Abstract
Haemophilus somnus isolates from cases of thrombotic meningoencephalitis, pneumonia, and other disease sites are capable of undergoing a high rate of phase variation in the oligosaccharide component of their lipooligosaccharides (LOS). In contrast, the LOS of commensal strains isolated from the normal reproductive tract phase vary little or not at all. In addition, the LOS of H. somnus shares conserved epitopes with LOS from Neisseria gonorrhoeae, Haemophilus influenzae, and other species that can incorporate sialic acid into their LOS. We now report that growth of disease isolates of H. somnus with CMP-N-acetylneuraminic acid (CMP-NeuAc) or NeuAc added to the medium resulted in incorporation of NeuAc into the LOS. However, NeuAc was not incorporated into the LOS of commensal isolates and one disease isolate following growth in medium containing CMP-NeuAc or NeuAc. Sialylated LOS was detected by an increase in the molecular size or an increase in the amount of the largest-molecular-size LOS electrophoretic bands, which disappeared following treatment with neuraminidase. Sialylated LOS could also be detected by reactivity with Limax flavus agglutinin lectin, which is specific for sialylated species, by dot blot assay; this reactivity was also reversed by neuraminidase treatment. H. somnus strain 2336 LOS was found to contain some sialic acid when grown in medium lacking CMP-NeuAc or NeuAc, although supplementation enhanced NeuAc incorporation. In contrast strain 738, an LOS phase variant of strain 2336, was less extensively sialylated when the growth medium was supplemented with CMP-NeuAc or NeuAc, as determined by electrophoretic profiles and electrospray mass spectrometry. The sialyltransferase of H. somnus strain 738 was confirmed to preferentially sialylate the Galβ-(1-3)-GlcNAc component of the lacto-N-tetraose structure by capillary electrophoresis assay. Enhanced sialylation of the strain 2336 LOS inhibited the binding of monoclonal antibodies to LOS by enzyme immunoassay and Western blotting. Furthermore, sialylation of the LOS enhanced the resistance of H. somnus to the bactericidal action of antiserum to LOS. Sialylation and increased resistance to killing by normal serum also occurred in a deletion mutant that was deficient in the terminal Gal-GlcNAc disaccharide. LOS sialylation may therefore be an important virulence mechanism to protect H. somnus against the host immune system.
Haemophilus somnus may be isolated as a commensal or pathogen from the genitourinary or respiratory tracts of cattle. Pathogenic strains may cause a wide variety of bovine diseases, including thrombotic meningoencephalitis, shipping fever pneumonia, abortion, arthritis, myocarditis, septicemia, and other infections (4, 16). Pathogenic strains of H. somnus have been shown to elaborate a variety of virulence factors designed to resist host immunity and enhance their survival in tissues and blood. Such factors include those for bacterial survival within phagocytic cells (1, 6, 11, 14), outer membrane proteins that bind the Fc region of immunoglobulins and contribute to resistance to serum (40, 43), endotoxin activity (20), and phase variation of epitopes of lipooligosaccharides (LOS) (18, 19, 27, 41). LOS phase variation appears to enable pathogenic strains to persist systemically by evading recognition by the host immune response (18). In contrast, commensal isolates of H. somnus from the prepuce either do not phase vary or do so at a greatly reduced rate, and they are less virulent than isolates recovered from disease sites (4, 18, 21). Clinical isolates of H. somnus also tend to be more resistant to the bactericidal effects of normal serum than urogenital isolates (3). Although LOS and outer membrane proteins have been proposed to play a role in resistance to serum (2, 18), other variables may also be involved in this complex virulence mechanism.
The LOS of Haemophilus influenzae, Neisseria gonorrhoeae, Neisseria meningitidis, and some other bacterial species are also capable of undergoing LOS phase variation (33), and their LOS are similar in composition and structure to H. somnus LOS (5, 42). Furthermore, H. somnus LOS epitopes react with monoclonal antibodies (MAb) to the LOS of Haemophilus aegyptius, H. influenzae, and N. gonorrhoeae (13, 19). The H. somnus genome also contains genes that share DNA homology with genes encoding LOS glycosyl transferases from H. influenzae (19, 27, 33, 41). The oligosaccharide components of H. influenzae, Haemophilus ducreyi, N. meningitidis, and N. gonorrhoeae LOS, and other surface structures of some bacteria, can be sialylated through one of several mechanisms (23-25, 28-30). In N. gonorrhoeae, N-acetylneuraminic acid (NeuAc) is transferred to the terminal Gal residue of lacto-N-neo-tetraose on its LOS from CMP-NeuAc by a surface sialyltransferase (7). Therefore, we sought to investigate whether H. somnus LOS could also be decorated with sialic acid. Our results demonstrate that some strains of H. somnus (i) are sialylated under all growth conditions; (ii) are sialylated predominantly only following growth in medium supplemented with CMP-NeuAc, NeuAc, or bovine serum; or (iii) cannot sialylate their LOS under any growth supplementation conditions. The resulting sialylation interferes with reactivity to LOS-specific antibodies and serum bactericidal activity.
MATERIALS AND METHODS
Bacteria and growth conditions.
Pathogenic H. somnus strains 649, 8025, and 2336; LOS phase variants 738, 797, and 813 (derived from strain 2336); and commensal strains 1P, 127P, and 129Pt have been described previously (3, 10). A mutant of strain 738 with a deletion in the phase-variable LOS biosynthesis gene lob2A (738-lob2A1::Km) has also been described, and the enzyme encoded by lob2A has been proposed to be a GlcNAc transferase (41). Strains were stored at −80°C in 10% skim milk, cultured onto Columbia blood agar, and incubated at 37°C in a candle jar for 24 to 36 h. One loopful of bacteria was inoculated into 10 ml of Columbia broth containing 0.1% Trizma base and 0.01% thiamine monophosphate (Sigma Chemical Co., St. Louis, Mo.) (CTT) and grown to 109 CFU/ml (for about 4 h) at 37°C with vigorous shaking (about 200 rpm). Alternatively, H. somnus strains were inoculated onto 30 sheep blood agar plates (Quelab Laboratories Columbia agar containing 5% sheep's blood) and incubated overnight at 37°C in a candle extinction jar, and the cells washed off the plates with phosphate-buffered saline, pH 7.4 (PBS).
For some experiments, bacteria were grown with CMP-NeuAc, NeuAc, bovine serum, or bovine red blood cell lysate. To 18 ml of fresh CTT, 2.25 ml of broth culture at 109 CFU/ml and 1 mg (50-μg/ml final concentration) of CMP-NeuAc or NeuAc (Sigma Chemical Co.) in sterile water were added. Control cultures had sterile water only added. The culture was incubated for 3 h at 37°C with shaking. In some cases, H. somnus was grown in 20 ml of supplemented Terrific broth (a less complex medium consisting of only a pancreatic digest of casein, yeast extract, and potassium salts) containing NeuAc (1.6 mg/ml) to 109 CFU/ml. Alternatively, 1 ml of bacteria at 109 CFU/ml was inoculated into 600 ml of a 1:1 mixture of fresh, sterile bovine serum and CTT and shaken for 3 h at 37°C. In place of bovine serum the same amount of bacterial inoculum was added to 200 ml of a 1:1 mixture of red blood cell lysate (29) and CTT and shaken for 3 h at 37°C.
Purification and O deacylation of LOS for mass spectrometry.
Small-scale extracts of LOS were prepared using phenol-water as previously described (15). In some cases LOS was further purified by treatment of the LOS solution with DNase and RNase for 2 h at 37°C followed by proteinase K for an additional 1 h at 37°C. Insoluble material was removed from the enzyme-treated LOS solution by centrifugation at 8,000 × g for 30 min, followed by sedimentation of the LOS by centrifugation at 55,000 × g for 16 h. The LOS pellet was resuspended in distilled water and lyophilized.
For O deacylation, LOS (2 to 10 mg) was treated with anhydrous hydrazine (1 to 2 ml) with stirring at 37°C for 30 min. The reaction mixture was cooled (0°C), cold acetone (−70°C, 10 ml) was added gradually to destroy excess hydrazine, and precipitated O-deacylated LOS (OdA LOS) was obtained by centrifugation (8,000 × g, 20 min.). The OdA LOS was washed twice with cold acetone, redissolved in water, and lyophilized.
Electrospray mass spectrometry (ES-MS) analysis.
The OdA LOS samples were analyzed on a VG Quattro triple-quadrupole mass spectrometer (Fisons Instruments) with an electrospray ion source. Samples were dissolved in an aqueous solvent containing 50% acetonitrile-0.1% formic acid. The electrospray tip voltage was 2.5 kV, and the mass spectrometer was scanned from m/z 150 to 2,500 with a scan time of 10 s.
Capillary electrophoresis assay for sialyltransferase activity.
Bacteria were grown with shaking in Columbia broth supplemented with vitamin B1 (thiamine HCl) at 10 μg/ml for 18 h at 37°C. After centrifugation of the broth culture, a cell suspension was made in 10 mM MOPS (morpholinepropanesulfonic acid) (pH 7.0), and cell extracts were made by passage of the cell suspension through an Emulsiflex C5 cell disruptor (Avestin, Ottawa, Canada). A protease inhibitor cocktail (Complete tablet; Roche) was added to the resulting lysate. Debris was removed by centrifugation, and the supernatant was then centrifuged at 100,000 × g for 30 min to collect the membrane fraction. Assays were performed with 0.2 mM aminopyrene trisulfonic acid (APTS)-labeled oligosaccharides as the acceptor molecule, which were prepared as previously described (8). The reaction mixture contained 50 mM HEPES (pH 7.0) with 10 mM MgCl2and 10 mM MnCl2, and the sugar nucleotide donor CMP-NeuAc (0.2 mM). Reactions were performed at room temperature or 37°C and were analyzed by capillary electrophoresis as previously described (8). The identity of the products was determined by comparison of the relative mobility against those of known standards (8).
Sera and antibodies.
Rabbit antiserum to purified LOS from H. somnus strains grown in CTT have been described previously (18). MAb 5F5.9 (the kind gift of Alan Lesse, Department of Veterans Affairs Western New York Healthcare System, State University of New York at Buffalo) was made to H. aegyptius LOS and is specific for the phosphorylcholine (ChoP) epitope (13). MAb 3F11 is specific for the Galβ-(1-4)-GlcNAc epitope of the lacto-N-neo-tetraose oligosaccharide on N. gonorrhoeae LOS and was kindly provided by Michael Apicella (University of Iowa College of Medicine, Iowa City).
Electrophoretic analysis and immunoblotting.
One microgram of LOS in water was boiled for 5 min with an equal volume of solubilization buffer and electrophoresed on a 14% discontinuous polyacrylamide gel (20). Gels were stained by periodate oxidation and ammoniacal silver (36). For some experiments, 1 μg of LOS was incubated with 0.005 U of Vibrio cholerae type III neuraminidase (Sigma) for 1 h at 37°C. For immunoblotting, the gel contents were transferred to nitrocellulose paper as described previously (18). Nonspecific sites on the blot were blocked with 1% nonfat dry milk in Tris-buffered saline, and a 1:10 dilution of convalescent bovine serum or undiluted MAb was added. The blot was washed with Tris-buffered saline, and horseradish peroxidase-conjugated goat anti-bovine immunoglobulin G or anti-mouse immunoglobulin G (Jackson Immunoresearch Laboratories, West Grove, Pa.) was added at a 1:2,000 dilution. After washing, blots were developed with 0.5% 4-chloro-1-naphthol (Bio-Rad, Richmond, Calif.) containing 0.002% H2O2.
Dot blots were performed as described above, except 1 μg of LOS was applied as a single spot onto nitrocellulose, and the paper was blocked and incubated with bovine serum, MAb, or the lectin Limax flavus (garden slug) agglutinin (LFA) conjugated to horseradish peroxidase (E.Y Laboratories, Inc., San Mateo, Calif..).
ELISA.
An enzyme-linked immunosorbent assay (ELISA) to measure binding of antibodies to extracted LOS was performed as previously described (20). Nonspecific binding of MAb to strain 2336 or 738 LOS was assessed by using strain-specific MAb 5D7 to strain 649 LOS as a control for both ELISA and immunoblotting (13).
Bactericidal assay.
The bactericidal assay was performed as previously described (17, 20) with the following modifications. Bacteria grown in CTT to 109 CFU/ml were diluted to 105 CFU/ml in CTT containing 30 μg of CMP-NeuAc and incubated for 2 h at 37°C. For some experiments, 20 mM pyruvate or lactate was added to the growth medium (26). The bacteria were diluted 1:10 into 15 or 30% rabbit antiserum to purified strain 738 LOS or various concentrations of normal bovine serum; precolostral calf serum was added as a complement source. Twenty microliters was spread onto Columbia blood agar plates immediately (time zero) and after 60 min of incubation at 37°C. Percent viability was determined by dividing the number of colonies present on plates after 60 min of incubation by the number of colonies present at time zero and multiplying by 100.
Sialyltransferase assay.
The sialyltransferase assay described by Parsons et al. (31) for N. gonorrhoeae was used. Alternatively, the ability of viable H. somnus cells to incorporate [14C]NeuAc from CMP-[14C]NeuAc was also assessed. H. somnus was grown in brain heart infusion broth supplemented with 0.1% Trizma base and 0.01% thiamine monophosphate to mid-log phase, and 100 μl of the culture was diluted 1:10 in the same broth in microcentrifuge tubes containing 0.04 μCi of CMP-[14C]NeuAc. The total CMP-NeuAc concentration tested varied from 36 to 81 μM. The Microfuge tubes were incubated at 37°C for 3 h, followed by washing of the bacteria three times in PBS. The bacteria were resuspended in 25 μl of PBS and spotted onto Whatman filter paper. The paper was air dried and placed into 3 ml of Ecoscint (National Diagnostics, Atlanta, Ga.), and the radioactivity was determined in a Beckman LS8100 liquid scintillation counter.
RESULTS
Lectin reactivity.
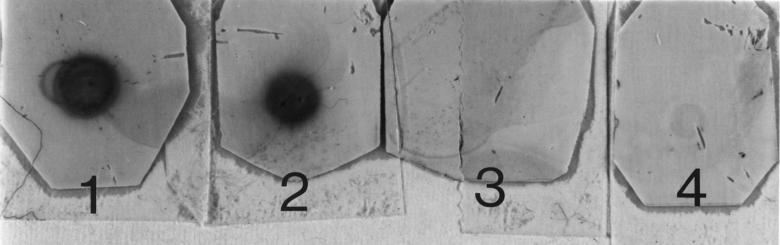
We sought to determine if the LOS of H. somnus strain 738 (an LOS phase variant of pneumonia isolate 2336) could be sialylated when the bacteria were grown in the presence of CMP-NeuAc or fresh bovine serum. To screen for sialylated glycoforms, a dot blot assay of extracted LOS with peroxidase-conjugated LFA, which is specific for sialic acid regardless of the linkage, was used. LFA was reactive with LOS from strain 738 grown in the presence of CMP-NeuAc or fresh bovine serum, but the LOS was nonreactive following treatment with neuraminidase or if the bacteria were grown in CTT only (Fig. 1).
FIG. 1.
Dot blot of extracted LOS from H. somnus strain 738 grown with or without CMP-NeuAc or fresh bovine serum with peroxidase-conjugated LFA. One microgram of each LOS was blotted onto nitrocellulose, and reactivity with LFA was determined as described in Materials and Methods. 1, LOS from bacteria grown in fresh bovine serum; 2, LOS from bacteria grown in CMP-NeuAc; 3, LOS from bacteria grown in CTT only; 4, LOS from bacteria grown in CMP-NeuAc and treated with V. cholerae neuraminidase.
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis analysis.
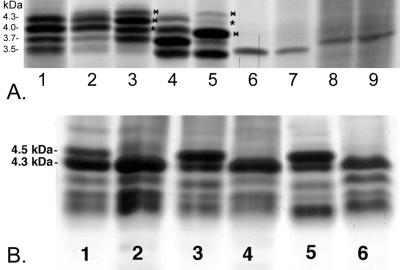
The electrophoretic profiles of LOS from strain 738, strain 738-lob2A1::Km (an allelic exchange mutant containing a mutation in lob2A, which encodes a putative GlcNAc transferase) (41), and commensal isolates 1P and 127P from the bovine prepuce, all grown with or without CMP-NeuAc, are shown in Fig. 2A. Strain 738 LOS resolved into four major bands of 4.3, 4.0, 3.7, and 3.5 kDa (lane 1). The three highest-molecular-mass LOS bands increased in size (lane 3) when the bacteria were grown with CMP-NeuAc. The increased shift in LOS molecular size could be reproduced by incubation of H. somnus in bovine serum or bovine red blood cell lysate (not shown). Neuraminidase treatment of LOS from bacteria grown with CMP-NeuAc resulted in a profile very similar to that of LOS from bacteria grown in CTT only (Fig. 2A, lane 2). Allelic exchange mutant 738-lob2A1::Km is deficient in the attachment of the terminal Gal-GlcNAc LOS disaccharide, which is sialylated in N. gonorrhoeae (24). Nonetheless, when 738-lob2A1::Km was grown in the presence of CMP-NeuAc, there was also an increase in the molecular mass of the predominant 3.7-kDa band as well as the faint 4.0- and 4.3-kDa bands (lanes 4 and 5). It is likely that the now-terminal galactose residue remaining on the truncated oligosaccharide chain in strain 738-lob2A1::Km LOS (5) is also capable of being sialylated. However, the LOS of preputial isolates 1P (Fig. 2A, lanes 6 and 7) and 127P (lanes 8 and 9) did not change in their electrophoretic profiles after the bacteria were grown without (lanes 6 and 8) or with (lanes 7 and 9) CMP-NeuAc.
FIG. 2.
Electrophoretic profiles of LOS from H. somnus strains 738, 738-lob2A1:: Km, 1P, and 127P grown in the presence or absence of CMP-NeuAc (A) and from strain 2336 grown in the presence or absence of CMP-NeuAc or NeuAc (B). (A) Lanes: 1, LOS from strain 738 grown in CTT only; 2, LOS from strain 738 grown in CTT plus CMP-NeuAc and treated with neuraminidase; 3, LOS from strain 738 grown in CTT plus CMP-NeuAc; 4 and 5, LOS from strain 738-lob2A1::Km grown in CTT only or in CTT plus CMP-NeuAc, respectively; 6 and 7, LOS from strain 1P grown in CTT only or in CTT plus CMP-NeuAc, respectively; 8 and 9, LOS from strain 127P grown in CTT only or in CTT plus CMP-NeuAc, respectively. Bands marked with asterisks on the right have increased in molecular mass, putatively due to the addition of NeuAc. (B) Lanes: 1, LOS from strain 2336 grown in CTT only; 2, LOS from strain 2336 grown in CTT and treated with neuraminidase; 3, LOS from strain 2336 grown in CTT plus CMP-NeuAc; 4, LOS from strain 2336 grown in CTT plus CMP-NeuAc and treated with neuraminidase; 5, LOS from strain 2336 grown in CTT plus NeuAc; 6, LOS from strain 2336 grown in CTT plus NeuAc and treated with neuraminidase.
To assess how phase variation affected LOS sialylation, we examined the electrophoretic profiles of LOS from strain 2336 grown in CTT with and without CMP-NeuAc or NeuAc (Fig. 2B). Unlike for strain 738 LOS, the 4.5-kDa band was present in strain 2336 LOS when the bacteria were grown in the absence of CMP-NeuAc but increased in intensity when the bacteria were grown in the presence of CMP-NeuAc (lanes 1 and 3, respectively). The 4.5-kDa band was also present in LOS from strain 2336 grown in supplemented Terrific broth, which lacks any tissue components (data not shown). These results suggested that CMP-NeuAc was not required for incorporation of NeuAc into H. somnus strain 2336 LOS. When H. somnus strain 2336 was grown in CTT containing NeuAc (Fig. 2B, lane 5), a similar increase in the intensity of the 4.5-kDa band occurred. Furthermore, when the LOS from strain 2336 grown in the presence or absence of either CMP-NeuAc or NeuAc was treated with neuraminidase, the entire 4.5-kDa band disappeared (lanes 2, 4, and 6, respectively). When strain 738 was grown with NeuAc, the 3.7-, 4.0-, and 4.3-kDa LOS bands increased in size in a manner similar to that when the bacteria were grown with CMP-NeuAc (data not shown). These results suggest that an exogenous source of CMP-NeuAc was not required for sialylation of LOS in some strains or phase variants of H. somnus but that enhanced sialylation could occur through utilization of free NeuAc.
An increase in the molecular mass of the largest LOS component was also seen in encephalitis isolate 8025, whereas an increase in the relative amount of the highest-molecular-mass LOS component occurred in strains 797 and 813, which are phase variants of strains 738 and 2336. However, the LOS of serum-sensitive preputial isolate 129Pt and abortion isolate 649 were not modified following incubation of the bacteria with CMP-NeuAc (data not shown).
ES-MS analysis of NeuAc in OdA LOS.
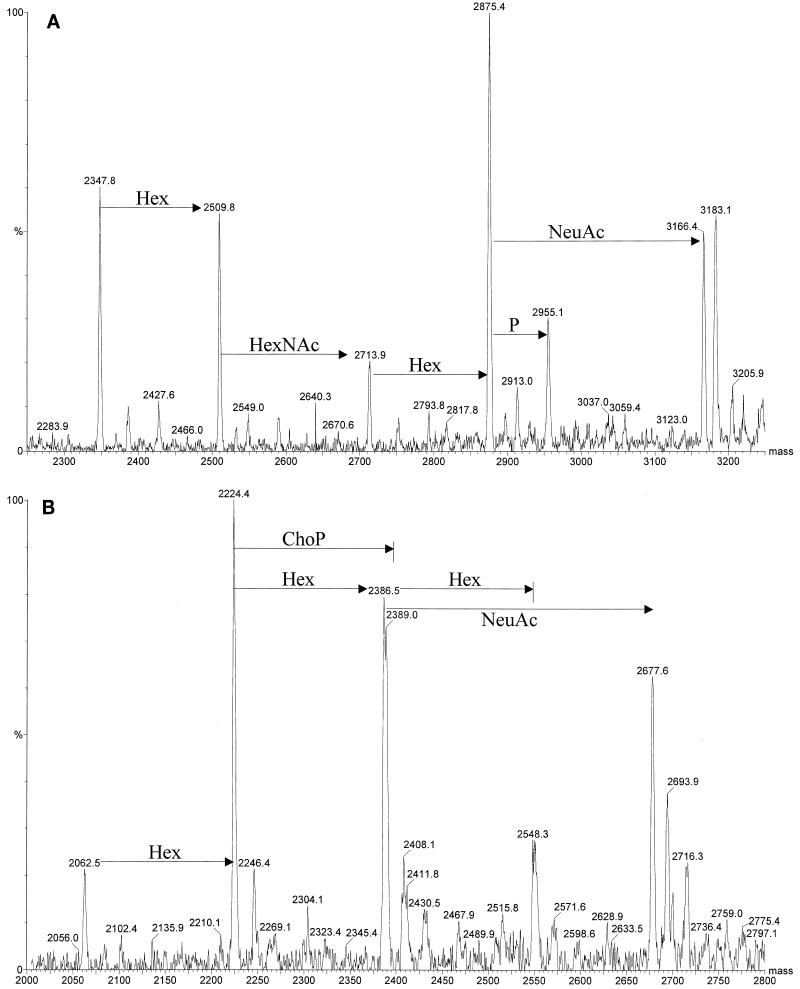
Relatively large amounts of NeuAc were detected in OdA LOS from strains 2336, 738, and 738-lob2A1::Km grown on blood agar plates without additional CMP-NeuAc, as determined by ES-MS (Table 1). However, NeuAc was also present, although in small amounts, in OdA LOS from strain 2336 grown in CTT, confirming that NeuAc was present in the LOS when the bacteria were grown without CMP-NeuAc. There was not a substantial increase in the amount of NeuAc present in strain 2336 or 738lob2A1::Km LOS when CMP-NeuAc was added to the blood agar (Fig. 3). Thus, it appeared that H. somnus could utilize a source of NeuAc from blood used in the agar plates for incorporation into their LOS. However, avirulent preputial isolate 1P had no detectable NeuAc in its LOS, even when grown on blood agar supplemented with CMP-NeuAc (data not shown).
TABLE 1.
Negative-ion ES-MS data and proposed compositions of OdA LOS from H. somnus strains 2336, 738, and 738-lob2A1::Km grown on blood agar or in CTT
| Strain (growth medium) | Observed ions (m/z)
|
Molecular Mass (Da)
|
Relative intensity | Proposed compositiona | ||
|---|---|---|---|---|---|---|
| (M − 2H)2− | (M − H)3− | Observed | Calculatedb | |||
| 1,172.8 | 2,347.6 | 2,347.1 | 18.2 | 2Hex, 2Hep, 2EtnP, 2Kdo, lipid A | ||
| 2336 (Blood agar) | 1,253.9 | 835.4 | 2,509.0 | 2,509.3 | 15.4 | 3Hex, 2Hep, 2EtnP, 2Kdo, lipid A |
| 1,355.9 | 903.1 | 2,713.8 | 2,712.4 | 5.2 | HexNAc, 3Hex, 2Hep, 2EtnP, 2Kdo, lipid A | |
| 1,436.7 | 957.4 | 2,875.3 | 2,874.6 | 30.6 | HexNAc, 4Hex, 2Hep, 2EtnP, 2Kdo, lipid A | |
| 1,582.0 | 1,054.6 | 3,166.4 | 3,165.6 | 15.2 | NeuAc, HexNAc, 4Hex, 2Hep, 2EtnP, 2Kdo, lipid A | |
| 2336 (CTT) | 1,172.8 | 2,347.6 | 2,347.1 | 70.9 | 2Hex, 2Hep, 2EtnP, 2Kdo, lipid A | |
| 1,253.6 | 2,509.5 | 2,509.3 | 7.1 | 3Hex, 2Hep, 2EtnP, 2Kdo, lipid A | ||
| 1,355.8 | 903.5 | 2,713.5 | 2,712.4 | 7.1 | HexNAc, 3Hex, 2Hep, 2EtnP, 2Kdo, lipid A | |
| 1,436.4 | 957.2 | 2,874.7 | 2,874.6 | 14.2 | HexNAc, 4Hex, 2Hep, 2EtnP, 2Kdo, lipid A | |
| 1,581.7 | 3,165.4 | 3,165.6 | 0.2 | NeuAc, HexNAc, 4Hex, 2Hep, 2EtnP, 2Kdo, lipid A | ||
| 738 (blood agar) | 1,111.2 | 2,224.5 | 2,224.1 | 10.1 | 2Hex, 2Hep, EtnP, 2Kdo, lipid A | |
| 1,192.3 | 2,387.3 | 2,386.2 | 4.7 | 3Hex, 2Hep, EtnP, 2Kdo, lipid A | ||
| 1,193.6 | 2,389.4 | 2,389.1 | 11.5 | ChoP, 2Hex, 2Hep, EtnP, 2Kdo, lipid A | ||
| 837.6 | 2,516.0 | 2,515.1 | 4.7 | NeuAc, 2Hex, 2Hep, EtnP, 2Kdo, lipid A | ||
| 1,293.6 | 2,589.9 | 2,589.4 | 10.1 | HexNAc, 3Hex, 2Hep, EtnP, 2Kdo, lipid A | ||
| 1,294.6 | 2,591.1 | 2,592.3 | 6.8 | ChoP, HexNAc, 2Hex, 2Hep, EtnP, 2Kdo, lipid A | ||
| 891.5 | 2,677.4 | 2,677.2 | 4.7 | NeuAc, 3Hex, 2Hep, EtnP, 2Kdo, lipid A | ||
| 1,376.8 | 2,752.1 | 2,751.5 | 6.8 | HexNAc, 4Hex, 2Hep, EtnP, 2Kdo, lipid A | ||
| 959.5 | 2,881.4 | 2,880.4 | 12.2 | NeuAc, HexNAc, 3Hex, 2Hep, EtnP, 2Kdo, lipid A | ||
| 1,457.9 | 2,917.9 | 2,916.6 | 13.5 | ChoP, HexNAc, 4Hex, 2Hep, EtnP, 2Kdo, lipid A | ||
| 1014.1 | 3,045.5 | 3,042.5 | 8.1 | NeuAc, HexNAc, 4Hex, 2Hep, EtnP, 2Kdo, lipid A | ||
| 1068.4 | 3,208.4 | 3,207.6 | 6.8 | NeuAc, ChoP, HexNAc, 4Hex, 2Hep, EtnP, 2Kdo, lipid A | ||
| 738-lob2A1::Km (blood agar) | 1,030.3 | 2,062.5 | 2,061.9 | 7.2 | Hex, 2Hep, EtnP, 2Kdo, lipid A | |
| 1,111.4 | 2,224.9 | 2,224.1 | 25.1 | 2Hex, 2Hep, EtnP, 2Kdo, lipid A | ||
| 1,192.7 | 2,387.4 | 2,386.2 | 9.7 | 3Hex, 2Hep, EtnP, 2Kdo, lipid A | ||
| 1,193.7 | 2,389.6 | 2,389.1 | 23.4 | ChoP, 2Hex, 2Hep, EtnP, 2Kdo, lipid A | ||
| 1,273.3 | 2,548.3 | 2,548.4 | 4.6 | 4Hex, 2Hep, EtnP, 2Kdo, lipid A | ||
| 1,274.8 | 2,551.9 | 2,551.3 | 9.8 | ChoP, 3Hex, 2Hep, EtnP, 2Kdo, lipid A | ||
| 1,337.9 | 891.6 | 2,678.0 | 2,677.2 | 16.2 | NeuAc, 3Hex, 2Hep, EtnP, 2Kdo, lipid A | |
| 946.6 | 2,843.0 | 2,842.3 | 4.0 | NeuAc, ChoP, 3Hex, 2Hep, EtnP, 2Kdo, lipid A | ||
Abbreviations: Hex, hexose; Hep, heptose; HexNAc, N-acetylhexosamine; Kdo, 3-deoxy-d-manno-2-octulosonic acid; EtnP, phosphatidylethanolamine; ChoP, phosphorylcholine; NeuAc, N-acetylneuraminic acid.
Average mass units were used for calculation of molecular weight based on proposed composition as follows: Hex, 162.15; Hep, 192.17; HexNAc, 203.19; Kdo, 220.18; EtnP, 123.05; ChoP, 165.05; NeuAc, 291.00
FIG. 3.
Negative-ion ES-MS of OdA LOS from H. somnus strain 2336 (A) or 738-lob2A1::Km (B). Addition of glycose units is indicated on the spectra. OdA LOS was isolated from cells grown on fresh sheep's blood agar plates supplemented with 50 μg of CMP-NeuAc per ml and analyzed as described in Materials and Methods.
Effect of sialylation on reactivity of LOS with specific antibodies.
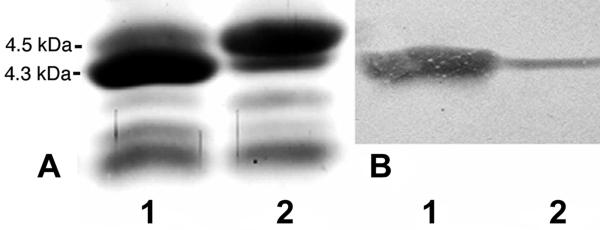
To determine if sialylation interfered with antibody binding, extracted LOS from strain 738 incubated with CMP-NeuAc was tested for reactivity with MAb 5F5.9 (specific for ChoP) by ELISA. Figure 4 shows that after incubation with CMP-NeuAc, strain 738 LOS was less reactive with MAb 5F5.9 than LOS from bacteria incubated in CTT only. However, after the sialylated LOS was treated with neuraminidase, reactivity to 5F5.9 increased to the same degree as reactivity of LOS from cells not incubated with CMP-NeuAc, suggesting that sialylation caused steric interference of the 5F5.9-specific epitope ChoP (13). The reactivity of strain 738 with MAb 5F5.9 is relatively weak (13), at least in part because ChoP is an internal epitope in the oligosaccharide chain (5). MAb 3F11 is specific for the terminal Galβ-(1-4)-GlcNAc epitope of N. gonorrhoeae strain F62 LOS (42). An important difference between H. somnus strain 738 and N. gonorrhoeae strain F62 LOS is that the terminal Galβ-GlcNAc linkage is 1-3 in strain 738, and its LOS is nonreactive with MAb 3F11 (5, 13). However, strain 2336 is reactive with MAb 3F11 (13). Recent nuclear magnetic resonance analysis confirmed that the Gal-GlcNAc linkage in strain 2336 is 1-4 (A. D. Cox, J. Li, M. D. Howard, and T. J. Inzana, submitted for publication). A clonal isolate of strain 2336 intensely reactive with MAb 3F11 (strain 2336-R) was selected following dot blotting and used for sialylation studies. Strain 2336-R LOS was strongly reactive with MAb 3F11 by ELISA, but following incubation of cells with CMP-NeuAc, the reactivity of MAb 3F11 with LOS was reduced to near background levels (Fig. 4). Following neuraminidase treatment, MAb 3F11 reactivity to LOS from cells grown with CMP-NeuAc was restored. Immunoblotting (Western blotting) further showed that MAb 3F11 reacted primarily with the 4.3-kDa band of strain 2336-R LOS and not at all with the 4.5-kDa band from cells grown with or without CMP-NeuAc (Fig. 5B). This indicated that NeuAc blocked accessibility of the 3F11 epitope in the 4.5-kDa band and confirmed that some LOS in strain 2336-R contained NeuAc in the absence of growth with exogenous CMP-NeuAc.
FIG. 4.
Reactivity of H. somnus strain 2336-R and phase variant 738 to LOS MAb determined by ELISA following growth with or without CMP-NeuAc. Bacteria were grown to 109 CFU/ml in CTT, diluted 1:10 in fresh CTT containing 50 μg of CMP-NeuAc per ml, and shaken for 3 h at 37°C. LOS was extracted, and 1 μg/well was used as antigen in the ELISA with MAb 5F5.9 (strain 738) or 3F11 (strain 2336-R). For some assays, 5 μg of LOS was pretreated with 5.5 U of V. cholerae neuraminidase for 1 h at 37°C. +S, LOS from cells grown with CMP-NeuAc; +N, LOS treated with neuraminidase; +S +N, LOS from cells grown with CMP-NeuAc and treated with neuraminidase. Error bars indicate standard deviations.
FIG. 5.
Electrophoretic profiles (A) and Western blots with MAb 3F11 (B) of LOS from H. somnus strain 2336-R grown in the absence (lanes 1) or presence (lanes 2) of CMP-NeuAc. There is no change in the profile, but note the increased quantity of the 4.5-kDa band when H. somnus is grown in the presence of CMP-NeuAc and the lack of reactivity of MAb 3F11 with the 4.5-kDa band.
Bactericidal activity.
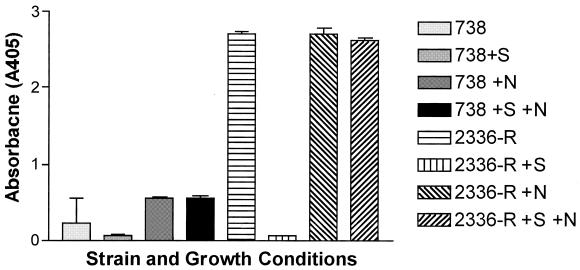
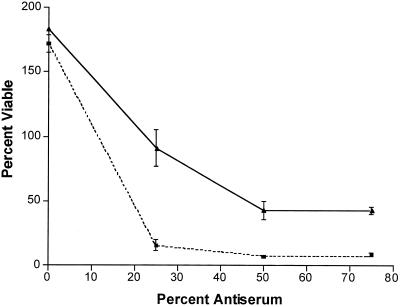
Bactericidal assays were used to determine if attachment of NeuAc to the LOS of H. somnus could contribute to resistance to serum. Preliminary assays showed that strains 2336-R and 738 were somewhat resistant to killing by normal serum but that the truncated mutant 738-lob2A1::Km was susceptible. Therefore, strain 738-lob2A1::Km was used for bactericidal assays involving normal serum. The bactericidal activity of normal bovine serum with strain 738-lob2A1::Km was dose variable and was significantly greater in 25% serum (P = 0.0074) and in 50% (or greater) serum (P = 0.0066) when strain 738-lob2A1::Km was grown in the absence of CMP-NeuAc than when CMP-NeuAc was present (Fig. 6). The preputial isolates were highly susceptible to killing by even small amounts of normal serum. However, the addition of CMP-NeuAc did not enhance the resistance of strain 1P or 127P to killing by antibody-deficient precolostral calf serum (P ≥ 0.1 for both strains). Following incubation in 10% precolostral calf serum only, 70% ± 4% of strain 1P cells grown in CTT only remained viable, compared with 54% ± 13% of those grown in CTT plus CMP-NeuAc. Strain 127P grown in CTT remained only 49% ± 3% viable in the presence of 10% precolostral calf serum, and when grown in CTT plus CMP-NeuAc, 55% ± 4% remained viable.
FIG. 6.
Bactericidal activity of normal bovine serum against H. somnus strain 738-lob2A1::Km, which is deficient in the terminal Galβ-(1-3)-GlcNAc disaccharide on its LOS. Log-phase bacteria were incubated for 3 h with (solid line) or without (dashed line) CMP-NeuAc and then incubated with various concentrations of normal calf serum for 1 h, as described in Materials and Methods. Bactericidal activity was determined by viable plate count at time zero and 60 min after incubation. Error bars indicate standard deviations.
In order to assess if LOS sialylation enhanced the viability of serum-resistant strains in normal serum, 15% antiserum to strain 738 LOS and 25% precolostral calf serum (as a complement source) were tested in bactericidal assays with strain 738. Furthermore, pyruvate (and to a lesser extent lactate) has been shown to enhance the effect of CMP-NeuAc in enhancing resistance to serum in gonococci (26). Therefore, 20 mM pyruvate or lactate was added with CMP-NeuAc during growth prior to some bactericidal assays. In the absence of CMP-NeuAc, 39.1% ± 5.4% of strain 738 cells remained viable, whereas no change in viability was noted when pyruvate only was added to the growth medium (40.3% ± 6.2% remained viable; P = 0.84). When CMP-NeuAc or CMP-NeuAc and pyruvate were added to the growth medium. 60.9% ± 7.2% and 61.7% ± 1.3% remained viable, respectively. Similar results were obtained when 20 mM lactate was added to growth medium with or without CMP-NeuAc (data not shown). Therefore, the addition of pyruvate or lactate to the medium did not significantly enhance bactericidal resistance (P = 0.26), but the addition of CMP-NeuAc did (P < 0.01). In other experiments when the percentage of antiserum was increased to 30% and that of precolostral calf serum was reduced to 15%, 12.9% ± 1.5% of strain 738 cells grown without CMP-NeuAc remained viable, whereas 86.5% ± 3.5% remained viable following growth in medium containing CMP-NeuAc (P < 0.0001). A similar difference was seen when phase variant strain 807 was grown in the presence or absence of CMP-NeuAc prior to the bactericidal assay (data not shown).
Sialyltransferase activity and capillary electrophoresis assay.
To further assess whether H. somnus LOS is sialylated due to the transfer of NeuAc from CMP-NeuAc, we used the CMP-[14C]NeuAc sialyltransferase assay described by Parsons et al. (31) for N. gonorrhoeae. A high level of [14C] was incorporated into purified H. somnus strain 2336-R and 738-lob2A1::Km LOS by Nonidet P-40 extracts of N. gonorrhoeae (Table 2). However, no transfer of [14C] was detected when cell extracts from H. somnus strain 2336-R, 738, or 738-lob2A1::Km were incubated with LOS from strain 738-lob2A1::Km or 2336-R, both of which can be well sialylated (Table 1). When CMP-[14C]NeuAc was incorporated into the growth medium with CMP-NeuAc, however, a high level of [14C] was detected in H. somnus strains 2336-R and 738-lob2A1::Km but [14C] was not detected in strain 1P (Table 2), supporting previous results that the LOS of the preputial isolates tested are not capable of being sialylated. These results also suggested that the sialyltransferase used by H. somnus is distinct from that of N. gonorrhoeae or at least is more sensitive to the detergent or extraction procedure.
TABLE 2.
Incorporation of [14C]NeuAc into LOS or cells by Nonidet P-40 extracts or by growth in CTT supplemented with CMP-[14C]NeuAc
| Extract or growth medium | Radioactivity (dpm) of:
|
||||||
|---|---|---|---|---|---|---|---|
| LOS
|
Cells
|
||||||
| None | 738-lob2A1::Km | 2336 | None | 738-lob2A1::Km | 2336 | 1P | |
| None extract | 27 | NDb | 39 | ||||
| N. gonorrhoeae | 573 | 4,788 | 7,177 | ||||
| H. somnus 738 | 51 | 38 | 29 | ||||
| H. somnus 738-lob2A1::Km | 24 | ND | 23 | ||||
| H. somnus 2336-R | 27 | ND | 26 | ||||
| CTT-CMP-[14C]NeuAc | 22 | 5,259 | 5,916 | 19 | |||
Nonidet P-40 cell extracts were prepared as described for measurement of N. gonorrhoeae sialyltransferase activity (31).
ND, not determined.
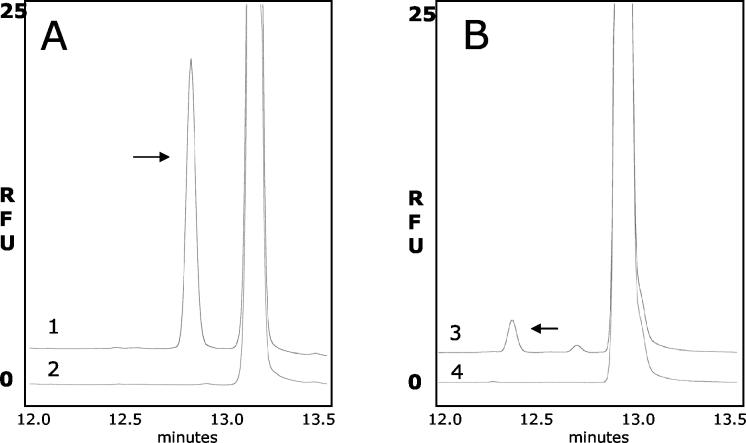
Sialyltransferase activity in H. somnus extracts was confirmed by capillary electrophoresis (Fig. 7). In the presence of CMP-NeuAc, NeuAc was preferentially incorporated onto the lacto-N-tetraose-APTS structure (Fig. 7A), as determined by the intensity of fluorescence, compared to the incorporation of APTS onto lacto-N-neo-tetraose (Fig. 7B). Thus, the H. somnus strain 738 sialyltransferase shows a greater affinity for Galβ-(1-3)-GlcNAc disaccharide than for Galβ-(1-4)-GlcNAc.
FIG. 7.
Capillary electrophoresis assay of sialyltransferase activity in H. somnus strain 738. The enzyme activity was measured with lacto-N-tetraose or lacto-N-neo-tetraose, which were labeled with APTS. (A) Electropherogram from the reaction of H. somnus strain 738 extract with lacto-N-tetraose-APTS. Trace 1 is from the complete enzyme reaction, and trace 2 is from a reaction without the donor sugar nucleotide CMP-NeuAc. (B) The same type of analysis with lacto-N-neo-tetraose-APTS. Trace 3 is from the complete reaction, and trace 4 is from the incomplete reaction. RFU, relative fluorescence units. The arrows show the product formed in the reaction.
DISCUSSION
Various bacteria are capable of incorporating NeuAc into their LOS or cell membrane or synthesize a capsule composed of NeuAc. H. influenzae, N. gonorrhoeae, and N. meningitidis typify bacteria that can sialylate their LOS, whereas some strains of Escherichia coli and N. meningitidis also produce capsular polysaccharides containing NeuAc (22). The mechanism by which microorganisms metabolize and incorporate NeuAc varies, however. Because the primary oligosaccharide chains of H. somnus strains 738 and 2336 LOS are similar to that of N. gonorrhoeae strain F62 LOS (5, 42), we sought to determine if H. somnus could also incorporate NeuAc into its LOS. N. gonorrhoeae cannot synthesize or activate NeuAc but utilizes a surface α-2,3-sialylatransferase to transfer NeuAc from CMP-NeuAc, which is present in human blood, to the terminal galactose residue of the Galβ-(1-4)-GlcNAc LOS component of lacto-N-neo-tetraose (7, 24). This modification results in an increase in molecular mass to a 4.5-kDa LOS component, as determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (24). We determined that H. somnus strain 738 could also sialylate its LOS when incubated with CMP-NeuAc or NeuAc under the same conditions used for N. gonorrhoeae, as determined by reactivity with LFA; an increase in the sizes of the 3.7-, 4.0-, and 4.3-kDa LOS components; and ES-MS. Furthermore, incubation of CMP-NeuAc with strain 738-lob2A1::Km, which is deficient in the attachment of the terminal Galβ-(1-3)-GlcNAc epitope of lacto-N-tetraose, also resulted in an increase in the size of primarily the 3.7-kDa LOS moiety. ES-MS analysis demonstrated that the three-hexose glycoform of 738-lob2A1::Km LOS is most efficiently sialylated. This indicated that the Gal glycose of the Galβ-(1-3)-Glcβ-(1-4)-Glc trisaccharide in the LOS of strain 738 can also be sialylated, as well as the two-hexose glycoform with the disaccharide acceptor Galβ-(1-4)-Glc (5) (Table 1). However, strain 2336 contained the 4.5-kDa LOS band in the absence of growth with CMP-NeuAc, although when CMP-NeuAc or NeuAc was added to the growth medium, the 4.5-kDa band increased substantially in intensity. Furthermore, when strain 2336 LOS was treated with neuraminidase, the 4.5-kDa moiety completely disappeared, as was the case for strain 738 LOS. These results indicate that H. somnus can utilize CMP-NeuAc or NeuAc from its growth medium for sialylation of its LOS. The differences in oligosaccharide structure and sialylation between strains 2336 and 738 are not clear, since 738 was derived from pneumonia isolate 2336 (10). However, 738 was a clonal isolate derived from 2336 passaged in another calf by intrabronchial inoculation. Therefore, if either the calf with the original infection or the calf used for passage was also colonized with another strain of H. somnus, then 738 and 2336 could be distinct strains.
It is apparent that strain 738 makes an α-2,3-sialyltransferase, which is found in the membrane fraction, like the α-2,3-sialyltransferases from H. influenzae, N. meningitidis, or N. gonorrhoeae (9, 12). The acceptor specificity was examined by the comparison of two tetrasaccharide acceptors, which differ only by the linkage of the terminal galactose residue. These acceptor molecules are very similar to those found in the outer core of H. somnus strain 738. The enzyme showed a marked preference for a terminal Galβ that is 1,3 linked to the adjacent GlcNAc. This is the opposite of the acceptor specificity that was observed for the α-2,3-sialyltransferase from N. meningitidis, where the preference was for the Galβ that is 1,4 linked to the adjacent GlcNAc (9). This may not reflect the preference in vivo, however, as only very small amounts of enzyme activity are required to sialylate LOS, and the sialylation of LOS is likely to be influenced by the conformation of the acceptor as well as the presence or absence of other modifications on the LOS, such as phosphoethanolamine or ChoP (38).
LOS from abortion isolate 649 and from all of the preputial isolates examined did not change in electrophoretic profile or in band intensity following incubation with CMP-NeuAc, nor was any NeuAc detected in these strains by LFA or ES-MS. Whether these strains cannot sialylate their LOS due to lack of the enzymes required for sialylation or due to an oligosaccharide chain that cannot accept NeuAc has yet to be determined. However, the present study and previous studies have shown that MAb to the LOS of strains 649 and 2336 do not cross-react with each other (Fig. 6) (13, 20), suggesting that the oligosaccharide of strain 649 LOS may be distinct from those of most other H. somnus disease isolates.
Unlike N. gonorrhoeae, H. influenzae and H. ducreyi can utilize and activate NeuAc directly from the growth medium to sialylate their LOS (24). Vimr et al. (37) demonstrated that H. influenzae is able to metabolize free NeuAc for nutritional purposes as well as for incorporation into its LOS by activation of NeuAc. Hypersialylation of the LOS by an aldolase-deficient mutant indicated that NeuAc was activated and incorporated directly into the LOS and was not incorporated through other metabolic pathways. Schilling et al. (35) showed that H. ducreyi also utilizes free NeuAc for incorporation into its LOS but that it can also incorporate N-glycolylneuraminc acid into its LOS. However, N-acetylmannosamine was not utilized to synthesize NeuAc, indicating that sialylation of LOS in H. ducreyi also does not occur through catabolic mechanisms. Although the capability of H. somnus to catabolize NeuAc or utilize other metabolites to generate NeuAc was not investigated, it was clear that H. somnus did not require CMP-NeuAc as a donor and could utilize NeuAc for incorporation into its LOS, presumably via the action of CMP-NeuAc synthetase. Thus, sialylation of H. somnus LOS appears to occur through pathways distinct from those used by N. gonorrhoeae, which was supported further by the failure of detergent extracts of H. somnus to incorporate [14C]NeuAc from CMP-[14C]NeuAc into LOS by a sialyltransferase assay. However, H. somnus LOS could serve as an acceptor molecule for [14C] NeuAc in the presence of the N. gonorrhoeae CMP-sialyltransferase. It is possible that the H. somnus sialyltransferase is inactivated by the Nonidet P-40 detergent or the extraction procedure used. Mandrell et al. found that extracts of H. influenzae and H. ducreyi also cannot sialylate LOS (33). Therefore, although the oligosaccharide structure of H. somnus LOS is very similar to that of N. gonorrhoeae, sialylation of the LOS appears to occur by a mechanism that may be more similar to that of other Haemophilus spp.
Both Western blotting and ELISA experiments demonstrated that the presence of NeuAc on H. somnus LOS could block binding of MAb 3F11 to the Galβ-(1-4)-GlcNAc epitope. Similar results were obtained in sialylation studies with N. gonorrhoeae (24, 32). Sialylation also inhibited MAb 5F5.9 binding to ChoP in an ELISA, indicating that sialylation may also block the binding of antibodies to epitopes that are not specifically sialylated.
Several distinctions have been made between clinical isolates and commensal, urogenital (particularly preputial) isolates of H. somnus. Unlike preputial isolates, clinical isolates can undergo LOS phase variation, may possess additional surface proteins, and are more virulent and serum resistant (2, 3, 19, 39). We now report that most clinical H. somnus isolates are capable of sialylating their LOS, whereas the preputial isolates tested cannot. Sialylation of the LOS was associated with reduced antibody binding and enhanced resistance to the bactericidal action of normal and immune sera. Therefore, lack of LOS sialylation may be a contributing factor to the susceptibility of some H. somnus isolates to serum. The mechanism of enhanced resistance to serum due to sialylation is currently under investigation, but it may be due to inhibition of complement activation and binding of complement components to the cell membrane (32, 34).
In summary, H. somnus was capable of incorporating NeuAc into its LOS through the utilization of CMP-NeuAc or NeuAc, and an H. somnus sialyltransferase was identified. LOS sialylation inhibited the binding of at least some antibodies to LOS and enhanced resistance to serum. Whether LOS sialylation enhances H. somnus virulence or general resistance to host immunity is currently under investigation in our laboratory.
Acknowledgments
We thank Becky Stormer for technical assistance, Don Krajcarski and Jianjun Li for ES-MS analysis, Doug Griffith for assistance with bacterial cell growth, Lynette Corbeil for supplying H. somnus strains, Herbert Schenieder for providing N. gonorrhoeae, Allan Lesse for providing MAb 5F5.9, and Michael Apicella for providing MAb 3F11 and for valuable discussions and suggestions.
This work was supported by National Research Initiative grant 99-35204-7670 from the U.S. Department of Agriculture/Cooperative State Research, Education, and Extension Service to T.J.I. and by HATCH formula funds to the Virginia State Agricultural Experiment Station.
Editor: R. N. Moore
REFERENCES
- 1.Chiang, Y.-W., M. L. Kaeberle, and J. A. Roth. 1986. Identification of suppressive components in “Haemophilus somnus” fractions which inhibit bovine polymorphonuclear leukocyte function. Infect. Immun. 52:792-797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cole, S. P., D. G. Guiney, and L. B. Corbeil. 1992. Two linked genes for outer membrane proteins are absent in four non-disease strains of Haemophilus somnus. Mol. Microbiol. 6:1895-1902. [DOI] [PubMed] [Google Scholar]
- 3.Corbeil, L. B., K. Blau, D. J. Prieur, and A. C. S. Ward. 1985. Serum susceptibility of Haemophilus somnus from bovine clinical cases and carriers. J. Clin. Microbiol. 22:192-198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Corbeil, L. B., R. P. Gogolewski, L. R. Stephens, and T. J. Inzana. 1995. Haemophilus somnus: antigen analysis and immune responses, p. 63-73. In W. Donachie, F. A. Lainson, and J. C. Hodgson (ed.), Haemophilus, Actinobacillus, and Pasteurella. Plenum Press, New York, N.Y.
- 5.Cox, A. D., M. D. Howard, J.-R. Brisson, M. Van Der Zwan, P. Thibault, M. B. Perry, and T. J. Inzana. 1998. Structural analysis of the phase-variable lipooligosaccharide from Haemophilus somnus strain 738. Eur. J. Biochem. 253:507-516. [DOI] [PubMed] [Google Scholar]
- 6.Czuprynski, C. J., and H. L. Hamilton. 1985. Bovine neutrophils ingest but do not kill Haemophilus somnus in vitro. Infect. Immun. 50:431-436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gao, L., L. Linden, N. J. Parsons, J. A. Cole, and H. Smith. 2000. Uptake of metabolites by gonococci grown with lactate in a medium containing glucose: evidence for a surface location of the sialyltransferase. Microb. Pathog. 28:257-266. [DOI] [PubMed] [Google Scholar]
- 8.Gilbert, M., A. M. Cunningham, D. C. Watson, A. Martin, J. C. Richards, and W. W. Wakarchuk. 1997. Characterization of a recombinant Neisseria meningitidis alpha-2,3-sialyltransferase and its acceptor specificity. Eur. J. Biochem. 249:187-194. [DOI] [PubMed] [Google Scholar]
- 9.Gilbert, M., D. C. Watson, A. M. Cunningham, M. P. Jennings, N. M. Young, and W. W. Wakarchuk. 1996. Cloning of the lipooligosaccharide α-2,3-sialyltransferase from the bacterial pathogens Neisseria meningitidis and Neisseria gonorrhoeae. J. Biol. Chem. 271:28271-28276. [DOI] [PubMed] [Google Scholar]
- 10.Gogolewski, R. P., D. C. Schaefer, S. K. Wasson, R. R. Corbeil, and L. B. Corbeil. 1989. Pulmonary persistence of Haemophilus somnus in the presence of specific antibody. J. Clin. Microbiol. 27:1767-1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gomis, S. M. 1998. Intracellular survival of Haemophilus somnus in bovine blood monocytes and alveolar macrophages. Microb. Pathog. 25:227-235. [DOI] [PubMed] [Google Scholar]
- 12.Hood, D. W., A. D. Cox, M. Gilbert, K. Makepeace, S. Walsh, M. E. Deadman, A. Cody, A. Martin, M. Månsson, E. K. H. Schweda, J. R. Brisson, J. C. Richards, E. R. Moxon, and W. W. Wakarchuk. 2001. Identification of a lipopolysaccharide α-2,3-sialyltransferase from Haemophilus influenzae. Mol. Microbiol. 39:341-350. [DOI] [PubMed] [Google Scholar]
- 13.Howard, M. D., A. D. Cox, J. N. Weiser, G. G. Schurig, and T. J. Inzana. 2000. Antigenic diversity of Haemophilus somnus lipooligosaccharide: phase-variable accessibility of the phosphorylcholine epitope. J. Clin. Microbiol. 38:4412-4419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hubbard, R. D., M. L. Kaeberle, J. A. Roth, and Y. W. Chang. 1986. Haemophilus somnus-induced interference with bovine neutrophil functions. Vet. Microbiol. 12:77-85. [DOI] [PubMed] [Google Scholar]
- 15.Inzana, T. J. 1983. Electrophoretic heterogeneity and interstrain variation of the lipopolysaccharide of Haemophilus influenzae. J. Infect. Dis. 148:492-499. [DOI] [PubMed] [Google Scholar]
- 16.Inzana, T. J. 1998. Haemophilus somnus infections, p. 358-361. In J. L. Howard and R. Smith (ed.), Current veterinary therapy: food animal practice 4, vol. 4. W. B. Saunders Company, Philadelphia, Pa.
- 17.Inzana, T. J., and P. Anderson. 1985. Serum factor-dependent resistance of Haemophilus influenzae type b to antibody to lipopolysaccharide. J. Infect. Dis. 151:869-877. [DOI] [PubMed] [Google Scholar]
- 18.Inzana, T. J., R. P. Gogolewski, and L. B. Corbeil. 1992. Phenotypic phase variation in Haemophilus somnus lipooligosaccharide during bovine pneumonia and after in vitro passage. Infect. Immun. 60:2943-2951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Inzana, T. J., J. Hensley, J. McQuiston, A. J. Lesse, A. A. Campagnari, S. M. Boyle, and M. A. Apicella. 1997. Phase variation and conservation of lipooligosaccharide epitopes in Haemophilus somnus. Infect. Immun. 65:4675-4681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Inzana, T. J., B. Iritani, R. P. Gogolewski, S. A. Kania, and L. B. Corbeil. 1988. Purification and characterization of lipooligosaccharides from four strains of “Haemophilus somnus.” Infect. Immun. 56:2830-2837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Inzana, T. J., and J. Todd. 1992. Immune response of cattle to an Haemophilus somnus lipid A-protein conjugate vaccine and efficacy in a mouse model. Am. J. Vet. Res. 53:175-179. [PubMed] [Google Scholar]
- 22.Kenne, L., and B. Lindberg. 1983. Bacterial polysaccharides, vol. 2. Academic Press, Inc., New York, N.Y.
- 23.Mandrell, R. E., J. J. Kim, C. M. John, B. W. Gibson, J. V. Sugai, M. A. Apicella, J. M. Griffiss, and R. Yamasaki. 1991. Endogenous sialylation of the lipooligosaccharides of Neisseria meningitidis. J. Bacteriol. 173:2823-2832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mandrell, R. E., A. J. Lesse, J. V. Sugai, M. Shero, J. M. Griffiss, J. A. Cole, N. J. Parsons, H. Smith, S. A. Morse, and M. A. Apicella. 1990. In vitro and in vivo modification of Neisseria gonorrhoeae lipooligosaccharide epitope structure by sialylation. J. Exp. Med. 171:1649-1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mandrell, R. E., R. McLaughlin, Y. A. Kwaik, A. Lesse, R. Yamasaki, B. Gibson, S. M. Spinola, and M. A. Apicella. 1992. Lipooligosaccharides (LOS) of some Haemophilus species mimic human glycosphingolipids, and some LOS are sialylated. Infect. Immun. 60:1322-1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McGee, D. J., and R. F. Rest. 1996. Regulation of gonococcal sialytransferase, lipooligosaccharide, and serum resistance by glucose, pyruvate, and lactate. Infect. Immun. 64:4630-4637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McQuiston, J. H., J. R. McQuiston, A. D. Cox, Y. Wu, S. M. Boyle, and T. J. Inzana. 2000. Characterization of a DNA region containing 5′-CAAT-3′ DNA sequences involved in lipooligosaccharide biosynthesis in Haemophilus somnus. Microb. Pathog. 28:301-312. [DOI] [PubMed] [Google Scholar]
- 28.Melaugh, W., B. W. Gibson, and A. A. Campagnari. 1996. The lipooligosaccharides of Haemophilus ducreyi are highly sialylated. J. Bacteriol. 178:564-570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nairn, C. A., J. A. Cole, P. V. Patel, N. J. Parsons, J. E. Fox, and H. Smith. 1988. Cytidine 5′-monophospho-N-acetylneuraminic acid or a related compound is the low Mr factor from human red blood cells that induces gonococcal resistance to killing by human serum. J. Gen. Microbiol. 134:3295-3306. [DOI] [PubMed] [Google Scholar]
- 30.Parsons, N. J., P. V. Patel, E. L. Tan, J. R. Andrade, C. A. Nairn, M. Goldner, J. A. Cole, and H. Smith. 1988. Cytidine 5′-monophospho-N-acetyl neuraminic acid and a low molecular weight factor from human blood cells induce lipopolysaccharide alteration in gonococci when conferring resistance to killing by human serum. Microb. Pathog. 5:303-309. [DOI] [PubMed] [Google Scholar]
- 31.Parsons, N. J., J.-P. Emond, M. Goldner, J. Bramley, H. Croke, J. A. Cole, and H. Smith. 1996. Lactate enhancement of sialylation of gonococcal lipopolysaccharide and of induction of serum resistance by CMP-NANA is not due to direct activation of the sialyltransferase: metabolic events are involved. Microb. Pathog. 21:193-204. [DOI] [PubMed] [Google Scholar]
- 32.Parsons, N. J., J. R. C. Andrade, P. V. Patel, J. A. Cole, and H. Smith. 1989. Sialylation of lipopolysaccharide and loss of absorption of bactericidal antibody during conversion of gonococci to serum resistance by cytidine 5′-monophospho-N-acetyl neuraminic acid. Microb. Pathog. 7:63-72. [DOI] [PubMed] [Google Scholar]
- 33.Preston, A., R. E. Mandrell, B. W. Gibson, and M. A. Apicella. 1996. The lipopolysaccharides of pathogenic gram-negative bacteria. Crit. Rev. Microbiol. 22:139-180. [DOI] [PubMed] [Google Scholar]
- 34.Ram, S., A. K. Sharma, S. D. Simpson, S. Gulati, D. P. McQuillen, M. K. Pangburn, and P. A. Rice. 1998. A novel sialic acid binding site on factor H mediates serum resistance of sialylated Neisseria gonorrhoeae. J. Exp. Med. 187:743-752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schilling, B., S. Goon, N. M. Samuels, S. P. Gaucher, J. A. Leary, C. R. Bertozzi, and B. W. Gibson. 2001. Biosynthesis of sialylated lipooligosaccharides in Haemophilus ducreyi is dependent on exogenous sialic acid and not mannosamine. Incorporation studies using N-acylmannosamine analogues, N-glycolylneuraminic acid, and 13C-labeled N-acetylneuraminic acid. Biochemistry 40:12666-12677. [DOI] [PubMed] [Google Scholar]
- 36.Tsai, C.-M., and C. E. Frasch. 1982. A sensitive silver stain for detecting lipopolysaccharides in polyacrylamide gels. Anal. Biochem. 119:115-119. [DOI] [PubMed] [Google Scholar]
- 37.Vimr, E., C. Lichtensteiger, and S. Steenbergen. 2000. Sialic acid metabolism's dual function in Haemophilus influenzae. Mol. Microbiol. 36:1113-1123. [DOI] [PubMed] [Google Scholar]
- 38.Wakarchuk, W. W., D. Watson, F. St. Michael, J. Li, Y. Wu, J. R. Brisson, N. M. Young, and M. Gilbert. 2001. Dependence of the bi-functional nature of a sialyltransferase from Neisseria meningitidis on a single amino acid substitution. J. Biol. Chem. 276:12785-12790. [DOI] [PubMed] [Google Scholar]
- 39.Widders, P. R., L. A. Dorrance, M. Yarnall, and L. B. Corbeil. 1989. Immunoglobulin-binding activity among pathogenic and carrier isolates of Haemophilus somnus. Infect. Immun. 57:639-642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Widders, P. R., J. W. Smith, M. Yarnall, T. C. McGuire, and L. B. Corbeil. 1988. Non-immune immunoglobulin binding of Haemophilus somnus. J. Med. Microbiol. 26:307-311. [DOI] [PubMed] [Google Scholar]
- 41.Wu, Y., J. H. McQuiston, A. Cox, T. D. Pack, and T. J. Inzana. 2000. Molecular cloning and mutagenesis of a DNA locus involved in lipooligosaccharide biosynthesis in Haemophilus somnus. Infect. Immun. 68:310-319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yamasaki, R., W. Nasholds, H. Schneider, and M. A. Apicella. 1991. Epitope expression and partial structural characterization of F62 lipooligosaccharide (LOS) of Neisseria gonorrhoeae. Mol. Immunol. 28:1233-1242. [DOI] [PubMed] [Google Scholar]
- 43.Yarnall, M., P. R. Widders, and L. B. Corbeil. 1988. Isolation and characterization of Fc receptors from Haemophilus somnus. Scand. J. Immunol. 28:129-137. [DOI] [PubMed] [Google Scholar]