Abstract
The bovine lactoferrin molecule and relatively long lactoferrin fragments containing residues 473 to 538 strongly inhibited adherence of Streptococcus mutans to saliva-coated hydroxyapatite beads. Each cysteine residue in Lf411 (residues 473 to 538) was replaced by a serine residue, and the mutants Lf411-C481S and Lf411-C532S strongly inhibited S. mutans adherence. These results suggest that the functional domain of lactoferrin that binds to a salivary film lies in residues 473 to 538 and that the region might be concealed by disulfide bond formation between Cys481 and Cys532 in the Lf411 fragment.
Streptococcus mutans has been implicated as the prime cause of dental caries, one of the most common diseases in humans (17, 18). Colonization of the tooth surface by S. mutans is initiated by attachment of the organism to salivary components adsorbed on tooth surfaces (7). A 190-kDa S. mutans surface protein antigen, variously designated as antigen I/II, B, IF, P1, SR, or MSL-1 (17), is known to be one of the factors that mediates the binding of the organism (2, 7, 9).
We recently demonstrated that bovine milk lactoferrin inhibits saliva-induced S. mutans aggregation by binding strongly to salivary components and that residues 473 to 538 of the molecule are important in this inhibition (12). There are two types of bacterial interaction with salivary components: saliva-induced bacterial aggregation in solution phase and bacterial adherence to salivary components adsorbed on the tooth surface. The mechanisms of these two types of interaction are different (5, 14), and therefore, we were unable to conclude that bovine milk lactoferrin inhibits the adherence of bacterial cells to a salivary film.
In this study, the effect of bovine milk lactoferrin on adherence of S. mutans to a salivary film was compared with the effects of other milk components. The inhibitory effect of lactoferrin fragments with residues 473 to 538 on S. mutans adherence to a salivary film was also investigated. To study the effect of mutation on S. mutans adherence, we used engineered bovine lactoferrin fragments in which each cysteine residue was substituted by site-directed mutagenesis.
Milk components tested for inhibition of S. mutans adherence to saliva-coated hydroxyapatite (S-HA).
Unstimulated whole saliva was collected from a single donor (male, 44 years of age) in an ice-chilled tube and clarified by centrifugation. Bovine α-casein, β-casein, κ-casein, lactalbumin, lactoferrin, and lactoperoxidase were purchased from Sigma Chemical Co. (St. Louis, Mo.). Bovine γ-casein was purchased from Research Organics (Cleveland, Ohio), and bovine lactoglobulin was purchased from ICN Biomedicals Inc. (Aurora, Ohio). Bovine immunoglobulin G was prepared from bovine milk, using affinity chromatography on a 5-ml HiTrap protein G column (Amersham Pharmacia Biotech, Uppsala, Sweden) (13).
For the adherence assay, 5 mg of spheroidal hydroxyapatite beads (BDH, Poole, England) was incubated with 200 μl of clarified whole saliva for 1 h at 37°C and washed three times with buffered KCl (4). S. mutans MT8148 (7) was labeled with 2′,7′-bis(2-carboxyethyl)-5 (6)-carboxyfluorescein (BCECF) as described previously (11). Bacterial cells were grown at 37°C for 18 h in brain heart infusion (Difco Laboratories, Detroit, Mich.) broth, and BCECF acetoxymethyl ester (Sigma) was added to the bacterial culture to a final concentration of 10 μM. The culture was incubated for an additional 30 min in the dark. After incubation, the cells were harvested by centrifugation and were washed three times with buffered KCl. To evaluate the inhibitory effects of milk components on S. mutans adherence to S-HA beads, BCECF-labeled bacteria (4 × 107) were allowed to react with S-HA beads (5 mg) in 200 μl of buffered KCl containing various amounts of milk components at 37°C for 3 h. After incubation, the beads were washed three times with buffered KCl, and the fluorescence intensity associated with the S-HA beads was determined with a Spectramax Gemini microplate reader (Molecular Devices, Sunnyvale, Calif.). The number of bacteria adsorbed was determined using the standard curve between the number of bacterial cells and the fluorescence intensity, and the interpolation was exactly performed (r2 = 0.99). Differences between control and test samples in adherence assays were determined using Student's t test.
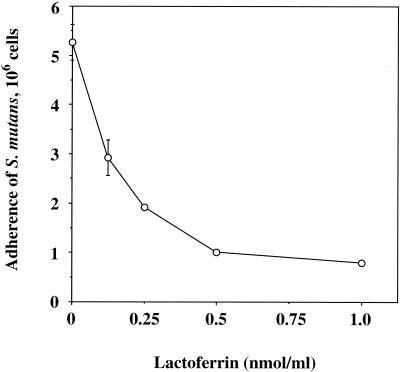
Of the milk components tested, bovine lactoferrin showed the strongest inhibitory activity (Table 1). Other components, such as lactoperoxidase and immunoglobulin G (IgG), showed moderate inhibitory activity. In a preliminary study, lactoferrin inhibited S. mutans adherence to S-HA beads in a dose-dependent manner (Fig. 1). The adherence was significantly inhibited by lactoferrin in the ranges equivalent to physiological levels in human saliva, which have been given as 8.5 to 24 μg/ml (0.11 to 0.3 nmol/ml) (21). Therefore, we used a sufficient concentration of 0.5 nmol/ml for the adherence assay.
TABLE 1.
Effects of various milk components on the adherence of S. mutans MT8148 cells to S-HA beads
| Milk component | No. of cells bound to S-HA (106)a | % Inhibitionb |
|---|---|---|
| Control | 5.26 ± 0.36 | |
| α-Casein | 4.86 ± 0.51 | 7.6 |
| β-Casein | 4.72 ± 0.58 | 10.3 |
| γ-Casein | 4.06 ± 0.75 | 22.8 |
| κ-Casein | 4.42 ± 0.51 | 16.0 |
| IgG | 3.25 ± 0.50c | 38.2 |
| Lactalbumin | 4.53 ± 0.50 | 13.9 |
| Lactoferrin | 1.01 ± 0.01d | 80.8 |
| Lactoglobulin | 4.30 ± 0.53 | 18.3 |
| Lactoperoxidase | 2.67 ± 0.56c | 49.2 |
The values were obtained at 0.5 nmol of milk component/ml and are expressed as the means ± standard deviations of triplicate assays.
Percent inhibition was calculated as follows: percent inhibition = 100 × [(a − b)/a], where a is the mean value without inhibitor (control) and b is the mean value with inhibitor.
P < 0.05 compared with the control.
P < 0.01 compared with the control.
FIG. 1.
Inhibition of adherence of S. mutans cells to S-HA beads by lactoferrin. Values are given as the means ± standard deviations of triplicate assays.
Effects of lactoferrin fragments on S. mutans adherence.
Truncated bovine lactoferrin fragments were prepared as six-His-tagged fusion proteins by cloning PCR-amplified lactoferrin gene fragments into the expression vector pQE-30 (Qiagen Inc., Chatworth, Calif.), as previously described (12). The primers used for amplification were also described in our previous study (12). The amplified DNAs were digested with BamHI and SalI restriction sites and inserted into the BamHI-SalI site of the pQE-30 plasmid. The ligated DNAs were then transformed into Escherichia coli M15(pREP4) (Qiagen), and the bacteria were cultured in 2× TY broth (8). The truncated lactoferrin fragments prepared were LfC, Lf4, Lf41, Lf42, Lf43, Lf44, Lf45, Lf46, Lf47, and Lf411, and the amino acid position of each fragment is listed in Table 2. As a control, six-His-tagged mouse dihydrofolate reductase (DHFR) fusion protein was produced as described previously (12). Lactoferrin and DHFR fusion proteins were purified from whole-cell extracts of E. coli M15(pREP4) containing the recombinant plasmids (12).
TABLE 2.
Effects of lactoferrin fragments on the adherence of S. mutans MT8148 cells to S-HA beads with or without dithiothreitol
| Lactoferrin fragment (amino acid position)b | No. of cells bound to S-HA (106)a
|
|
|---|---|---|
| Without DTT | With DDT (0.1 M) | |
| Control | 5.26 ± 0.36 | 5.18 ± 0.27 |
| Lactoferrin (1-689) | 1.01 ± 0.01 (80.8)c | 1.15 ± 0.09 (78.1)c |
| LfC (345-689) | 1.20 ± 0.15 (77.2)c | NDf |
| Lf4 (345-571) | 1.41 ± 0.21 (73.2)c | ND |
| Lf41 (345-538) | 1.78 ± 0.20 (66.2)c | ND |
| Lf42 (345-505) | 2.98 ± 0.18 (43.3)d | ND |
| Lf43 (345-472) | 5.04 ± 0.45 (4.2) | 4.66 ± 0.18 (11.4) |
| Lf44 (345-439) | 5.16 ± 0.39 (1.9) | 5.50 ± 0.48 (0) |
| Lf45 (366-571) | 5.03 ± 0.27 (4.4) | 3.51 ± 0.15 (33.3)d |
| Lf46 (399-571) | 4.34 ± 0.19 (17.5) | 2.57 ± 0.07 (51.1)c |
| Lf47 (432-571) | 5.61 ± 0.03 (0) | 3.46 ± 0.01 (34.2)d |
| Lf411 (473-538) | 4.41 ± 0.40 (16.2) | 3.81 ± 0.32 (27.6)e |
| DHFR | 4.89 ± 0.35 (7.0) | 4.78 ± 0.38 (9.1) |
The values were obtained at 0.5 nmol of lactoferrin fragment/ml, and are expressed as the means ± standard deviations of triplicate assays. The values in the parentheses are the percent inhibition, calculated as described in Table 1.
Amino acid numbering is according to Goodman and Schanbacher (6).
P < 0.001 compared with the control.
P < 0.01 compared with the control.
P < 0.05 compared with the control.
ND, not determined.
The longer fragments containing residues 473 to 538, such as LfC, Lf4, and Lf41, strongly inhibited adherence of S. mutans to S-HA beads in the absence of dithiothreitol (DTT) (Table 2). However, shorter fragments such as Lf43, Lf44, Lf45, Lf46, Lf47, and Lf411, did not inhibit adherence in the absence of DTT. The inhibitory effect was recovered for the shorter fragments containing residues 473 to 538, such as Lf45, Lf46, Lf47, and Lf411, when DTT was added to the assay, and the difference in the level of adherence were significant between the control and each such shorter fragment. The six-His-tagged DHFR showed no adherence inhibition in either the presence or the absence of DTT.
Effects of Lf411 mutants on S. mutans adherence.
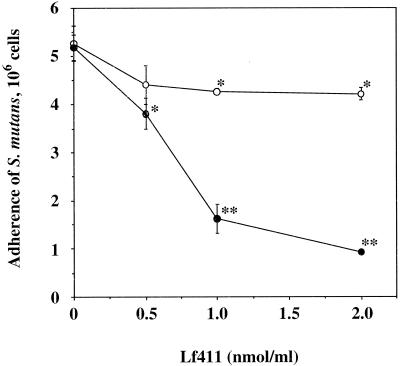
Since the addition of DTT to the S. mutans adherence assay enhanced the inhibitory effect of several lactoferrin fragments, mutant Lf411 genes were constructed using a QuikChange site-directed mutagenesis kit (Stratagene, La Jolla, Calif.). Lf411 fragment originally contains six cysteine residues, and the role of them in the fragments in producing a conformational change was then examined. For the individual mutations of each cysteine residue, the cysteine codons were modified to serine codons using the oligonucleotides listed in Table 3. The nucleotide sequence was confirmed by the dideoxy chain termination technique (19) with a BigDye terminator cycle sequencing Kit, FS, and an ABI PRISM 310 genetic analyzer (Perkin-Elmer Applied Biosystems, Foster City, Calif.). The expression vector pQE-30, which contains a DNA fragment encoding the mutant Lf411, was transformed into E. coli M15(pREP4), and the Lf411 mutants were purified as described above. The Lf411 fragment strongly inhibited S. mutans adherence to S-HA beads in the presence of DTT in a dose-dependent manner but showed weak inhibition in the absence of DTT (Fig. 2). The mutants Lf411-C481S and Lf411-C532S strongly inhibited S. mutans adherence to S-HA beads, whereas other mutants showed very weak inhibition (Table 4).
TABLE 3.
Oligonucleotide sequences used for the mutations of each cysteine residue in the Lf411 fragment
| Lf411 mutant | Sequencea |
|---|---|
| Lf411-C481S (Cys481 to Ser) | 5′-AACCAGACAGGCTCCTCCGCATTTGATGAATTC-3′ |
| Lf411-C491S (Cys491 to Ser) | 5′-TTCTTTAGTCAGAGCTCTGCCCCTGGGGCTGAC-3′ |
| Lf411-C502S (Cys502 to Ser) | 5′-CCGAAATCCAGACTCTCTGCCTTGTGTGCTGGC-3′ |
| Lf411-C505S (Cys505 to Ser) | 5′-AGACTCTGTGCCTTGTCTGCTGGCGATGACCAG-3′ |
| Lf411-C515S (Cys515 to Ser) | 5′-CAGGGCCTGGACAAGTCTGTGCCCAACTCTAAG-3′ |
| Lf411-C532S (Cys532 to Ser) | 5′-ACCGGGGCTTTCAGGTCCCTGGCTGAGGACGTT-3′ |
Modified codons are underlined.
FIG. 2.
Inhibition of adherence of S. mutans cells to S-HA beads by the lactoferrin fragment Lf411 in the absence (○) or presence (•) of DTT (0.1 M). Values are given as the means ± standard deviations of triplicate assays. ∗, P < 0.05; ∗∗, P < 0.001 (compared with control).
TABLE 4.
Effects of Lf411 mutants on the adherence of S. mutans MT8148 cells to S-HA beads
| Mutant Lf411 | No. of cells bound to S-HA (106)a | % Inhibitionb |
|---|---|---|
| Control | 5.26 ± 0.36 | |
| Lf411 | 4.26 ± 0.06c | 19.0 |
| Lf411-C481S | 1.65 ± 0.25d | 68.6 |
| Lf411-C491S | 4.63 ± 0.43 | 15.8 |
| Lf411-C502S | 4.51 ± 0.61 | 14.3 |
| Lf411-C505S | 4.89 ± 0.63 | 7.0 |
| Lf411-C515S | 4.71 ± 0.14 | 10.5 |
| Lf411-C532S | 1.92 ± 0.13d | 63.5 |
The values were obtained at 1.0 nmol of mutant Lf411/ml, and are expressed as the means ± standard deviations of triplicate assays.
Percent inhibition was calculated as described in Table 1.
P < 0.05 compared with control.
P < 0.001 compared with control.
Human saliva in solution phase induces bacterial aggregation, and bacteria adhere to saliva in solid phase. Gibbons and Hay (5), and Raj et al. (14) reported that proline-rich proteins and statherin are pellicle receptors for some streptococcal strains but do not induce aggregation of the organisms in suspension. Biesbrock et al. (1) also showed that Staphylococcus aureus and Pseudomonas aeruginosa bind heterotypic complex of salivary mucin and secretory IgA in solution but not in solid phase. Based on these findings, Gibbons (3) proposed a model that an apparent conformational change occurs when salivary components bind to hydoxyapatite, which exposes the binding sites for bacterial adhesin. This explains the difference between bacterial aggregation and adherence. In this study, we have shown that bovine milk lactoferrin strongly inhibits S. mutans adherence to a salivary film. Therefore, it is likely that conformational changes in salivary protein adsorbed on hydroxyapatite have no influence on the exposure of the binding epitope for bacterial adhesin.
To identify the lactoferrin functional domain that binds to salivary protein in solid phase, we prepared a series of truncated lactoferrin fragments and examined their effect on S. mutans adherence to S-HA beads. Of the fragments tested, longer fragments containing residues 473 to 538 strongly inhibited adherence. In contrast, shorter fragments did not show any significant inhibitory effect, although inhibition was recovered by treatment of fragments containing residues 473 to 538 with DTT. This suggests that the lactoferrin functional domain that binds to a salivary film lies in residues 473 to 538, which is the same region responsible for aggregation-inhibition in solution phase (12). In shorter fragments, conformational changes may conceal the saliva-binding epitope on the lactoferrin fragment, thus preventing salivary receptor binding. To clarify the role of cysteine residues in the shorter fragments, Lf411 (the shortest fragment, residues 473 to 538) was selected as a representative fragment and several mutants were constructed by substituting each cysteine residue for a serine residue. Mutants Lf411-C481S and Lf411-C532S strongly inhibited S. mutans adherence to S-HA beads, while other mutants showed weak inhibition. These results suggest that a disulfide bond might be formed between Cys481 and Cys532, causing a conformational change that hinders the saliva-binding epitope.
Studies of the control of dental caries have focused on bacterial interaction with salivary proteins, saliva-induced bacterial aggregation, and bacterial adherence to a salivary film. Rosan et al. (15) reported a significant increase in S. mutans-aggregating activity in the saliva of a caries-resistant group compared to a caries-susceptible group and a significant decrease in S. mutans adhesion-promoting activity in the saliva of the caries-resistant group. Furthermore, Slomiany et al. (20) reported that the bacterium-aggregating epitope of salivary protein is expressed to a greater extent in caries-resistant individuals than in caries-susceptible individuals. Therefore, S. mutans aggregation by salivary proteins may be advantageous for dental caries prevention, since it may clear the organisms from the oral cavity by forming clumps of bacteria that are swallowed. Assays of S. mutans adherence to salivary films are essential to evaluate the inhibition of dental caries initiation (10, 16).
In conclusion, we demonstrated that bovine lactoferrin inhibits the adherence of S. mutans cells to a salivary film and that residues 473 to 538 are important for the inhibition. Lf411-C481S and Lf411-C532S mutants could be used as inhibitors of S. mutans adherence to salivary films. Further studies are necessary to identify the saliva-binding region of lactoferrin at the peptide level.
Acknowledgments
We thank Kei-ichi Shimazaki and Ichiro Nakamura of Hokkaido University, Sapporo, Japan, for the gift of bovine lactoferrin cDNA. We also thank Firoz Rahemtulla of The University of Alabama at Birmingham for the gift of BDH hydroxyapatite beads.
This work was supported in part by Grants-in-Aid for Developmental Scientific Research (A)12357013 (T.K.) and (C)13672158 (T.O.) from the Ministry of Education, Science, Sports and Culture of Japan.
Editor: V. J. DiRita
Footnotes
This paper is dedicated to the memory of Toshihiko Koga.
REFERENCES
- 1.Biesbrock, A. R., M. S. Reddy, and M. J. Levine. 1991. Interaction of a salivary mucin-secretory immunoglobulin A complex with mucosal pathogens. Infect. Immun. 59:3492-3497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bowen, W. H., K. Schilling, E. Giertsen, S. Pearson, S. F. Lee, A. Bleiweis, and D. Beeman. 1991. Role of a cell surface-associated protein in adherence and dental caries. Infect. Immun. 59:4606-4609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gibbons, R. J. 1989. Bacterial adhesion to oral tissues: a model for infectious diseases. J. Dent. Res. 68:750-760. [DOI] [PubMed] [Google Scholar]
- 4.Gibbons, R. J., and D. I. Hay. 1989. Adsorbed salivary acidic proline-rich proteins contribute to the adhesion of Streptococcus mutans JBP to apatitic surfaces. J. Dent. Res. 68:1303-1307. [DOI] [PubMed] [Google Scholar]
- 5.Gibbons, R. J., and D. I. Hay. 1988. Human salivary acidic proline-rich proteins and statherin promote the attachment of Actinomyces viscosus LY7 to apatitic surfaces. Infect. Immun. 56:439-445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goodman, R. E., and F. L. Schanbacher. 1991. Bovine lactoferrin mRNA: sequence, analysis, and expression in the mammary gland. Biochem. Biophys. Res. Commun. 180:75-84. [DOI] [PubMed] [Google Scholar]
- 7.Koga, T., N. Okahashi, I. Takahashi, T. Kanamoto, H. Asakawa, and M. Iwaki. 1990. Surface hydrophobicity, adherence, and aggregation of cell surface protein antigen mutants of Streptococcus mutans serotype c. Infect. Immun. 58:289-296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Laloi, P., C. L. Munro, K. R. Jones, and F. L. Macrina. 1996. Immunologic characteristics of a Streptococcus mutans glucosyltransferase B sucrose-binding site peptide-cholera toxin B-subunit chimeric protein. Infect. Immun. 64:28-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee, S. F., A. Progulske-Fox, G. W. Erdos, D. A. Piacentini, G. Y. Ayakawa, P. J. Crowley, and A. S. Bleiweis. 1989. Construction and characterization of isogenic mutants of Streptococcus mutans deficient in major surface protein antigen P1 (I/II). Infect. Immun. 57:3306-3313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liljemark, W. F., and C. Bloomquist. 1996. Human oral microbial ecology and dental caries and periodontal diseases. Crit. Rev. Oral Biol. Med. 7:180-198. [DOI] [PubMed] [Google Scholar]
- 11.Martin, E., and S. Bhakdi. 1992. Flow cytometric assay for quantifying opsonophagocytosis and killing of Staphylococcus aureus by peripheral blood leukocytes. J. Clin. Microbiol. 30:2246-2255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mitoma, M., T. Oho, Y. Shimazaki, and T. Koga. 2001. Inhibitory effect of bovine milk lactoferrin on the interaction between a streptococcal surface protein antigen and human salivary agglutinin. J. Biol. Chem. 276:18060-18065. [DOI] [PubMed] [Google Scholar]
- 13.Oho, T., Y. Shimazaki, M. Mitoma, M. Yoshimura, Y. Yamashita, K. Okano, Y. Nakano, H. Kawagoe, M. Fukuyama, N. Fujihara, and T. Koga. 1999. Bovine milk antibodies against cell surface protein antigen PAc- glucosyltransferase fusion protein suppress cell adhesion and alter glucan synthesis of Streptococcus mutans. J. Nutr. 129:1836-1841. [DOI] [PubMed] [Google Scholar]
- 14.Raj, P. A., M. Johnsson, M. J. Levine, and G. H. Nancollas. 1992. Salivary statherin. Dependence on sequence, charge, hydrogen bonding potency, and helical conformation for adsorption to hydroxyapatite and inhibition of mineralization. J. Biol. Chem. 267:5968-5976. [PubMed] [Google Scholar]
- 15.Rosan, B., B. Appelbaum, E. Golub, D. Malamud, and I. D. Mandel. 1982. Enhanced saliva-mediated bacterial aggregation and decreased bacterial adhesion in caries-resistant versus caries-susceptible individuals. Infect. Immun. 38:1056-1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rudney, J. D., K. L. Hickey, and Z. Ji. 1999. Cumulative correlations of lysozyme, lactoferrin, peroxidase, S-IgA, amylase, and total protein concentrations with adherence of oral viridans streptococci to microplates coated with human saliva. J. Dent. Res. 78:759-768. [DOI] [PubMed] [Google Scholar]
- 17.Russell, M. W. 1992. Immunization against dental caries. Curr. Opin. Dent. 2:72-80. [PubMed] [Google Scholar]
- 18.Russell, R. R. 1994. The application of molecular genetics to the microbiology of dental caries. Caries Res. 28:69-82. [DOI] [PubMed] [Google Scholar]
- 19.Sanger, F., S. Nicklen, and A. R. Coulson. 1977. DNA sequencing with chain-terminating inhibitors. Proc. Natl. Acad. Sci. USA 74:5463-5467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Slomiany, B. L., J. Piotrowski, A. Czajkowski, F. E. Shovlin, and A. Slomiany. 1993. Differential expression of salivary mucin bacterial aggregating activity with caries status. Int. J. Biochem. 25:935-940. [DOI] [PubMed] [Google Scholar]
- 21.Tenovuo, J. O. 1989. Nonimmunoglobulin defense factors in human saliva, p. 55-91. In J. O. Tenovuo (ed.), Human saliva: clinical chemistry and microbiology. CRC Press, Inc., Boca Raton, Fla.