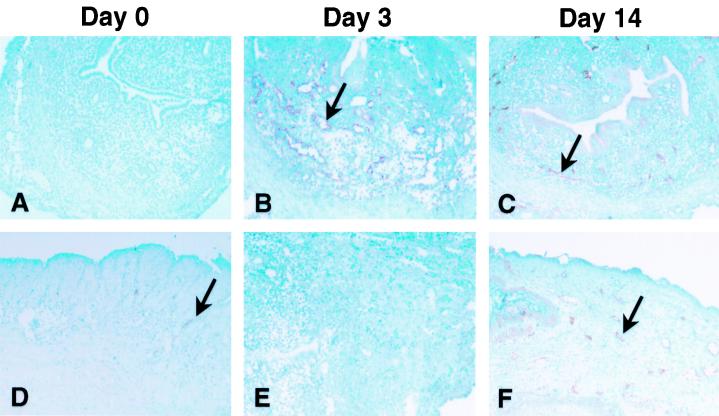
We have previously reported that the ligand for LPAM-1, mucosal addressin cell adhesion molecule 1 (MAdCAM-1), is induced in the GT following MoPn infection (18, 20). To establish a role for PSGL-1 in T-cell migration to the GT, we examined the expression of CD62P in the GT during infection. As shown in Fig. 5, CD62P was not expressed in the upper GT (uterine horn) of uninfected mice, but few CD62P-staining vessels could be found in the cervical-vaginal region. This finding was similar to the data obtained for CD54 (18). After MoPn vaginal infection, CD62P expression was induced on venules in the upper GT and increased in the cervical-vaginal region. The early expression of CD62P, noted 3 days after infection, preceded expression of MAdCAM-1 in the upper GT. This is not surprising since CD62P is stored in granules within endothelial cells, known as Weibel-Palade bodies, whereas MAdCAM-1 must be synthesized (3). We also examined CD62E expression in the GT but did not observe staining on venules before or after infection (data not shown). Thus, the induction of CD62P on the GT endothelium during Chlamydia infection suggests that PSGL-1 also contributes to the homing potential of anti-chlamydial T cells.
FIG. 5.
Expression of CD62P in the GT during MoPn infection. Frozen sections of the upper GT (uterine horn) (A to C and E) or the cervical-vaginal region (D and F) were stained for expression of CD62P (A to D and F) or an irrelevant control antibody (E). The arrows indicate endothelial venules that express CD62P. Magnification, ×400.
DISCUSSION
In this study we evaluated the contribution of Th2 CD4 cells in anti-chlamydial immunity. Protective immunity against infection with the bacterium C. trachomatis is maximal when both CD4 and B cells respond (27). Although the role of Chlamydia-specific antibody and/or B cells has not been elucidated yet, Th2 cells are very effective in providing help for B-cell maturation and differentiation. We found that adoptive transfer of clone Th2-MoPn was not able to eradicate chlamydiae from the GT of nude mice. However, we found that this clone was functionally active, as shown by its ability to provide B-cell help and facilitate antibody production in vivo.
Typically, Th1 and Th2 dominant responses are also reflected in the IgG isotype produced (1). Antibody isotypes associated with a Th2 response appear to be more beneficial in reducing chlamydial infection than isotypes associated with a Th1 response. For instance, IgA in vaginal secretions has been associated with resolution of infection (25). Also, differences in neutralization of infection in vitro were observed between anti-MoPn IgG2b (Th1) and IgG1 (Th2) isotypes when FcRγIII-expressing HeLa cells were used (33). IgG2b was shown to neutralize infectivity in cells lacking FcRγIII but enhanced infection in HeLa cells that expressed FcRγIII. IgG1 has a weak affinity for FcRγIII, and neutralization with this isotype was not affected by Fc receptor expression. Likewise, a similar effect was observed in vivo; under these conditions a slight reduction in the incidence of infected mice was observed when anti-MoPn IgG1 was passively transferred with IgG1 prior to infection (32). Although serum antibody against chlamydiae was detected both in mice that received the Th1 clone and in mice that received the Th2 clone, we found differences in the immunoglobulin isotypes produced in response to infection. Likewise, we noted that mice that received Th1-MoPn developed anti-MoPn-IgG2a but not IgG1 antibodies, while the reverse was observed in mice that received Th2-MoPn. However, in this study, we did not observe a decrease in chlamydial burden over an 80-day period in mice that received Th2-MoPn, despite the fact that they had significantly greater serum antibody titers than mice given Th1-MoPn. These data support recent studies (26, 27) showing that antibodies may not be effective once an infection is established but may be important in reducing the infection load when they are present before the infection is initiated.
While clone Th2-MoPn is a clone and may not represent a polyclonal Th2 response, adoptive transfer of this clone resulted in the production of antibodies against multiple chlamydial antigens (36). This suggests that a single chlamydia-specific clone may interact with multiple B-cell clones to facilitate production of antibody to multiple chlamydial antigens. In a different system, it has been shown that T cells specific for one epitope can provide help for B cells with specificity for widely separated epitopes in the same protein (22). This concept of linked recognition requires that the T- and B-cell epitopes are physically linked, like the epitopes in a complex protein or organism (16). This may also occur during Chlamydia infection since Ortiz et al. (28) demonstrated that T-cell responses to major outer membrane protein are predominately directed against regions that are constant across chlamydial strains. Also, Ramsey et al. (34) showed that mice previously infected with a murine Chlamydia biovar were protected against subsequent infection with the human biovar and visa versa. In addition, antibodies were found that reacted with both biovars of Chlamydia. However, it is also possible that a single B-cell clone could produce cross-reactive antibody against different chlamydial antigens.
We also found that although both Th2-MoPn and Th1-MoPn are specific for chlamydiae, there is a significant difference in the abilities of these clones to migrate to the infected GT. We found that Th2-MoPn did not bind to CD62P as readily as Th1-MoPn. CD62P has been shown to influence differences in the homing potentials of Th1 and Th2 populations, with CD62P binding necessary for recruitment of Th1 cells into sites of tissue inflammation (2, 44). As expected, we found that clone Th2-MoPn expressed lower levels of PSGL-1. Also, CD62P was detected on the endothelium of MoPn-infected GT. Thus, diminished expression of PSGL-1 could conceivably contribute to the reduced capacity of Th2-MoPn to migrate to the GT. Experiments are under way to examine the influence of CD62P on chlamydial infection in knockout mice.
Acknowledgments
We thank Anthony W. Butch for helpful discussions and for reading the manuscript.
This work was supported by National Institutes of Health grant AI-26328 (to K.A.K.).
Editor: J. D. Clements
REFERENCES
- 1.Abbas, A. K., K. M. Murphy, and A. Sher. 1996. Functional diversity of helper T lymphocytes. Nature 383:787-793. [DOI] [PubMed] [Google Scholar]
- 2.Austrup, F., D. Vestweber, E. Borges, M. Lohning, R. Brauer, U. Herz, H. Renz, R. Hallmann, A. Scheffold, A. Radbruch, and A. Hamann. 1997. P- and E-selectin mediate recruitment of T helper 1 but not T helper 2 cells into inflamed tissues. Nature 385:81-83. [DOI] [PubMed] [Google Scholar]
- 3.Bevilacqua, M. P. 1993. Endothelial-leukocyte adhesion molecules Annu. Rev. Immunol. 11:767-804. [DOI] [PubMed] [Google Scholar]
- 4.Borges, E., W. Tietz, M. Steegmaier, T. Moll, R. Hallmann, A. Hamann, and D. Vestweber. 1997. P-selectin glycoprotein ligand-1 (PSGL-1) on T helper 1 but not on T helper 2 cells binds to P-selectin and supports migration into inflamed skin. J. Exp. Med. 185:573-578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Butcher, E. C., and L. J. Picker. 1996. Lymphocyte homing and homeostasis. Science 272:60-66. [DOI] [PubMed] [Google Scholar]
- 6.Byrne, G. I., L. K. Lehmann, and G. J. Landry. 1986. Induction of tryptophan catabolism is the mechanism for gamma interferon-mediated inhibition of intracellular Chlamydia psittaci replication in T24 cells. Infect. Immun. 53:347-351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cain, T. K., and R. G. Rank. 1995. Local Th1-like responses are induced by intravaginal infection of mice with the mouse pneumonitis biovar of Chlamydia trachomatis. Infect. Immun. 63:1784-1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention. 1997. Chlamydia trachomatis genital infections—United States, 1995. JAMA 277:952-953. [PubMed] [Google Scholar]
- 9.Cotter, T. W., Q. Meng, Z. L. Shen, Y. X. Zhang, H. Su, and H. D. Caldwell. 1995. Protective efficacy of major outer membrane protein-specific immunoglobulin A (IgA) and IgG monoclonal antibodies in a murine model of Chlamydia trachomatis genital tract infection. Infect. Immun. 63:4704-4714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fuhlbrigge, R. C., J. D. Kieffer, D. Armerding, and T. S. Kupper. 1997. Cutaneous lymphocyte antigen is a specialized form of PSGL-1 expressed on skin-homing T cells. Nature 389:978-981. [DOI] [PubMed] [Google Scholar]
- 11.Hawkins, R. A., R. G. Rank, and K. A. Kelly. 2000. Expression of mucosal homing receptor α4β7 is associated with enhanced migration to the Chlamydia-infected murine genital mucosa in vivo. Infect. Immun. 68:5587-5594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hirata, T., G. Merrill-Skoloff, M. Aab, J. Yang, B. C. Furie, and B. Furie. 2000. P-selectin glycoprotein ligand 1 (PSGL-1) is a physiological ligand for E-selectin in mediating T helper 1 lymphocyte migration. J. Exp. Med. 192:1669-1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Igietseme, J. U. 1996. Molecular mechanism of T-cell control of Chlamydia in mice: role of nitric oxide in vivo. Immunology 88:1-5. [PMC free article] [PubMed] [Google Scholar]
- 14.Igietseme, J. U., G. A. Ananaba, J. Bolier, S. Bowers, T. Moore, T. Belay, F. O. Eko, D. Lyn, and C. M. Black. 2000. Suppression of endogenous IL-10 gene expression in dendritic cells enhances antigen presentation for specific Th1 induction: potential for cellular vaccine development. J. Immunol. 164:4212-4219. [DOI] [PubMed] [Google Scholar]
- 15.Igietseme, J. U., K. H. Ramsey, D. M. Magee, D. M. Williams, T. J. Kincy, and R. G. Rank. 1993. Resolution of murine chlamydial genital infection by the adoptive transfer of a biovar-specific TH1 lymphocyte clone. Reg. Immunol. 5:317-324. [PubMed] [Google Scholar]
- 16.Janeway, C. A., P. Travers, M. Walport, and M. Sholmchik (ed.). 2001. Immunobiology, 5th ed., p. 341-380. Garland Publishing, New York, N.Y.
- 17.Johansson, M., M. Ward, and N. Lycke. 1997. B-cell-deficient mice develop complete immune protection against genital tract infection with Chlamydia trachomatis. Immunology 92:422-428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kelly, K. A., and R. G. Rank. 1997. Identification of homing receptors that mediate the recruitment of CD4 T cells to the genital tract following intravaginal infection with Chlamydia trachomatis. Infect. Immun. 65:5198-5208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kelly, K. A., E. A. Robinson, and R. G. Rank. 1996. Initial route of antigen administration alters the T-cell cytokine profile produced in response to the mouse pneumonitis biovar of Chlamydia trachomatis following genital infection. Infect. Immun. 64:4976-4983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kelly, K. A., J. C. Walker, S. H. Jameel, H. L. Gray, and R. G. Rank. 2000. Differential regulation of CD4 lymphocyte recruitment between the upper and lower regions of the genital tract during Chlamyida infection. Infect. Immun. 68:1519-1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kutteh, W. H., S. J. Prince, K. R. Hammond, C. C. Kutteh, and J. Mestecky. 1996. Variations in immunoglobulins and IgA subclasses of human uterine cervical secretions around the time of ovulation. Clin. Exp. Immunol. 104:538-542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lowenadler, B., and N. Lycke. 1994. Fusion proteins with heterologous T helper epitopes. Recombinant E. coli heat-stable enterotoxin proteins. Int. Rev. Immunol. 11:103-111. [DOI] [PubMed] [Google Scholar]
- 23.Mackay, C. R. 1992. Migration pathways and immunologic memory among T lymphocytes. Semin. Immunol. 4:51-58. [PubMed] [Google Scholar]
- 24.Masson, P. L., J. F. Heremans, and J. Ferin. 1969. Clinical importance of the biochemical changes in the female genital tract. I. Studies on the proteins of cervical mucus. Int. J. Fertil. 14:1-7. [PubMed] [Google Scholar]
- 25.Morrison, R. P., K. Feilzer, and D. B. Tumas. 1995. Gene knockout mice establish a primary protective role for major histocompatibility complex class II-restricted responses in Chlamydia trachomatis genital tract infection. Infect. Immun. 63:4661-4668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Morrison, S. G., and R. P. Morrison. 2001. Resolution of secondary Chlamydia trachomatis genital tract infection in immune mice with depletion of both CD4+ and CD8+ T cells. Infect. Immun. 69:2643-2649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morrison, S. G., H. Su, H. D. Caldwell, and R. P. Morrison. 2000. Immunity to murine Chlamydia trachomatis genital tract reinfection involves B cells and CD4+ T cells but not CD8+ T cells. Infect. Immun. 68:6979-6987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ortiz, L., K. P. Demick, J. W. Petersen, M. Polka, R. A. Rudersdorf, B. Van der Pol, R. Jones, M. Angevine, and R. DeMars. 1996. Chlamydia trachomatis major outer membrane protein (MOMP) epitopes that activate HLA class II-restricted T cells from infected humans. J. Immunol. 157:4554-4567. [PubMed] [Google Scholar]
- 29.Pal, S., I. Theodor, E. M. Peterson, and L. M. de la Maza. 1997. Monoclonal immunoglobulin A antibody to the major outer membrane protein of the Chlamydia trachomatis mouse pneumonitis biovar protects mice against a chlamydial genital challenge. Vaccine 15:575-582. [DOI] [PubMed] [Google Scholar]
- 30.Parr, M. B., L. Kepple, M. R. McDermott, M. D. Drew, J. J. Bozzola, and E. L. Parr. 1994. A mouse model for studies of mucosal immunity to vaginal infection by herpes simplex virus type 2. Lab. Investig. 70:369-380. [PubMed] [Google Scholar]
- 31.Perry, L. L., K. Feilzer, and H. D. Caldwell. 1997. Immunity to Chlamydia trachomatis is mediated by T helper 1 cells through IFN-gamma-dependent and -independent pathways. J. Immunol. 158:3344-3352. [PubMed] [Google Scholar]
- 32.Peterson, E. M., X. Cheng, V. L. Motin, and L. M. de la Maza. 1997. Effect of immunoglobulin G isotype on the infectivity of Chlamydia trachomatis in a mouse model of intravaginal infection. Infect. Immun. 65:2693-2699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Peterson, E. M., X. Cheng, S. Pal, and L. M. de la Maza. 1993. Effects of antibody isotype and host cell type on in vitro neutralization of Chlamydia trachomatis. Infect. Immun. 61:498-503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ramsey, K. H., T. W. Cotter, R. D. Salyer, G. S. Miranpuri, M. A. Yanez, C. E. Poulsen, J. L. DeWolfe, and G. I. Byrne. 1999. Prior genital tract infection with a murine or human biovar of Chlamydia trachomatis protects mice against heterotypic challenge infection. Infect. Immun. 67:3019-3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ramsey, K. H., G. S. Miranpuri, C. E. Poulsen, N. B. Marthakis, L. M. Braune, and G. I. Byrne. 1998. Inducible nitric oxide synthase does not affect resolution of murine chlamydial genital tract infections or eradication of chlamydiae in primary murine cell culture. Infect. Immun. 66:835-838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ramsey, K. H., W. J. Newhall, and R. G. Rank. 1989. Humoral immune response to chlamydial genital infection of mice with the agent of mouse pneumonitis. Infect. Immun. 57:2441-2446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ramsey, K. H., L. S. F. Soderberg, and R. G. Rank. 1988. Resolution of chlamydial genital infection in B-cell-deficient mice and immunity to reinfection. Infect. Immun. 56:1320-1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rank, R. G., and B. E. Batteiger. 1989. Protective role of serum antibody in immunity to chlamydial genital infection. Infect. Immun. 57:299-301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rank, R. G., K. H. Ramsey, E. A. Pack, and D. M. Williams. 1992. Effect of gamma interferon on resolution of murine chlamydial genital infection. Infect. Immun. 60:4427-4429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rank, R. G., L. S. F. Soderberg, and A. L. Barron. 1985. Chronic chlamydial genital infection in congenitally athymic nude mice Infect. Immun. 48:847-849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stamm, W. E. 1999. Chlamydia trachomatis infections: progress and problems. J. Infect. Dis. 179(Suppl. 2):S380-S383. [DOI] [PubMed] [Google Scholar]
- 42.Su, H., and H. D. Caldwell. 1995. CD4+ T cells play a significant role in adoptive immunity to Chlamydia trachomatis infection of the mouse genital tract. Infect. Immun. 63:3302-3308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Su, H., K. Feilzer, H. D. Caldwell, and R. P. Morrison. 1997. Chlamydia trachomatis genital tract infection of antibody-deficient gene knockout mice. Infect. Immun. 65:1993-1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tietz, W., Y. Allemand, E. Borges, D. von Laer, R. Hallmann, D. Vestweber, and A. Hamann. 1998. CD4+ T cells migrate into inflamed skin only if they express ligands for E- and P-selectin. J. Immunol. 161:963-970. [PubMed] [Google Scholar]
- 45.van Wely, C. A., A. D. Blanchard, and C. J. Britten. 1998. Differential expression of alpha3 fucosyltransferases in Th1 and Th2 cells correlates with their ability to bind P-selectin. Biochem. Biophys. Res. Commun. 247:307-311. [DOI] [PubMed] [Google Scholar]
- 46.Westrom, L., R. Joesoef, G. Reynolds, A. Hagdu, and S. E. Thompson. 1992. Pelvic inflammatory disease and fertility. A cohort study of 1,844 women with laparoscopically verified disease and 657 control women with normal laparoscopic results. Sex. Transm. Dis. 19:185-192. [PubMed] [Google Scholar]
- 47.Williams, D. M., B. Grubbs, K. A. Kelly, and R. G. Rank. 1997. Humoral and cellular immunity in secondary infection due to murine Chlamydia trachomatis. Infect. Immun. 65:2876-2882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yang, X., and R. C. Brunham. 1998. Gene knockout B cell-deficient mice demonstrate that B cells play an important role in the initiation of T cell responses to Chlamydia trachomatis (mouse pneumonitis) lung infection. J. Immunol. 161:1439-1446. [PubMed] [Google Scholar]
- 49.Yang, X., J. Gartner, L. Zhu, S. Wang, and R. C. Brunham. 1999. IL-10 gene knockout mice show enhanced Th1-like protective immunity and absent granuloma formation following Chlamydia trachomatis lung infection. J. Immunol. 162:1010-1017. [PubMed] [Google Scholar]
- 50.Yang, X., K. T. Hayglass, and R. C. Brunham. 1996. Genetically determined differences in IL-10 and IFN-γ responses correlate with clearance of Chlamydia trachomatis mouse pneumonitis infection. J. Immunol. 156:4338-4344. [PubMed] [Google Scholar]