Abstract
Nitric oxide is a recognized cytotoxic effector against facultative and obligate intracellular bacteria. This study examined the effect of nitric oxide produced by inducible nitric oxide synthase (iNOS) up-regulated in response to cytokine stimulation, or by a synthetic nitric oxide donor, on replication of obligately intracellular Coxiella burnetii in murine L-929 cells. Immunoblotting and nitrite assays revealed that C. burnetii infection of L-929 cells augments expression of iNOS up-regulated in response to gamma interferon (IFN-γ) and tumor necrosis factor alpha (TNF-α). Infection in the absence of cytokine stimulation did not result in demonstrable up-regulation of iNOS expression or in increased nitrite production. Nitrite production by cytokine-treated cells was significantly inhibited by the iNOS inhibitor S-methylisothiourea (SMT). Treatment of infected cells with IFN-γ and TNF-α or the synthetic nitric oxide donor 2,2′-(hydroxynitrosohydrazino)bis-ethanamine (DETA/NONOate) had a bacteriostatic effect on C. burnetii replication. Inhibition of replication was reversed upon addition of SMT to the culture medium of cytokine-treated cells. Microscopic analysis of infected cells revealed that nitric oxide (either cytokine induced or donor derived) inhibited formation of the mature (large) parasitophorous vacuole that is characteristic of C. burnetii infection of host cells. Instead, exposure of infected cells to nitric oxide resulted in the formation of multiple small, acidic vacuoles usually containing one C. burnetii cell. Removal of nitrosative stress resulted in the coalescence of small vacuoles to form a large vacuole harboring multiple C. burnetii cells. These experiments demonstrate that nitric oxide reversibly inhibits replication of C. burnetii and formation of the parasitophorous vacuole.
Coxiella burnetii is a bacterial obligate intracellular pathogen and the etiological agent of the zoonosis Q fever. The reservoir host range of C. burnetii is extensive and includes livestock, pets, and wildlife. Infected mammals rarely show signs of disease even when shedding large numbers of organisms (25). The primary route of human infection is via inhalation of contaminated aerosols generated by domestic livestock operations. Acute Q fever normally manifests as a flu-like illness with a characteristic severe preorbital headache and cyclic fever (2). This illness is generally self-limiting and is treatable with antibiotics. Rare cases of chronic Q fever occur and usually present as a life-threatening endocarditis that is refractory to antibiotic treatment (26).
Aerosol transmission of C. burnetii results in uptake by alveolar macrophages. There, C. burnetii replicates within a parasitophorous vacuole (PV) with lysosomal characteristics (6, 20). C. burnetii has a unique adaptation to life in this niche, as the moderately acidic pH (∼4.5) of the lysosome is required to activate its metabolism (18). In this normally bactericidal-bacteriostatic environment, C. burnetii replicates to high numbers within an expanding PV. Host cell lysis releases organisms that can disseminate to infect a variety of tissues (26).
The specific and nonspecific host defenses that control acute C. burnetii infection and possible progression to chronic disease are ill defined. C. burnetii infection usually results in vigorous humoral and cell-mediated immune responses that lead to effective control of the bacterium and long-lived immunity (26). The alveolar macrophage is considered a first-line nonspecific defense against respiratory pathogens such as C. burnetii. An antimicrobic mechanism of macrophages and other cell types is the production of nitric oxide (29). Nitric oxide is a freely diffusible gas that is synthesized from l-arginine and oxygen by nitric oxide synthases (NOS). When transiently produced at low levels, it serves as a critical signaling molecule (4). When produced at high levels for an extended period, nitric oxide and/or resulting reactive nitrogen intermediates are cytotoxic and can inhibit the replication of a variety of intracellular parasites in animal models of infection, including the aerosol-borne bacterial pathogens Mycobacterium tuberculosis (7) and Legionella pneumophila (31). Accumulating evidence also suggests that nitric oxide controls human infection by intracellular parasites, with the most compelling evidence coming from studies of infection by mycobacteria (24, 30). Inducible NOS (iNOS), encoded by nos2, is the NOS isoform responsible for producing sustained bactericidal-bacteriostatic levels of nitric oxide in macrophages and other cell types (21). Expression of iNOS is up-regulated by proinflammatory cytokines, such as tumor necrosis factor alpha (TNF-α) and gamma interferon (IFN-γ), as well as by microbial products, such as lipopolysaccharide (LPS) and lipoteichoic acid (28).
There have been few studies investigating the potential of nitric oxide to control the replication of C. burnetii. An early study demonstrated that IFN-γ used in combination with a crude lymphokine preparation inhibits replication of C. burnetii in L-929 mouse fibroblast cells and that inhibition is suppressed by treatment of cells with cycloheximide (38). Because IFN-γ is now known to induce production of iNOS in L-929 cells and cycloheximide effectively inhibits iNOS production, it is reasonable to suspect that the reported inhibition of replication was due to nitric oxide (11). In contrast, the results of a recent study employing primary murine alveolar macrophages suggested that nitric oxide is not involved in the control of C. burnetii infection (42).
In this study, we employed murine L-929 fibroblasts to assess the effects of nitric oxide on the infectious cycle of C. burnetii. This is a commonly used host cell for C. burnetii propagation that is also resistant to long-term exposure to nitric oxide (3). Moreover, the proinflammatory cytokines TNF-α and IFN-γ induce expression of iNOS without detectable indoleamine 2,3-deoxygenase up-regulation and concomitant intracellular tryptophan depletion, which can be bacteriostatic (35, 39). This study demonstrates that nitric oxide, whether endogenously produced by cytokine-induced iNOS or exogenously released from a synthetic nitric oxide donor, reversibly inhibits the replication of C. burnetii. Moreover, growth inhibition is coupled with the lack of maturation of the C. burnetii PV.
MATERIALS AND METHODS
Coxiella.
C. burnetii (Nine Mile strain in phase II) was propagated in African green monkey kidney (Vero) fibroblasts (CCL-81; American Type Culture Collection). The organisms were purified by Renografin density gradient centrifugation as previously described (17) and stored at −80oC.
Cytokines, iNOS inhibitor treatments, and nitric oxide donor.
All experiments evaluating the effect of nitric oxide on the replication of C. burnetii were conducted on infected murine L-929 cells (CCL-1; American Type Culture Collection). L-929 cells were cultivated in M199 medium (Invitrogen, Carlsbad, Calif.) supplemented with 10% fetal bovine serum (FBS) (Invitrogen) at 37°C in an atmosphere containing 5% CO2. L-929 cells cultivated in six-well plates (2 × 105 cells/well) were infected with C. burnetii suspended in M199 medium at a multiplicity of infection (MOI) of approximately 10 for 2 h at room temperature with gentle rocking. After infection, the cells were washed once and incubation continued in M199 medium containing 2% FBS supplemented with cytokines, iNOS inhibitor, or a nitric oxide donor as needed. TNF-α (final concentrations, 250 to 1,000 U/ml) and IFN-γ (final concentration, 100 U/ml) (R&D Systems, Minneapolis, Minn.) were added to the culture medium to induce iNOS expression. S-Methylisothiourea (SMT) (Sigma-Aldrich, St. Louis, Mo.) was added to the culture medium at a final concentration of 100 μM to inhibit iNOS activity (16). To expose the infected cells to nitric oxide independent of iNOS expression, DETA/NONOate (Calbiochem, San Diego, Calif.), which releases nitric oxide and has a half-life of 56.6 h, was added to the culture medium (final concentrations, 100 to 500 μM).
FFU assay.
The effects of various treatments on C. burnetii replication and viability were quantified by a fluorescent infectious focus-forming unit (FFU) assay on Vero cell monolayers. Media with supplements overlaying infected L-929 cells were replaced every 24 h, and at specified time points, cells were scraped from individual wells of a six-well plate. Cell suspensions were disrupted with gentle sonication. Sonicates were serially diluted in M199 medium supplemented with 2% FBS and used to inoculate Vero cell monolayers on 12-mm-diameter glass coverslips in a 24-well plate. The cells were infected for 2 h with 200 μl of diluted sonicate containing C. burnetii, washed, and incubated in fresh M199 supplemented with 2% FBS. At 48 h postinfection, Vero cells were fixed with cold 100% methanol. Fluorescent staining of infectious foci was accomplished by indirect immunofluorescence employing polyclonal rabbit antisera generated against formalin-killed C. burnetii and FITC-conjugated goat anti-immunoglobulin G serum (Molecular Probes, Eugene, Oreg.). The average number of FFU in 50 fields was determined for each sample by fluorescence microscopy at ×1,000 magnification using a Nikon Diaphot inverted microscope.
Immunoblotting.
Polyacrylamide gel electrophoresis and immunoblotting were conducted as previously described (19). Briefly, cells in individual wells of a six-well plate were lysed in situ with sample buffer prior to electrophoresis. Membranes containing transferred proteins were probed with monoclonal antibodies directed against iNOS (clone 54; Transduction Laboratories, Lexington, Ky.) and β-actin (clone AC-74; Sigma-Aldrich). Bound antibodies were detected using horseradish peroxidase-conjugated anti-immunoglobulin G (Pierce, Rockford, Ill.) and enhanced chemiluminesence (Amersham, Cleveland, Ohio). Images were analyzed by scanning densitometry using a Chemi Doc imaging system and Quantity One software (Bio-Rad, Hercules, Calif.).
Phase-contrast and laser scanning confocal microscopy.
Infected L-929 cells were cultured in 35-mm-diameter coverslip-bottom petri dishes (MatTek, Ashland, Mass.). Phase-contrast microscopy was conducted using a Nikon Diaphot inverted microscope. Images were captured using a Dage cooled charge-coupled device video camera (MTI, Inc., Michigan City, Ind.) and QED software (QED Imaging Inc., Pittsburgh, Pa.). Laser scanning confocal microscopy was conducted using a Leica TCS 4D CLSM confocal microscope equipped with a krypton-argon laser illuminator. All images were processed using Photoshop 5.0 (Adobe Systems Inc., San Jose, Calif.).
Acridine orange staining.
The acidity of bacterium-containing vacuoles was qualitatively determined by acridine orange staining (20). Infected L-929 cell monolayers were incubated for 48 h in the presence or absence of 500 μM 2,2′-(hydroxynitrosohydrazino)bis-ethanamine DETA/NONOate. Intracellular C. burnetii cells were stained first by incubating infected cells in culture medium containing the membrane-permeant fluorescent DNA-RNA stain Syto 12 (Molecular Probes) at a final concentration of 20 μM for 15 min at 37°C. The medium containing Syto 12 was then removed, and the monolayer was washed with fresh medium. The acidic vesicles were then stained with acridine orange at a final concentration of 5 μg/ml in culture medium. After 15 min at 37°C, the stain was removed, the monolayer was washed three times with phosphate-buffered saline (150 mM NaCl, 10 mM NaPO4, pH 7.2), and the cells were imaged by confocal microscopy.
Nitrite measurements.
The relative level of nitric oxide generated by L-929 cell cultures was determined by measuring the nitrite concentration in culture supernatants using a nitric oxide assay kit (Calbiochem) that is based on the Griess reaction. Briefly, nitrate in clarified culture supernatants was first converted to nitrite by nitrate reductase. The nitrite was reacted with sulfanilamide and N-(1-naphthyl) ethylenediamine and detected chromogenically by measuring the optical density at 540 nm using a Bio-Rad microplate reader model 550. The concentration of nitrite was calculated by linear regression. A standard curve was established using sodium nitrite at concentrations from 0 to 200 μM.
RESULTS
Infection with C. burnetii augments IFN-γ and TNF-α up-regulation of iNOS expression in L-929 cells.
Immunoblotting and quantification of nitrite concentrations were used to assess iNOS expression and nitric oxide production, respectively, by L-929 cells treated with the proinflammatory cytokines TNF-α and IFN-γ. These cytokines act synergistically in this cell line to up-regulate the nos2 promoter, with concomitant expression of iNOS (37). TNF-α induced iNOS expression in a dose-dependent manner (Fig. 1). For example, increasing the concentration of TNF-α from 500 to 1,000 U/ml in conjunction with an IFN-γ concentration of 100 U/ml increased iNOS expression by approximately fourfold as determined by scanning densitometry. Infection with C. burnetii at an MOI of 10 enhanced iNOS expression in cells simultaneously treated with IFN-γ and TNF-α. This was clearly evident in L-929 cells treated with 100 U of IFN-γ/ml and 250 U of TNF-α/ml, where iNOS expression is almost undetectable in uninfected cells but easily detectable in C. burnetii-infected cells. MOIs of 20 and 1 up-regulated iNOS to a similar degree in cytokine-treated cells (data not shown). Up-regulation of iNOS expression was not observed in cells infected with C. burnetii without IFN-γ and TNF-α treatment.
FIG. 1.
Infection of L-929 cells by C. burnetii up-regulates iNOS expression in cells that are costimulated with TNF-α and IFN-γ. Cells were infected with C. burnetii at an MOI of 10 for 24 h and simultaneously treated with cytokines. The cell lysates were analyzed for iNOS and β-actin expression by immunoblotting. +, infected; −, uninfected.
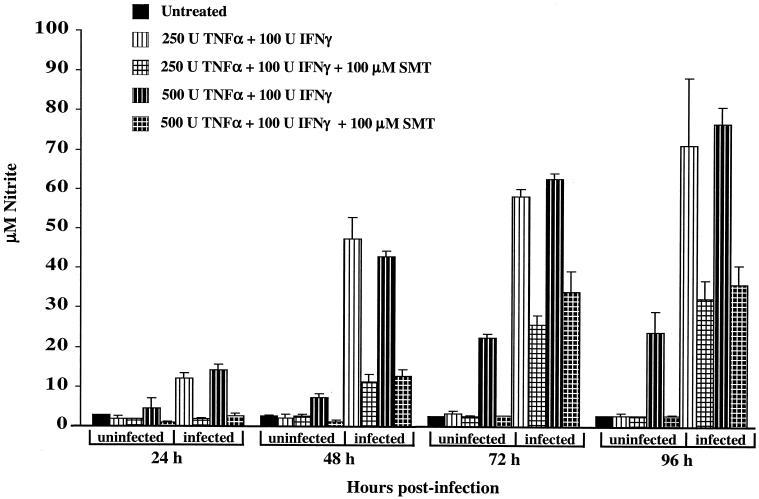
Nitric oxide levels (measured as nitrite concentration) in L-929 culture supernatants were consistent with the up-regulation of iNOS expression measured by immunoblotting (Fig. 2). For instance, significant production of nitrite by L-929 cells treated for 24 h with cytokines was observed only for cells coincidently infected with C. burnetii. The nitrite levels in the culture supernatants of infected L-929 cells treated with 500 U of TNF-α/ml and 100 U of IFN-γ/ml, or with 250 U of TNF-α/ml and 100 U of IFN-γ/ml, were not significantly different from each other at each time point. For example, after 96 h of incubation, the nitrite concentration of supernatants corresponding to each cytokine treatment had increased approximately 27-fold over the background level of nitrite in the supernatants of untreated, uninfected cells (∼2.5 μM), to approximately 74 μM.
FIG. 2.
Nitrite levels in culture supernatants of L-929 cells stimulated with cytokines reflect iNOS expression levels measured by immunoblotting. Infected and uninfected cells were simultaneously treated with cytokines in the presence or absence of the iNOS inhibitor SMT (100 μM). Nitric oxide produced by iNOS was measured indirectly by measuring the nitrite concentration in culture supernatants. The results are expressed as the mean of three representative experiments, with error bars representing the standard deviation.
To confirm that nitric oxide was being produced by iNOS, the iNOS inhibitor SMT was added to infected and uninfected L-929 cells treated with cytokines. The culture supernatant nitrite levels of cytokine-treated cells infected with C. burnetii for 24 h and concomitantly treated with 100 μM SMT were approximately the same as those of supernatants from untreated, uninfected cells (∼2.5 μM). Coincident SMT treatment of cytokine-treated infected cells for 48, 72, and 96 h diminished nitric oxide production by approximately 75, 55, and 50%, respectively. These results indicate that nitric oxide synthesis is due to the up-regulation of iNOS expression.
Nitric oxide reversibly inhibits C. burnetii replication.
The effect of nitric oxide on the replication of C. burnetii was assessed by treating infected L-929 cells with IFN-γ and TNF-α or with the synthetic nitric oxide donor DETA/NONOate. C. burnetii cells were subsequently released from treated cells at 24-h intervals and used to infect fresh Vero cell monolayers. Infectious FFU representing defined PVs were visualized by indirect immunofluorescence at 48 h postinfection and enumerated. Nitric oxide reversibly inhibited C. burnetii replication, with this inhibition most clearly evident at the 96-h postinfection time point (Fig. 3). Whereas substantial replication (approximately 15-fold increase in recoverable FFU relative to the starting inoculum) was observed in untreated L-929 cells, slight declines in FFU were observed for C. burnetii harvested from cells treated during the incubation with 100 U of IFN-γ/ml and 250 or 500 U of TNF-α/ml or with 250 μM DETA/NONOate. Cotreatment of infected cells treated with 100 U of IFN-γ/ml and 250 or 500 U of TNF-α/ml with 100 μM SMT resulted in nine- and twofold increases in FFU, respectively. Collectively, these data suggest that nitric oxide has a bacteriostatic effect on the replication of C. burnetii and that growth inhibition by TNF-α and IFN-γ is partially due to the induction of iNOS expression.
FIG. 3.
Replication of C. burnetii in L-929 cells is inhibited by nitric oxide endogenously produced by cytokine-induced iNOS or exogenously released from the synthetic nitric oxide donor DETA/NONOate. L-929 cells were infected with C. burnetii at an MOI of 10 and simultaneously treated as indicated. C. burnetii cells were harvested from infected cell cultures at 0, 24, 48, 72, and 96 h postinfection by gentle sonication. The viability of C. burnetii in sonicates was quantified by an immunofluorescent FFU assay on Vero cell monolayers. FFU in 50 fields were counted at each time point for three independent experiments, and the results were averaged. The number of FFU recovered from untreated cells at 96 h postinfection was significantly different from those recovered from all treated cultures at the same time point except those treated with 250 U of TNF-α/ml, 100 U of IFN-γ/ml, and 100 μM iNOS inhibitor SMT (P < 0.05).
Nitric oxide inhibits C. burnetii PV maturation.
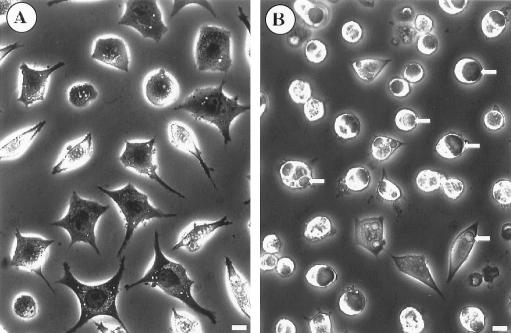
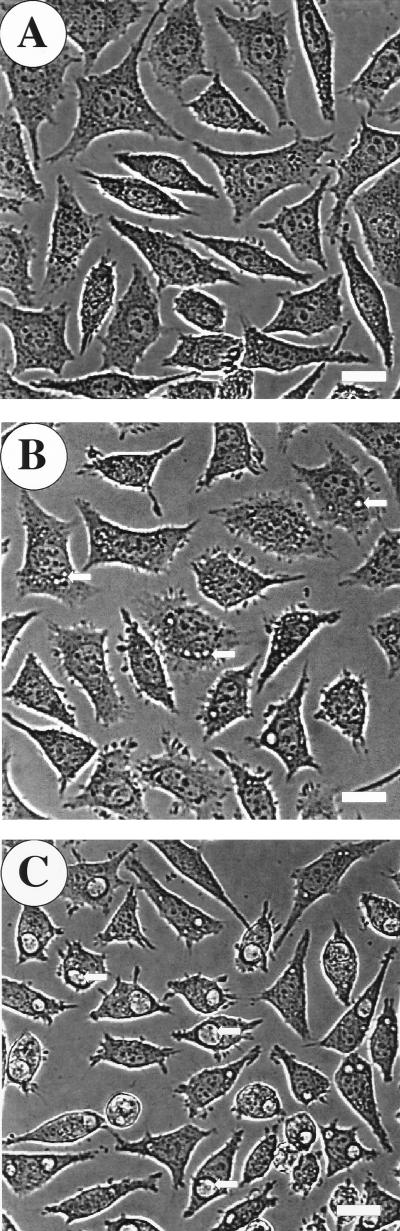
To further investigate the anti-C. burnetii activity of nitric oxide, we examined the formation of C. burnetii PVs in infected L-929 cells treated with iNOS-inducing cytokines or DETA/NONOate. Individual L-929 cells infected with C. burnetii for 48 h contain one or occasionally two C. burnetii-laden PVs that are easily discernible by phase-contrast microscopy. No PVs were observed in L-929 cells infected for 48 h and simultaneously treated with 250 U of TNF-α/ml and 100 U of IFN-γ/ml (Fig. 4A). However, if 100 μM SMT was included in the culture medium during the 48-h incubation period, large, mature PVs were observed (Fig. 4B). A dose-dependent effect of nitric oxide on PV maturation was demonstrated by simultaneously treating infected L-929 cells during a 48-h incubation period with increasing concentrations of DETA/NONOate. In stark contrast to the large, usually singular PVs observed in untreated cells (Fig. 5C), infected cells treated with 100 μM DETA/NONOate produced multiple small PVs (Fig. 5B), while infected cells treated with 250 μM DETA/NONOate produced no visible PVs (Fig. 5A). These data suggest that nitric oxide may inhibit C. burnetii replication by preventing the formation of a PV that supports C. burnetii growth.
FIG. 4.
Treatment of C. burnetii-infected L-929 cells with IFN-γ and TNF-α prevents PV maturation. L-929 cells were infected with C. burnetii for 48 h and simultaneously treated with 100 U of IFN-γ/ml and 250 U of TNF-α/ml without (A) or with (B) the iNOS inhibitor SMT (100 μM). Live cells were viewed by phase-contrast light microscopy. Large phase translucent PVs (arrows) were observed only in cytokine-treated L-929 cells that were simultaneously treated with SMT. Bars, 20 μm.
FIG. 5.
Nitric oxide inhibits C. burnetii PV maturation in a dose-dependent manner. (A) PVs were not observed in infected L-929 cells treated with 250 μM DETA/NONOate. (B) Multiple small PVs (arrows) were observed in L-929 cells infected for 48 h and simultaneously treated with 100 μM DETA/NONOate. (C) Mature (large) PVs (arrows) were observed in untreated cells. Bars, 20 μm.
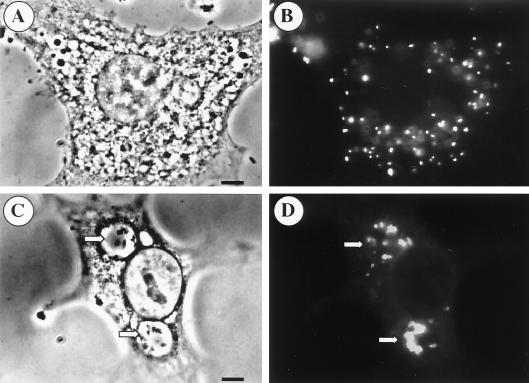
Like the effect of nitric oxide on C. burnetii replication, the inhibition of PV formation was reversible. Indirect-immunofluorescence microscopy of L-929 cells infected for 48 h and simultaneously treated with 500 μM DETA/NONOate demonstrated single organisms, presumably bound by a vacuolar membrane, scattered throughout the cytoplasm (Fig. 6A and B). When DETA/NONOate was removed and incubation was continued for 48 h, the individual small vacuoles coalesced to form one or two clearly visible large PVs containing multiple C. burnetii cells (Fig. 6C and D).
FIG. 6.
Nitric oxide inhibition of C. burnetii PV maturation is reversible. L-929 cells were infected with C. burnetii at an MOI of 10 for 48 h and simultaneously treated with 500 μM DETA/NONOate. (A) Phase-contrast micrograph depicting a single infected cell without a discernible PV. (B) Parallel fluorescent image of the cell in panel A with C. burnetii stained by indirect immunofluorescence. Individual C. burnetii cells within vacuoles are disbursed throughout the cytoplasm. (C) Infected L-929 cells where the DETA/NONOate was removed, the cells were washed, and incubation continued for an additional 48 h followed by microscopy. Two large PVs are evident in the phase-contrast micrograph (arrows). (D) Parallel immunofluorescence micrograph of the PVs in panel C. Bars, 5 μm.
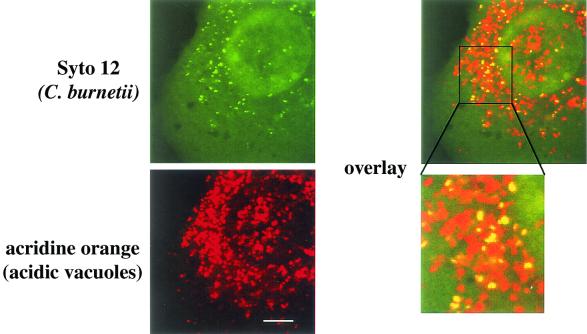
C. burnetii is a mild acidophile and is metabolically active only at a pH of approximately 4.5 (18). This pH is found in the mature C. burnetii PV, which has the characteristics of a secondary lysosome (6, 20). Nitric oxide has been demonstrated to inhibit the vacuolar H+-ATPase responsible for acidifying cellular organelles and vesicles (14). To determine whether the inhibition of C. burnetii PV coalescence and maturation by nitric oxide correlated with a lack of vacuolar acidification, we qualitatively determined the acidity of small C. burnetii-containing PVs in L-929 cells treated with 500 μM DETA/NONOate by acridine orange staining. Acridine orange is an acidotropic base that accumulates in acidic vesicles and fluoresces orange to red when illuminated at 543 nm (20). Live infected L-929 cells were dual labeled with acridine orange and Syto 12, a green fluorescent nucleic acid stain that stained intracellular C. burnetii. Laser scanning confocal microscopy revealed nearly complete overlap of acridine orange and Syto 12 fluorescences (Fig. 7), indicating that C. burnetii in nitric oxide-treated cells resides in acidic vacuoles. Mature PVs in untreated cells displayed a similar bright orange-red fluorescence as previously described (20). These results suggest that nitric oxide inhibition of C. burnetii PV maturation is not due to the failure of vacuoles to acidify to a level that is likely sufficient to activate C. burnetii metabolism.
FIG. 7.
Immature (small) C. burnetii PVs in DETA/NONOate-treated L-929 cells are acidic. L-929 cells were infected for 48 h with C. burnetii and simultaneously treated with 500 μM DETA/NONOate. Acidic vacuoles were stained red with the acidotropic base acridine orange, and C. burnetii cells were stained green with Syto 12, a DNA-RNA stain. Live cells were viewed by laser scanning confocal microscopy. Individual C. burnetii cells in acidic vacuoles are indicated as a yellow signal in the overlay. Bar, 5 μm.
DISCUSSION
Clinical Q fever typically presents as a self-limiting, nondescript flu-like illness that spontaneously resolves without antibiotic intervention (26). C. burnetii infection is frequently asymptomatic, as documented in a study by Raoult et al. (32), who showed that only 1,383 of 17,989 patients seropositive for C. burnetii had been previously diagnosed with clinical Q fever. Chronic Q fever (disease lasting longer than 6 months) occurs in about 5% of patients who have experienced acute disease and can manifest as a life-threatening endocarditis (15). Chronic disease can occur several years after the acute infection, suggesting reactivation of a latent C. burnetii infection (26, 27). Although C. burnetii infection induces both vigorous humoral and cell-mediated immune responses, T-cell activation of macrophages (the host cell of C. burnetii) by proinflammatory cytokines is thought to be largely responsible for clearance of the organism (22, 23, 27, 38, 41). Activated macrophages from guinea pigs and mouse fibroblasts stimulated with IFN-γ and TNF-α have been shown to inhibit C. burnetii replication (23, 38). Although the precise mechanism of growth inhibition was not determined in these studies, induction of iNOS expression and nitric oxide production may have contributed.
In this study, we demonstrate that nitric oxide, exogenously generated from a synthetic nitric oxide donor or generated by cytokine-induced iNOS, reversibly inhibits the replication of C. burnetii. These findings are consistent with those of numerous studies showing bactericidal-bacteriostatic effects of nitric oxide on the replication of facultative and obligate intracellular bacterial pathogens, such as Listeria monocytogenes (5), M. tuberculosis (7), Salmonella enterica serovar Typhimurium (40), Rickettsia conorii (12), Rickettsia prowazekii (37), and Brucella melitensis (9). Immunoblotting demonstrated that iNOS expression in L-929 cells is up-regulated by TNF-α in a dose-dependent manner and that C. burnetii infection further enhanced iNOS expression. The mechanism by which C. burnetii infection augments iNOS expression likely involves LPS. Purified phase I (smooth) and phase II (rough) C. burnetii LPSs have been shown to stimulate the release of TNF-α from murine and human macrophages (8, 36), and in this study, purified phase I C. burnetii LPS increased iNOS expression in cytokine-treated L-929 cells when added to the culture medium (data not shown). Replication of C. burnetii was completely arrested in L-929 cells infected for 96 h and treated with either 250 or 500 U of TNF-α/ml and 100 U of IFN-γ/ml or with a 250-μM concentration of the synthetic nitric oxide donor DETA/NONOate. Although the iNOS inhibitor SMT reduced nitrite levels in the culture supernatants of cytokine-treated cells infected for 96 h to approximately equal levels, it allowed C. burnetii replication at 96 h postinfection to near that of untreated cultures only in cells treated with 250 U of TNF-α/ml and 100 U of IFN-γ/ml. This result suggests that the inhibition of C. burnetii replication observed in infected cells treated with the lower TNF-α concentration (250 U/ml) is largely mediated through the induction of iNOS expression and the production of nitric oxide. In cells treated with the higher concentration of TNF-α (500 U/ml), SMT cotreatment allowed only 9% of the replication seen in untreated cells, suggesting that cytokine effects distinct from nitric oxide production may be inhibiting C. burnetii replication. Nonetheless, an inhibitory effect of nitric oxide on C. burnetii replication is clearly supported by the observation that replication is inhibited in cells exposed to the synthetic nitric oxide donor DETA/NONOate.
In addition to reversibly inhibiting C. burnetii replication, nitric oxide also reversibly inhibited maturation of the C. burnetii PV. Exposure of infected cells to nitric oxide resulted in multiple small PVs, usually containing one C. burnetii cell, scattered throughout the cytoplasm. Normal PV maturation ensued in cytokine-treated cells if iNOS activity was inhibited by SMT. Furthermore, coalescence of multiple small PVs into usually one large PV occurred in DETA/NONOate-treated cells if the nitric oxide donor was removed and incubation continued for an additional 48 h. Acridine orange accumulation in PVs of cells exposed to nitric oxide indicates that the vacuoles are acidic. Thus, it is unlikely that the lack of PV maturation and C. burnetii replication in cells exposed to nitric oxide is due to inadequate vacuole acidification and failure to acid activate C. burnetii metabolism. It is interesting to speculate that nitric oxide may inhibit PV maturation by inhibiting the production or function of a C. burnetii protein(s) that is secreted and localized to the PV membrane to facilitate homotypic fusion of C. burnetii-containing vacuoles. Indeed, genes encoding proteins comprising a putative type IV secretion apparatus have recently been identified in C. burnetii, and type IV secretion of effector proteins is essential for proper maturation of PVs harboring L. pneumophila, a close relative of C. burnetii (33, 34).
Phagocytosis of C. burnetii by professional phagocytes does not result in a demonstrable respiratory burst and generation of superoxide anion (1). Therefore, intracellular C. burnetii cells are probably not exposed to toxic peroxynitrite, a reaction product of superoxide anion and nitric oxide (29). Thus, the reversible bacteriostatic effect of nitric oxide on C. burnetii replication is more likely due to direct interactions of nitric oxide with proteins, such as S-nitrosylation of cysteine sulfhydryls, or inactivation of iron-sulfur centers or heme-prosthetic groups of metabolic enzymes (10). Either mechanism could conceivably affect C. burnetii protein synthesis by directly inhibiting various aspects of the transcription-translation machinery or indirectly by affecting C. burnetii energy charge.
Q fever may present as a chronic disease (15). Reactivation of latent C. burnetii cells acquired during acute infection is thought to cause some cases of chronic disease (27). Indeed, immunosuppression is associated with an increased risk of chronic Q fever (32). Nitric oxide appears to play a role in maintaining latent infections. For example, in a murine model of experimental tuberculosis, reactivation of latent tuberculosis infection occurs if mice are treated with the iNOS inhibitor aminoguanidine (13). In this study, we demonstrate that in vitro C. burnetii resides in a quiescent, nonreplicating state when subjected to nitric oxide stress and that proper PV maturation and C. burnetii replication ensues after removal of nitric oxide. Thus, an in vivo parallel may exist for nitric oxide in the maintenance of latent C. burnetii infection.
Acknowledgments
We thank Scott Boitano, Shelly Robertson, and Scott Bohle for review of the manuscript.
This work was supported by National Institutes of Health grant RR-15553 (R.A.H.).
Editor: A. D. O'Brien
REFERENCES
- 1.Akporiaye, E. T., D. Stefanovich, V. Tsosie, and G. Baca. 1990. Coxiella burnetii fails to stimulate human neutrophil superoxide anion production. Acta Virol. 34:64-70. [PubMed] [Google Scholar]
- 2.Baca, O. G., and D. Paretsky. 1983. Q fever and Coxiella burnetii: a model for host-parasite interactions. Microbiol. Rev. 47:127-149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berendji, D., V. Kolb-Bachofen, K. L. Meyer, and K. D. Kroncke. 1999. Influence of nitric oxide on the intracellular reduced glutathione pool: different cellular capacities and strategies to encounter nitric oxide-mediated stress. Free Radic. Biol. Med. 27:773-780. [DOI] [PubMed] [Google Scholar]
- 4.Bogdan, C. 2001. Nitric oxide and the regulation of gene expression. Trends Cell Biol. 11:66-75. [DOI] [PubMed] [Google Scholar]
- 5.Boockvar, K. S., D. L. Granger, R. M. Poston, M. Maybodi, M. K. Washington, J. B. Hibbs, Jr., and R. L. Kurlander. 1994. Nitric oxide produced during murine listeriosis is protective. Infect. Immun. 62:1089-1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burton, P. R., N. Kordova, and D. Paretsky. 1971. Electron microscopic studies of the rickettsia Coxiella burnetii: entry, lysosomal response, and fate of rickettsial DNA in L-cells. Can. J. Microbiol. 17:143-150. [DOI] [PubMed] [Google Scholar]
- 7.Chan, J., K. Tanaka, D. Carroll, J. Flynn, and B. R. Bloom. 1995. Effects of nitric oxide synthase inhibitors on murine infection with Mycobacterium tuberculosis. Infect. Immun. 63:736-740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dellacasagrande, J., E. Ghigo, S. M. Hammami, R. Toman, D. Raoult, C. Capo, and J. L. Mege. 2000. αvβ3 integrin and bacterial lipopolysaccharide are involved in Coxiella burnetii-stimulated production of tumor necrosis factor by human monocytes. Infect. Immun. 68:5673-5678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eze, M. O., L. Yuan, R. M. Crawford, C. M. Paranavitana, T. L. Hadfield, A. K. Bhattacharjee, R. L. Warren, and D. L. Hoover. 2000. Effects of opsonization and gamma interferon on growth of Brucella melitensis 16M in mouse peritoneal macrophages in vitro. Infect. Immun. 68:257-263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fang, F. C. 1997. Mechanisms of nitric oxide related antimicrobial activity. J. Clin. Investig. 99:2818-2825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Feng, H. M., and D. H. Walker. 1993. Interferon-gamma and tumor necrosis factor-alpha exert their antirickettsial effect via induction of synthesis of nitric oxide. Am. J. Pathol. 143:1016-1023. [PMC free article] [PubMed] [Google Scholar]
- 12.Feng, H. M., and D. H. Walker. 2000. Mechanisms of intracellular killing of Rickettsia conorii in infected human endothelial cells, hepatocytes, and macrophages. Infect. Immun. 68:6729-6736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Flynn, J. L., C. A. Scanga, K. E. Tanaka, and J. Chan. 1998. Effects of aminoguanidine on latent murine tuberculosis. J. Immunol. 160:1796-1803. [PubMed] [Google Scholar]
- 14.Forgac, M. 1999. The vacuolar H+-ATPase of clathrin-coated vesicles is reversibly inhibited by S-nitrosoglutathione. J. Biol. Chem. 274:1301-1305. [DOI] [PubMed] [Google Scholar]
- 15.Fournier, P. E., T. J. Marrie, and D. Raoult. 1998. Diagnosis of Q fever. J. Clin. Microbiol. 36:1823-1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Garvey, E. P., J. A. Oplinger, G. J. Tanoury, P. A. Sherman, M. Fowler, S. Marshall, M. F. Harmon, J. E. Paith, and E. S. Furfine. 1994. Potent and selective inhibition of human nitric oxide synthases. Inhibition by non-amino acid isothioureas. J. Biol. Chem. 269:26669-26676. [PubMed] [Google Scholar]
- 17.Hackstadt, T., R. Messer, W. Cieplak, and M. G. Peacock. 1992. Evidence for proteolytic cleavage of the 120-kilodalton outer membrane protein of rickettsiae: identification of an avirulent mutant deficient in processing. Infect. Immun. 60:159-165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hackstadt, T., and J. C. Williams. 1981. Biochemical stratagem for obligate parasitism of eukaryotic cells by Coxiella burnetii. Proc. Natl. Acad. Sci. USA 78:3240-3244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Heinzen, R. A., D. Howe, L. P. Mallavia, D. D. Rockey, and T. Hackstadt. 1996. Developmentally regulated synthesis of an unusually small, basic peptide by Coxiella burnetii. Mol. Microbiol. 22:9-19. [DOI] [PubMed] [Google Scholar]
- 20.Heinzen, R. A., M. A. Scidmore, D. D. Rockey, and T. Hackstadt. 1996. Differential interaction with endocytic and exocytic pathways distinguish parasitophorous vacuoles of Coxiella burnetii and Chlamydia trachomatis. Infect. Immun. 64:796-809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hibbs, J. B., and N. R. Bastian. 1999. The discovery of the biological synthesis of nitric oxide, p. 13-36. In F. C. Fang (ed.), Nitric oxide and infection. Kluwer Academic/Plenum Publishers, Denver, Colo.
- 22.Kishimoto, R. A., H. Rozmiarek, and E. W. Larson. 1978. Experimental Q fever infection in congenitally athymic nude mice. Infect. Immun. 22:69-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kishimoto, R. A., B. J. Veltri, F. G. Shirey, P. G. Canonico, and J. S. Walker. 1977. Fate of Coxiella burnetii in macrophages from immune guinea pigs. Infect. Immun. 15:601-607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kwon, O. J., J. H. Kim, H. C. Kim, G. Y. Suh, J. W. Park, M. P. Chung, H. Kim, and C. H. Rhee. 1998. Nitric oxide expression in airway epithelial cells in response to tubercle bacilli stimulation. Respirology 3:119-124. [DOI] [PubMed] [Google Scholar]
- 25.Lang, G. H. 1990. Coxiellosis (Q fever) in animals, p. 23-48. In T. J. Marrie (ed.), Q fever; the disease. CRC Press, Boca Raton, Fla.
- 26.Maurin, M., and D. Raoult. 1999. Q fever. Clin. Microbiol. Rev. 12:518-553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mege, J. L., M. Maurin, C. Capo, and D. Raoult. 1997. Coxiella burnetii: the ′query' fever bacterium. A model of immune subversion by a strictly intracellular microorganism. FEMS Microbiol. Rev. 19:209-217. [DOI] [PubMed] [Google Scholar]
- 28.Muhl, H., and C. A. Dinarello. 1999. Cytokine regulation of nitric oxide production, p. 77-94. In F. C. Fang (ed.), Nitric oxide and infection. Kluwer Academic/Plenum Publishers, Denver, Colo.
- 29.Nathan, C., and M. U. Shiloh. 2000. Reactive oxygen and nitrogen intermediates in the relationship between mammalian hosts and microbial pathogens. Proc. Natl. Acad. Sci. USA 97:8841-8848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nozaki, Y., Y. Hasegawa, S. Ichiyama, I. Nakashima, and K. Shimokata. 1997. Mechanism of nitric oxide-dependent killing of Mycobacterium bovis BCG in human alveolar macrophages. Infect. Immun. 65:3644-3647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rajagopalan-Levasseur, P., D. Lecointe, G. Bertrand, M. Fay, and M. A. Gougerot-Pocidalo. 1996. Differential nitric oxide (NO) production by macrophages from mice and guinea pigs infected with virulent and avirulent Legionella pneumophila serogroup 1. Clin. Exp. Immunol. 104:48-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Raoult, D., H. Tissot-Dupont, C. Foucault, J. Gouvernet, P. E. Fournier, E. Bernit, A. Stein, M. Nesri, J. R. Harle, and P. J. Weiller. 2000. Q fever 1985-1998. Clinical and epidemiologic features of 1,383 infections. Medicine (Baltimore) 79:109-123. [DOI] [PubMed] [Google Scholar]
- 33.Roy, C. R., K. H. Berger, and R. R. Isberg. 1998. Legionella pneumophila DotA protein is required for early phagosome trafficking decisions that occur within minutes of bacterial uptake. Mol. Microbiol. 28:663-674. [DOI] [PubMed] [Google Scholar]
- 34.Segal, G., and H. A. Shuman. 1999. Possible origin of the Legionella pneumophila virulence genes and their relation to Coxiella burnetii. Mol. Microbiol. 33:669-670. [DOI] [PubMed] [Google Scholar]
- 35.Taylor, M. W., and G. S. Feng. 1991. Relationship between interferon-gamma, indoleamine 2,3-dioxygenase, and tryptophan catabolism. FASEB J. 5:2516-2522. [PubMed] [Google Scholar]
- 36.Tujulin, E., B. Lilliehook, A. Macellaro, A. Sjostedt, and L. Norlander. 1999. Early cytokine induction in mouse P388D1 macrophages infected by Coxiella burnetii. Vet. Immunol. Immunopathol. 68:159-168. [DOI] [PubMed] [Google Scholar]
- 37.Turco, J., H. Liu, S. F. Gottlieb, and H. H. Winkler. 1998. Nitric oxide-mediated inhibition of the ability of Rickettsia prowazekii to infect mouse fibroblasts and mouse macrophagelike cells. Infect. Immun. 66:558-566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Turco, J., H. A. Thompson, and H. H. Winkler. 1984. Interferon-gamma inhibits growth of Coxiella burnetii in mouse fibroblasts. Infect. Immun. 45:781-783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Turco, J., and H. H. Winkler. 1986. Gamma-interferon-induced inhibition of the growth of Rickettsia prowazekii in fibroblasts cannot be explained by the degradation of tryptophan or other amino acids. Infect. Immun. 53:38-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vazquez-Torres, A., J. Jones-Carson, P. Mastroeni, H. Ischiropoulos, and F. C. Fang. 2000. Antimicrobial actions of the NADPH phagocyte oxidase and inducible nitric oxide synthase in experimental salmonellosis. I. Effects on microbial killing by activated peritoneal macrophages in vitro. J. Exp. Med. 192:227-236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Waag, D. M., C. R. Bolt, T. J. Marrie, and J. C. Williams. 1991. Immune responses of humans to Coxiella burnetii: specific antibody and cell-mediated responses after vaccination or infection, p. 157-174. In J. C. Williams (ed.), Q fever: the biology of Coxiella burnetii. CRC Press, Boca Raton, Fla.
- 42.Yoshiie, K., S. Matayoshi, T. Fujimura, N. Maeno, and H. Oda. 1999. Induced production of nitric oxide and sensitivity of alveolar macrophages derived from mice with different sensitivity to Coxiella burnetii. Acta Virol. 43:273-278. [PubMed] [Google Scholar]