Abstract
The plethora of changes associated with immunosenescence radically alters virtually all aspects of immune responsiveness. How this transformation effects resolution of an infectious challenge is addressed in this study. A well-established infection model was used; Trichuris muris, a cecum-dwelling helminth, is natural to mice, and infection in different strains results in clearly polarized responses. A dominating T helper 2 (Th2) response orchestrates immunity, whereas a Th1 response will result in susceptibility. Mice between 19 and 28 months old were more susceptible to infection, whereas 3-month-old mice of the same strain demonstrated the resistant phenotype. The cytokine response made by these aged mice was clearly altered at the site of infection, and within the local draining lymph nodes higher Th1 and lower Th2 cytokine levels were found, both at the protein and RNA level. Confirming these changes, aged mice also showed a delayed parasite-specific immunoglobulin G1 response and intestinal mastocytosis, both of which are driven by Th2 cytokines. To address possible causes of the observed immune deviation, purified CD4 cells from both young and aged mice were stimulated in vitro. Cells from aged mice did not respond to stimulation via CD28 and in vitro were less able to proliferate and polarize into Th2 cells; Th1 polarization was found to be normal. Together these data suggest that changes in cytokine phenotype, particularly CD4 cells, contribute to the observed age-associated switch from T. muris resistance to susceptibility.
Understanding changes occurring within an ageing immune system is essential if public heath authorities are to be equipped to manage an ageing population. Specifically, knowledge of altered immune responses to infectious agents is required if rational clinical interventions are to be tailored to these ageing individuals.
The catalogue of changes occurring within the immune system of the ageing individual is extensive, with major changes in T-cell responses (reviewed in reference 36), including increasing frequency of memory phenotype cells (19, 50), clonal exhaustion (52, 53; reviewed in reference 41), thymic involution (45), and disrupted costimulation (9, 18). Interleukin-2 (IL-2) production is reduced (40), and the Th1-Th2 cytokine balance is disrupted. Previous work studying T-cell cytokine responses from aged individuals has largely been done in vitro, often resulting in conflicting data (27, 33).
The in vivo balance between Th1 and Th2 cytokines is critical with respect to the control of certain diseases; models of allergic asthma (35, 49) and rheumatoid arthritis (47) have demonstrated that the deregulation of cytokine balance can lead to increased autoimmune pathology. Studies manipulating Th1, Th2 cytokines within parasitic disease models have provided major insights into the pivotal role cytokine phenotype can play in the regulation of disease severity (reviewed in reference 4). A dominating type 2 response is required to expel intestinal parasites Trichinella spiralis, Nippostrogylus brasiliensis, Heligmosomoides polygyrus, and Trichuris muris, with type 1 cytokines being associated with increased susceptibility in mice. In the T. muris infection model (24), a dominating type 1 response, whether natural to the mouse strain or induced in a resistant strain by the addition of anti-IL-4 receptor monoclonal antibodies (MAbs) (14) or murine recombinant IL-12 (3), results in chronic infection. Thus, an age-associated deregulation of T-helper-cell subsets and subsequent changes within the large intestine may have serious consequences for the control of infection, particularly parasitic disease.
Here we use this well-defined in vivo intestinal parasite infection model to address this issue, helping to define which T-helper response is initiated in response to an infectious agent in the ageing animal and establishing the consequences of any changes in terms of immunological and immunopathological responses to the parasite. Using in vitro techniques we investigate possible causes of any observed age-related changes in host parasite immunity.
Data presented here demonstrate the ageing mouse is phenotypically different from the young animal, with increased susceptibility to chronic T. muris infection, due largely to a reduced Th2 and increased Th1 cytokine response. These changes are clearly evident within both the large intestine (immunologically and pathologically) and the associated draining lymph nodes with associated systemic antibody effects. The naïve gut environment of the ageing animal was also found to be more proinflammatory, with higher IL-18, tumor necrosis factor (TNF) alpha, and gamma interferon (IFN-γ) mRNA levels. In vitro data demonstrate a defect in ageing T-cell costimulation and the subsequent ability of the cells to differentiate into Th2 effector cells.
MATERIALS AND METHODS
Mice.
A colony of ageing C57BL/Icrfat mice are maintained as a pure inbred line at the University of Manchester; the survival characteristics of these animals have been described in reference 12. Mice 19 to 28 months old were used with sex-matched 3-month-old mice; all experiments used group sizes between four and eight. The colony of ageing mice has been kept under pathogen-free conditions and monitored for the last 28 years. All aspects of physiology and health are constantly assessed, and the animals are currently housed in individual ventilated cage racking systems. Animals were screened for abnormal gross pathology such as lymphomas prior to inclusion in the study; all work was performed under the regulations of the Home Office Scientific Procedures Act (1986).
Parasites.
T. muris was maintained as previously described (51). Mice were orally infected with ∼150 infective eggs on day 0, and parasite burdens were assessed on days 11, 21, and 35 postinfection (p.i.) as described previously (16). T. muris excretory-secretory antigen (ES Ag) was prepared from adult worms following 4 h in in vitro culture; the resulting supernatant was concentrated, sterilized, and assayed for protein concentration by the Lowry assay (38).
Cell culture, antibody, and cytokine reagents.
Mesenteric lymph nodes (MLN) were removed aseptically, and a single-cell suspension was created in RPMI 1640 (supplemented with 10% fetal calf serum, 2 mM l-glutamine, penicillin [100 U/ml], streptomycin [100 μg/ml; GIBCO BRL], and 60 μM monothioglycerol [Sigma Chemical Co.]). MLN cells at 5 × 106 cells/ml were cultured in the presence of of T. muris ES Ag (50 μg/ml) at 37°C for 24 h. Anti-IL-4 receptor (M1) MAb (5 μg/ml) was added to aid detection of IL-4 within the supernatants (C. Maliszewski, Immunex Co., Seattle, Wash.). Cells were pelleted, and supernatants was stored at −20°C.
Each supernatant was assayed by sandwich enzyme-linked immunosorbent assay (ELISA) for the presence cytokines: IL-4, using MAb BVC4-1D11 and BVD6-24G2.3; IL-5, using MAb TRFK.5 and TRFK.4 (PharMingen); IL-9, using MAb 229.4 (E. Schmitt, University of Mainz) and 2C12 (J. Van Snick, Ludwig Institute of Cancer Research, Brussels, Belgium); IL-12, using MAb C15.6 and C17.8 (G. Trinchieri, Wistar Institute, Philadelphia, Pa.); and IFN-γ, using MAb R46A2 and XMG1.2 (PharMingen). A sample was considered positive if the optical density was more than the mean + 3 standard deviations of 16 control wells containing medium alone, quantified using commercially available recombinant murine cytokine standards.
Serum was assayed by capture ELISA for parasite-specific immunoglobulin G1 (IgG1) and IgG2a as described in reference 17. Briefly, Immulon 96-well plates (Dynex) were coated with 5-μg/ml T. muris ES Ag and incubated with serum diluted through eight serial 2-fold dilutions from 1:20 to 1:2,560. Parasite-specific Ig was detected using biotinylated anti-murine IgG1 (Serotec) and IgG2a (PharMingen).
Histology and immunohistochemistry.
For quantifying mastocytosis within the large intestine, ∼3 mm of gut was taken from the cecal tip of each animal and fixed in Carnoys fluid (39). This tissue was embedded in wax, sectioned at 5 μm, dewaxed, and stained in toluidine blue (BDH) for 24 h. Mastocytosis was assessed as the number of mast cells per 20 crypt units; mast cells were counted in two sections per animal.
To visualize IFN-γ within the gut, cecal snip specimens ∼7 mm in length were washed in phosphate-buffered saline, embedded in OCT (Sakura Finetechnical Co.), snap frozen in isopentane (BDH), cooled in liquid nitrogen, and stored at −80°C. Frozen sections were cut at 3 μm, dried, stained using biotinylated anti-mouse IFN-γ MAb (XMG1.2 PharMingen), and visualized with 3,3′-diaminobenzidine (Dako). Sections were counterstained with Haemalum (Mayer's) (DBH), dehydrated, and mounted using DePeX (DBH). Positive staining intensity range was identified and quantified using Scion Image software.
Cell proliferation.
Analysis of in vitro cell proliferation was carried out in 96-well tissue culture plates (Nunc) using MLN cells, isolated as described above. Cells were cultured at 2 × 104 cells/well (to reduce cellular interactions) in the presence or absence of plate-bound anti-mouse CD3ɛ (PharMingen) at 3 μg/ml and soluble anti-mouse CD28 (PharMingen) at 10 μg/ml for 72 h. [6-3H] thymidine was added after 48 h. Fluorescence-activated cell sorter (FACS) analysis was performed both before and after in vitro stimulation using MAbs for murine anti-CD4, anti-CD8, and anti-CD28 (PharMingen).
CD4+ purification, 5-6-carboxyfluorescein diacetate, succinimidyl ester (CFSE) labeling, and Th1 Th2 polarization.
CD4+ cell purification was carried out by negative beading; MLN cells were isolated as described above, resuspended at 2 × 107 cells/ml, and incubated on ice for 30 min with anti-CD8 and anti-B220 MAbs (10 μg/ml; PharMingen). Cells were then washed and resuspended with goat anti-rat IgG magnetic particles (Dynex), and beaded cells were removed magnetically. Remaining cells were incubated for 30 min in standard tissue culture flasks, and adherent cells discarded. The CD4+ purity of the remaining cells was ascertained using FACS analysis with murine anti-CD4 phycoerythrin-conjugated MAb; 90 to 98% CD4+ populations were typically obtained.
CFSE labeling (54) was performed at 37°C on cells resuspended in phosphate-buffered saline at 107 cells/ml. A 5 μM concentration of CFSE was added for 15 min, fetal calf serum was added to stop the reaction, and the cells were washed and resuspended at the required concentration.
Th1 and Th2 cultures were set up with young and aged CFSE-labeled CD4+ cells at 105 cells/ml. Plate-bound anti-CD3ɛ was used at 3 μg/ml, and soluble anti-CD28 was used at 10 μg/ml. Th1 polarization was induced using recombinant murine IL-12 (5 ng/ml) and anti-murine IL-4 MAb (11B11) (20 μg/ml). Th2 polarization was induced by adding recombinant murine IL-4 (50 ng/ml) and anti-murine IFN-γ MAb (XMG1.2) (50 μg/ml). Cocultures were harvested on days 2, 3, 4, 5, and 6 postisolation for FACS analysis, and additional cells harvested on day 3 were washed and restimulated; supernatants were removed after a further 24 h and assayed for archetypal Th1/Th2 cytokines.
RNA purification and RPA.
Cecal tissue snap frozen in Trizol (Life Technologies) was thawed and homogenized. RNA was precipitated using chloroform and isopropanol; the pellet was then washed with 75% ethanol and redissolved. To ensure high purity, RNA was reprecipitated with phenol-chloroform (GIBCO), mixed with 100% ethanol and 3 M sodium acetate (pH 5.5), freeze-thawed, redissolved in water, quantified, and stored at −80°C. RNA was checked for two intact ribosomal bands on a 2% agarose-ethidium bromide gel. For RNase protection assay (RPA) a custom-made Riboquant template (BD PharMingen) was used to assay for mRNA levels of IL-4, IL-9, IL-12, IL-12Rβ2, IL-13, IL-18, TNF, IFN-γ, and glyceraldehyde-3-phosphate dehydrogenase. Probe synthesis was done using 32P-labeled UPT (Amersham Pharmacia Biotech) and a Riboprobe kit and T7 polymerase (Promega). A 10-μg of sample RNA was hybridized with radiolabeled antisense probe at 56°C over night, digested with RNases, and purified, and protected radiolabeled RNA was resolved on a denaturing sequencing gel. Exposing the dried gel to a phosphorimaging screen and visualizing fragments using a Molecular Imager FX System (Bio-Rad Laboratories) enabled protected mRNA to be quantified. All samples were normalized with respect to the intensity of the housekeeping gene glyceraldehyde-3-phosphate dehydrogenase.
Statistics.
Significant differences (P < 0.05) between two experimental groups were determined using the Student's t test.
RESULTS
Young C57BL/Icrfat mice expel T. muris.
We have previously demonstrated that C57BL/6 mice expel the intestinal parasite T. muris by day 35 p.i., with restimulated MLN cells producing a strong type 2 cytokine response with high levels of IL-4, IL-5, IL-9, and IL-13 and low levels of IL-12 and IFN-γ (5). Here we investigate whether young C57BL/Icrfat mice, originally derived from the C57BL/6 inbred line more than 25 years ago, still maintain the C57BL/6 phenotype.
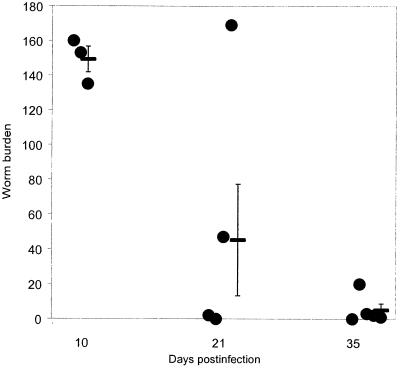
Three-month-old C57BL/Icrfat mice infected with T. muris exhibit normal worm expulsion kinetics (Fig. 1), with only residual worms remaining within the cecum by day 35 p.i.. Worm expulsion correlated with a typical resistant phenotype; increases in type 2 cytokines, parasite-specific IgG1, and total IgE; and, within the cecum, mastocytosis, eosinophilia, and goblet cell hyperplasia (data not shown). These data suggest young C57BL/Icrfat and C57BL/6 mice do not significantly differ in their response to T. muris infection.
FIG. 1.
Mean worm burden ± SEM in 3-month-old C57BL/6icrfat mice (-). Mice were infected with ∼150 T. muris eggs on day 0 and numbers of intestinal worms determined for individual mice (•) days 10, 21, and 35 p.i.
Worm expulsion is delayed in ageing C57BL/Icrfat mice.
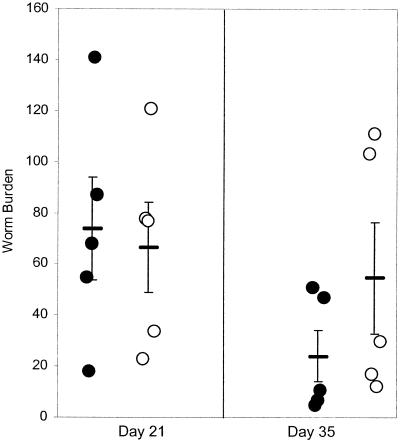
To determine whether ageing affects the immune response we investigated the ageing host's ability to respond to the intestinal parasite. Rates of establishment of L1 or L2 T. muris larvae 10 days p.i. in both young (3-month-old) and aged (19-month-old) mice were comparable (data not shown); similar numbers of worms also remained within both groups 21 days p.i. (Fig. 2). By day 35 p.i. young mice, in comparison to aged mice, had a reduced number of worms remaining within the cecum. Despite the difference in worm number not being significant, there was a clear difference in worm morphology; worms remaining within young animals showed damage (stunted growth), indicative of immunologically driven expulsion (also noted in the previous experiment), whereas a number of aged mice had chronically infected guts with in excess of 100 undamaged sexually mature worms.
FIG. 2.
Worm burden in 3 (•)- and 19 (○)-month-old C57BL/6icrfat mice; values represent means ± standard errors of the means (error bars) with five animals per group. Mice were infected with ∼150 T. muris eggs on day 0, and worm numbers within the large intestine were determined days 21 and 35 p.i.
Altered cytokine production within both MLN and large intestine of aged mice.
A chronic T. muris infection is associated with a Th1-dominated phenotype (3). Using MLN cells taken at day 21 p.i., restimulated with T. muris ES Ag, and cecal tissue snap frozen at the time of autopsy, we investigated whether the observed higher worm burdens seen in aged mice correlated with an altered cytokine response.
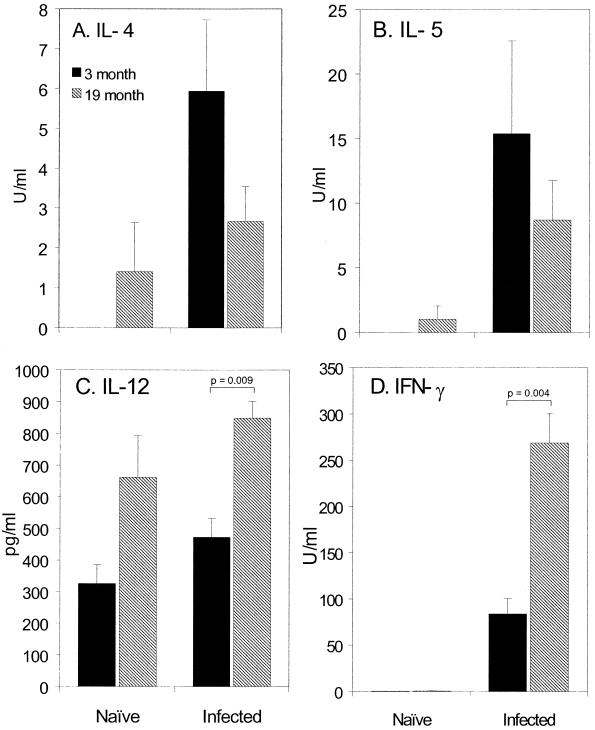
Analysis of Ag-specific cytokine production day 21 p.i. reveals 19-month-old C57BL/Icrfat mice produce a reduced IL-4 and IL-5 response. In contrast the type 1 response indicative of host susceptibly is enhanced in aged mice, with significantly higher levels of IL-12 p40/p75 and IFN-γ (Fig. 3). Naïve controls show a mixed phenotype, with MLN cells from aged mice spontaneously producing higher levels of IL-4, IL-5, and IL-12.
FIG. 3.
Cytokine production from naïve or T. muris-infected 3 (closed bars)- and 19 (hatched bars)-month-old C57BL/6icrfat mice. MLN cells were taken at day 21 p.i. and stimulated in vitro with T. muris ES Ag for 24 h. IL-4 (A), IL-5 (B), IL-12 (C), and IFN-γ (D) within the supernatants were assayed by sandwich ELISA; values represent means ± standard errors of the means (error bars) with five to eight animals per group.
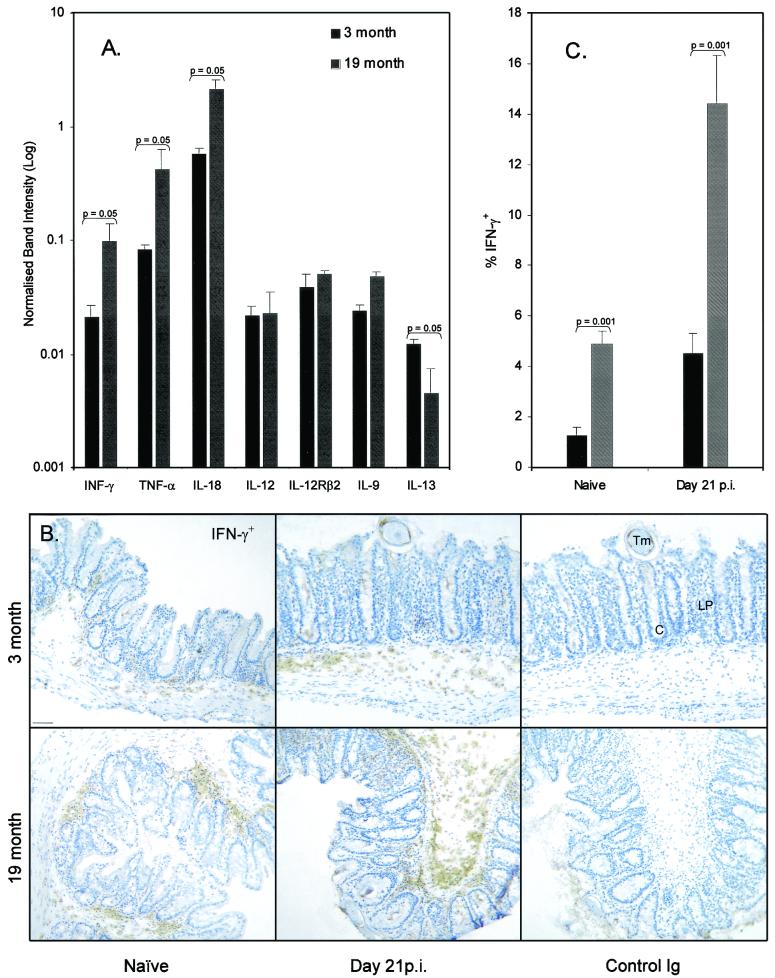
Quantification of RNA isolated from cecal tissue using RPA (Fig. 4A) indicates a proinflammatory environment in the naïve ageing gut, with significantly higher levels of IFN-γ and TNF alpha and a 3.7-fold increase in IL-18 mRNA; the type 2 cytokine IL-13 was significantly reduced, IL-9 was unchanged, and IL-4 mRNA was undetectable. Tissue from day 21 p.i. showed a 2.2-fold increase in IL-18 mRNA in aged mice; all other cytokines were not significantly different (data not shown).
FIG. 4.
(A) Cytokine RNA isolated from cecum from both 3- and 19-month-old C57BL/Icrfat mice, quantified using RPA. Observed cytokine band intensity is normalized to housekeeping gene intensity. Values represent means ± standard errors of the means (error bars) of three animals per group. (B) IFN-γ+ immunostaining within the large intestine of naïve or T. muris-infected young or old mice. Sections from snap frozen cecum were immunostained with an anti-IFN-γ MAb or control Ig and visualized with 3,3′-diaminobenzidine. Representative plates from groups of three animals. Abbreviations: Tm, T. muris; LP, lamina propria; C, cecal crypts. Bar = 50 μm. (C) Image analysis was performed using Scion image software. Values are percent of gut imaged staining IFN-γ+ and represent means ± standard errors of the means (error bars) of 10 images per group.
By using immunohistochemical staining for IFN-γ on sectioned cecum, it was possible to visualize a type 1 cytokine in host tissue that is directly associated with the parasite (Fig. 4B). In naïve 19-month-old C57BL/Icrfat mice, levels of IFN-γ staining within the cecum were significantly greater than those in 3-month-old controls, mirroring the observed difference seen in cecal IFN-γ mRNA (Fig. 4C). Interestingly, staining for IFN-γ protein was also significantly higher in the infected ageing animal, despite IFN-γ mRNA levels remaining similar to those in young controls. Together these data indicate a reduced Th2 response within the draining lymph nodes and an enhanced Th1 cytokine response occurring in both the cecum and the MLN of the infected ageing host.
Parasite-specific antibody production and intestinal mastocytosis were delayed and reduced in aged mice.
An altered cytokine response to T. muris has previously been shown to have downstream effects on the parasite-specific antibody isotype generated, with IgG1 production indicating a polarized type 2 response (8). Intestinal mastocytosis, though not essential for expulsion (7) is also indicative of type 2 cytokines (20). Here we investigate whether the observed age-associated shift in cytokine phenotype and subsequent altered expulsion of T. muris affected serum parasite-specific antibody isotype and the mast cell infiltrate within the large intestine.
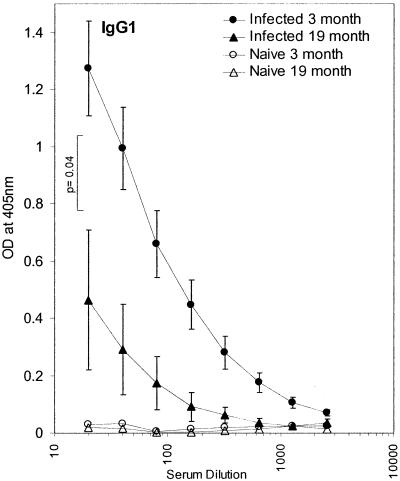
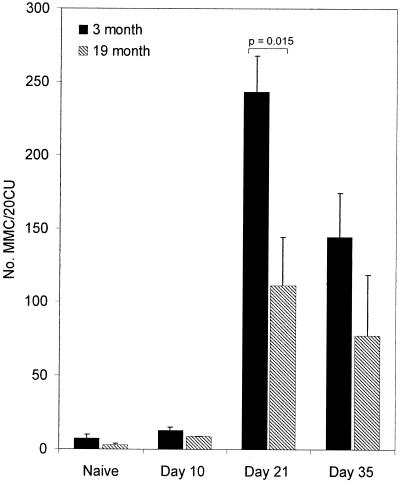
By day 21 p.i. 19-month-old mice produced significantly less parasite-specific IgG1 than young control C57BL/Icrfat mice (Fig. 5), corroborating the observed evidence for a reduction in type 2 cytokines seen in these animals at this time point. By day 35 p.i. there was no significant age-related difference in both parasite-specific IgG1 and IgG2a (data not shown), indicating that in aged mice the Ig response was delayed rather than reduced. Cecal mastocytosis was also significantly reduced within infected 19-month-old mice by day 21 p.i. (Fig. 6); this trend was still evident day 35 p.i..
FIG. 5.
Levels of T. muris ES Ag-specific IgG1 in serum from naïve 3 (○)- and 19 (▵)-month-old and T. muris-infected young (•) and old (▴) C57BL/Icrfat mice. Serum was collected on day 21 p.i. and assayed by ELISA for IgG1. Optical density (OD) values are shown from serially diluted sera, with the mean ± standard errors of the means (error bars) generated from groups of five to eight mice.
FIG. 6.
Mucosal mastocytosis within the large intestine of 3 (closed bars)- and 19 (hatched bars)-month-old C57BL/Icrfat mice, naïve or T. muris-infected, on days 10, 21, and 35 p.i. Mast cells were quantified per 20 crypt units, as described in Materials and Methods; values are means ± standard errors of the means (error bars) generated from groups of five to eight mice.
Effects of age on αCD3/CD28-induced cell proliferation.
Previously it has been shown that both murine and human T cells have a possible defect in CD28-mediated costimulation as they age. Human cells become CD28 negative (45), whereas mice maintain the cell surface protein but become unresponsive to anti-CD28 MAb costimulation (9). Several in vivo murine studies have indicated that reduced CD28 signaling in activated T cells profoundly affects maturation and the resulting cytokine production, with zero or weak signaling being associated with elevated type 1 cytokines (29, 46). Here we investigate whether lymphocytes from our colony of ageing C57BL/Icrfat mice show a similar phenotype to that seen in other ageing mice (9).
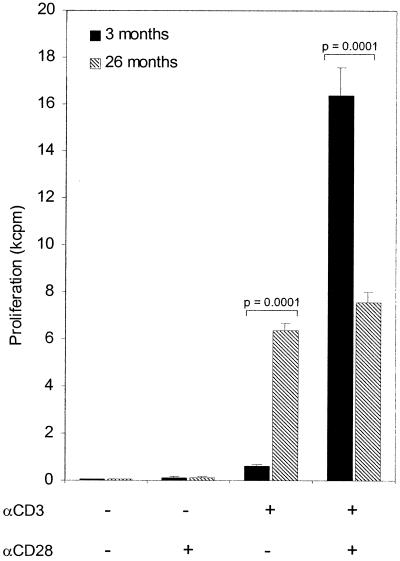
Plate-bound anti-CD3ɛ MAb stimulated MLN cells from naïve 26-month-old C57BL/Icrfat mice showed no significant increase in proliferation rate with the addition of soluble anti-CD28 MAb; however, proliferation rate induced by anti-CD3ɛ MAb alone was significantly higher than that seen in young cells. In contrast, MLN cells from naïve 3-month-old C57BL/Icrfat mice receiving anti-CD3ɛ/CD28 stimulation showed a 27-fold increase over proliferation induced with anti-CD3ɛ MAb alone (Fig. 7). These data suggest aged cells from C57BL/Icrfat mice become unresponsive to CD28 costimulation but are compensated to some degree by an increased ability to proliferate via anti-CD3ɛ-mediated stimulation alone.
FIG. 7.
In vitro stimulation of MLN cells from naïve 3 (closed bars)- and 26 (hatched bars)-month-old C57BL/Icrfat mice in the presence or absence of plate-bound anti-CD3ɛ (10 μg/ml) and soluble anti-CD28 (10 μg/ml). Proliferation was assayed as overnight incorporation of [3-H6]thymidine; data represent means ± standard errors of the means (error bars) of six culture wells.
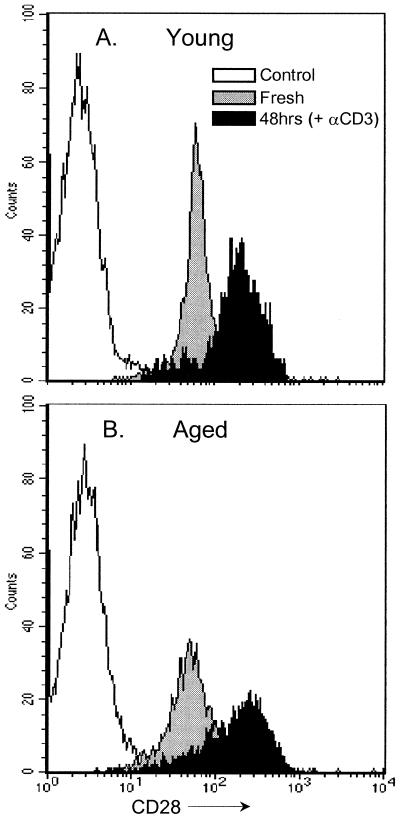
Unresponsiveness to anti-CD28 MAb seen in aged cells was not due to reduced expression of surface CD28 on MLN cells. Both young and aged CD4+ (Fig. 8) and CD8+ cells (data not shown) stained with similar intensity for CD28, with both age groups showing a similar up regulation in CD28 staining intensity after 48 h of in vitro anti-CD3ɛ stimulation.
FIG. 8.
CD28 expression on CD4+ cells isolated from the MLN of naïve 3 (A)- and 26 (B)-month-old C57BL/Icrfat mice. FACS histogram plots show intensity of CD28 staining on freshly isolated CD4+-gated cells (grey) and cells stimulated in vitro with anti-CD3ɛ MAb for 48 h (black) against the total control stained CD4+ population (white). Cells are from the same animals as analyzed in Fig. 7.
Altered cellular growth kinetics of aged cells under type 2 polarizing culture conditions.
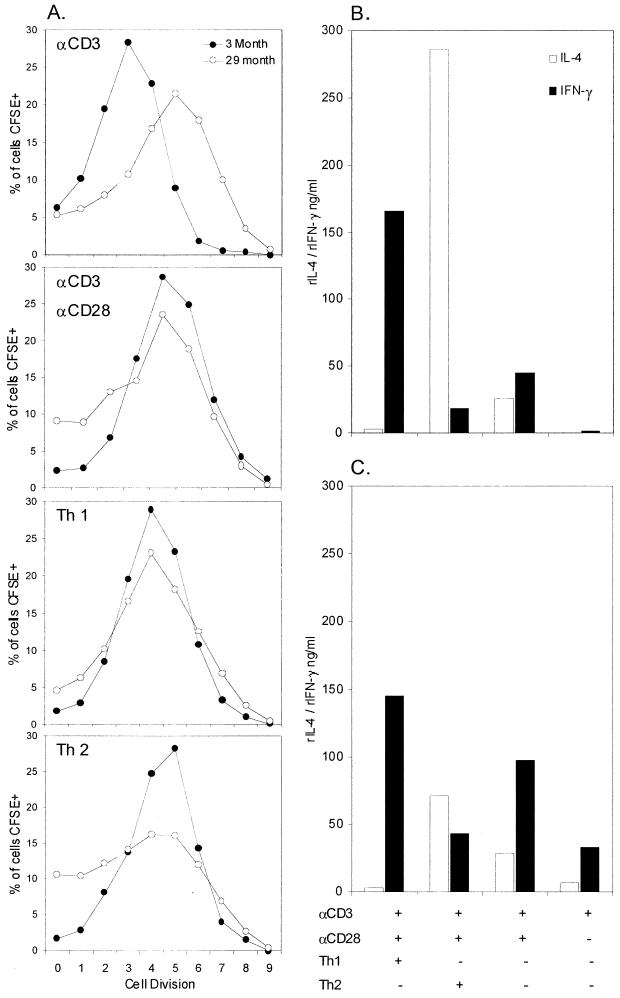
In an attempt to assess whether the observed deficiency in anti-CD28 costimulation has any effect on the ability of aged CD4+ cells to proliferate and polarize into Th1 or Th2 cells, we CFSE labeled purified aged CD4+ MLN cells and stimulated them under polarizing culture conditions. Aged cells at day 4 postisolation, when stimulated with plate-bound anti-CD3ɛ MAb alone, showed a higher proliferative rate, with increased numbers of CFSE-positive cells undergoing five, six, and seven divisions (Fig. 9A). This was similar to results obtained with [6-3H]thymidine incorporation in the previous experiment. With the addition of anti-CD28 MAb, the difference in proliferative rate between young and aged cells is less clear; however, larger populations of aged cells at division cycles 0, 1, and 2 indicate a subpopulation of slower-dividing cells. The addition of anti-CD28 had little effect on the division rate of aged cells again reflecting what was found in the previous experiment.
FIG. 9.
(A) FACS analysis of in vitro cell division kinetics, 4 days postisolation. CFSE-labeled CD4+ purified MLN cells from naïve 3 (•)- and 28 (○)-month-old C57BL/Icrfat mice, stimulated in the presence or absence of plate-bound anti-CD3ɛ (3 μg/ml) or soluble anti-CD28 (10 μg/ml), under Th1 or Th2 polarizing culture conditions. Cytokines produced from concurrent cultures 4 days postisolation in 3 (B)- and 28 (C)-month-old C57BL/Icrfat mice. rIL-4, recombinant IL-4; rIFN-γ, recombinant IFN-γ.
Under Th1 polarizing culture conditions, both young and aged CD4+ cells divide at a comparable rate, with similar numbers of CFSE-positive cells in all division subgroups. A Th2 culture environment induced fewer aged cells to divide, with lower numbers of CFSE-positive cells undergoing four, five, and six divisions and larger populations remaining at the zero-, one-, or two-cell-division stage. These different trends in proliferative rate found in aged and young cells were also evident at days 3 and 5 postisolation (data not shown).
Cytokine release from cocultures also at day 4 postisolation shows young CD4+ cells (Fig. 9B) were clearly able to polarize, becoming Th1 or Th2 cells under the correct culture conditions. The addition of anti-CD3ɛ and anti-CD28 alone generated young cells of an intermediate phenotype, producing equal amounts of IL-4 and IFN-γ. Aged CD4+ cells (Fig. 9C) clearly polarized towards the Th1-cell lineage, producing a similar pattern of high IFN-γ and low IL-4, as seen in young cells. However aged cells grown under Th2 culture conditions were less able to effectively differentiate into Th2 like cells, with lower IL-4 production than young controls and higher IFN-γ production. When stimulated with anti-CD3ɛ and anti-CD28 or anti-CD3ɛ alone, aged cells also produced higher levels of IFN-γ than young controls, implying a predisposition of aged CD4+ cells to the Th1 lineage. Taken together these data indicate that aged CD4+ MLN cells are less able to differentiate into Th2 cells, proliferating at a lower rate and producing an intermediate cytokine profile when grown in Th2 polarizing culture conditions. Aged Th1 cells show no such deficiency, with cell proliferation and cytokine polarization being similar to young controls.
DISCUSSION
Using both in vivo and in vitro investigative tools, this study demonstrates that age-associated immunological change within the gut mucosa and associated lymphatics is of critical significance with respect the control of intestinal parasitic disease. Furthermore, we identify defects in the development of Th2 polarization and an over expression of Th1 type cytokines within the gut as possible causes of the observed increase in susceptibility.
Resistance to T. muris is genetically determined (13), with different inbred mouse strains exhibiting a range of responses from highly resistant through to complete susceptibility. Typical for the C57/BL6 strain, young animals from an independently maintained colony of ageing C57/BL6icrfat mice (12) expelled the parasite, whereas genetically identical animals 19 to 28 months old demonstrated increased susceptibility. Thus, ageing, within the same inbred strain, has caused the animal to mount either an attenuated or misdirected immune response to infection.
In order to expel T. muris from the gut, previous investigators (15) have highlighted the importance of a Th2 response, with associated cytokines IL-4 and IL-13 (5) playing critical roles, whereas a Th1 response with high levels of IL-12 and IFN-γ enables adult worms to establish within the cecum (3). The fate of the Th1/Th2 cytokine balance within the ageing animal has been addressed in a growing number of studies. Work with both humans and mice shows ageing T cells can produce more IFN-γ (27, 31,) and less IL-4 (34, 43, 48), others have indicated the opposite, with reductions in IFN-γ (1, 25, 37) and more or the same IL-4 (27, 42). A possible cause of these inconsistencies in the literature is differences in the methods used for in vitro stimulation. To help resolve this issue, the present study provides in vivo data on age-associated T-cell changes in the context of a well-established infection model, supplying direct measurements of changes in cytokine balance within host tissue. In accordance with the observed increase in susceptibility, T. muris-infected aged mice switched cytokine phenotype, with MLN Ag-restimulated cells producing more Th1 type cytokines (IL-12 and IFN-γ) and less IL-4. Interestingly cells from aged naïve control animals within the same experiment appear more activated, spontaneously producing higher levels of IL-4, IL-5 and IL-12.
These data from naïve cells match some observations previously recorded in the literature, but not others (27, 42). One possible factor accounting for these variations in cytokine responses may be differences in animal husbandry experienced by these animals during the course of their extended life spans. However, upon infection with T. muris the background cytokine production is overridden, with ageing animals clearly producing an MLN cellular population dominated by Th1 cytokines, whereas young controls produce Th2 cytokines.
Studies within the ageing GALT have indicated that this lymphatic compartment is less susceptible to age-associated changes. Populations of CD4+ and CD8+ T cells within the Peyer's patches, naïve CD4+ cells within the MLN, and IL-2 production do not show the same age-associated decline as seen elsewhere (6, 11, 21, 32). However, reduced numbers of Th2 cells and lower levels of Ag-specific IgG and IgA have been noted within the GALT (22, 28). To ascertain whether these reported changes within the GALT influence the immune response occurring in the context of parasite invasion of intestinal mucosa and to confirm whether the altered cytokine milieu seen is real and not an artifact of ex vivo restimulation we looked directly at cytokine protein and mRNA production within cecum.
Using image analysis of immunohistochemically stained cecal tissue, we verified that within both the ageing naïve and infected mouse there were higher levels of IFN-γ, mirroring the observed increase found in the MLN. Quantitative RPA from homogenized whole-gut tissue further confirmed the proinflammatory environment of the ageing naïve gut, with higher mRNA levels found for IFN-γ, tumor necrosis factor alpha, and IL-18. Interestingly, levels of IL-12 and IL-12Rβ2 message within both young and aged gut tissue were identical, suggesting the observed age-associated high levels of cecal IFN-γ protein and mRNA may be due to the significantly higher levels of IL-18 and not IL-12.
By examining antibody production and cecal pathology we can assess age-associated changes in the T-cell response to the parasite. Our data here demonstrate that during infection, an ageing animal produces a delayed parasite-specific IgG1 response and a reduced intestinal mastocytosis, both of which are regulated by type 2 cytokines. It is not possible at present to rule out other unrelated age-associated causes for these data. For example a previous study has called into question the ability of the ageing spleen to act as an adhesive substratum (2); this may also be true of the high endothelial venules within the ageing gut, thus accounting for the reduced number of infiltrating mast cells. Also, despite B-cell number and total serum Ig remaining constant within the ageing animal (55), the level of Ag-specific antibody (44) declines with age. These reports could account for the reduced parasite-specific IgG1 observed here; however, in this case the Ag-specific IgG response did recover at a later time point. Despite these findings from other authors the most consistent explanation for these data remains the observed changes in cytokine environment. Further work will be required to confirm this.
It has been known for some time in both mice and humans that CD28-driven T-cell proliferation becomes defective with increasing age (9, 45). Data presented here confirm that this occurs in CD4+ cells from aged C57BL/Icrfat mice, despite CD28 cell surface expression and up regulation being comparable to those in young controls. It has been suggested that strength of CD28 signaling in response to certain antigenic stimuli can alter the subsequent differentiation of Th2 cell populations (23). In the case of in vivo parasite infection studies using N. brasiliensis (26), Leishmania major (10), Schistosoma mansoni (30), and T. muris (unpublished data), a dependence on CD28 signaling for effective Th2-cell generation has been demonstrated. Here we have shown that aged CD4+ T cells have inherent differences in their Th1 and Th2 polarization. Cells grown under Th2 culture conditions stimulated via CD3 and CD28 show a reduced proliferative rate and an inability to effectively produce the archetypal Th2 cytokine profile; these defects were not found in aged cells grown under Th1 conditions. Reduced CD28 costimulation can cause a weakened Th2 polarization and such a correlation appears to occur in these data from aged T cells. Thus, the age-related in vitro defect in CD28 costimulation and subsequent Th2 polarization may in vivo alter the initiation of a T-cell response to T. muris and result in the altered cytokine phenotype seen in infected aged mice.
This study consolidates the concept that an ageing animal is less able to generate a successful Th2 type response to an infectious challenge. Our data also for the first time extend this trend to the ageing gut environment with increased Th1 cytokines found in cecal tissue. Proliferative responses to CD28 costimulation and resulting differences in cytokine profile in Th1/Th2 polarizing culture conditions suggests that ageing T cells in vitro are also less able to differentiate into Th2 cells. Thus, in the ageing host this switch in cytokine response together with reduced Ag-specific antibody and intestinal mastocytosis results in greater susceptibility to the natural enteric pathogen T. muris.
Acknowledgments
We thank I. Davies (University of Manchester, Manchester, United Kingdom) for advice on the C57BL/Icrfat mouse colony, Helena Helmby for technical help, and Allison J. Bancroft for critical reading of the manuscript.
This work is supported by BBSRC grant 34/SAG09972.
Editor: R. N. Moore
REFERENCES
- 1.Albright, J. W., and J. F. Albright. 1994. Ageing alters the competence of the immune system to control parasitic infection. Immunol. Lett. 40:279-285. [DOI] [PubMed] [Google Scholar]
- 2.Albright, J. W., R. C. Mease, C. Lambert, and J. F. Albright. 1998. Effects of aging on the dynamics of lymphocyte organ distribution in mice: use of a radioiodinated cell membrane probe. Mech. Ageing Dev. 101:197-211. [DOI] [PubMed] [Google Scholar]
- 3.Bancroft, A. J., K. J. Else, J. P. Sypek, and R. K. Grencis. 1997. Interleukin-12 promotes a chronic intestinal nematode infection. Eur. J. Immunol. 27:866-870. [DOI] [PubMed] [Google Scholar]
- 4.Bancroft, A. J., and R. K. Grencis. 1998. Th1 and Th2 cells and immunity to intestinal helminths. Chem. Immunol. 71:192-208. [DOI] [PubMed] [Google Scholar]
- 5.Bancroft, A. J., A. N. McKenzie, and R. K. Grencis. 1998. A critical role for IL-13 in resistance to intestinal nematode infection. J. Immunol. 160:3453-3461. [PubMed] [Google Scholar]
- 6.Banerjee, M., J. D. Sanderson, J. Spencer, and D. K. Dunn-Walters. 2000. Immunohistochemical analysis of ageing human B and T cell populations reveals an age-related decline of CD8 T cells in spleen but not gut-associated lymphoid tissue (GALT). Mech. Ageing Dev. 115:85-99. [DOI] [PubMed] [Google Scholar]
- 7.Betts, C. J., and K. J. Else. 1999. Mast cells, eosinophils and antibody-mediated cellular cytotoxicity are not critical in resistance to Trichuris muris. Parasite Immunol. 21:45-52. [DOI] [PubMed] [Google Scholar]
- 8.Boom, W. H., D. Liano, and A. K. Abbas. 1988. Heterogeneity of helper/inducer T lymphocytes. II. Effects of interleukin 4- and interleukin 2-producing T cell clones on resting B lymphocytes. J. Exp. Med. 167:1350-1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boucher, N., T. Dufeu-Duchesne, E. Vicaut, D. Farge, R. B. Effros, and F. Schachter. 1998. CD28 expression in T cell aging and human longevity. Exp. Gerontol. 33:267-282. [DOI] [PubMed] [Google Scholar]
- 10.Corry, D. B., S. L. Reiner, P. S. Linsley, and R. M. Locksley. 1994. Differential effects of blockade of CD28-B7 on the development of Th1 or Th2 effector cells in experimental leishmaniasis. J. Immunol. 153:4142-4148. [PubMed] [Google Scholar]
- 11.Daniels, C. K., P. Perez, and D. L. Schmucker. 1993. Alterations in CD8+ cell distribution in gut-associated lymphoid tissues (GALT) of the aging Fischer 344 rat: a correlated immunohistochemical and flow cytometric analysis. Exp. Gerontol. 28:549-555. [DOI] [PubMed] [Google Scholar]
- 12.Davies, I., and J. D. Schofield. 1980. Connective tissue ageing: the influence of a lathyrogen (β-aminopropionitrile) on the lifespan of female C57BL/Icrfat mice. Exp. Gerontol. 15:487-494. [DOI] [PubMed] [Google Scholar]
- 13.Else, K., and D. Wakelin. 1988. The effects of H-2 and non-H-2 genes on the expulsion of the nematode Trichuris muris from inbred and congenic mice. Parasitology 96:543-550. [DOI] [PubMed] [Google Scholar]
- 14.Else, K. J., F. D. Finkelman, C. R. Maliszewski, R. K. Grencis. 1994. Cytokine-mediated regulation of chronic intestinal helminth infection. J. Exp. Med. 179:347-351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Else, K. J., and R. K. Grencis. 1991. Cellular immune responses to the murine nematode parasite Trichuris muris. I. Differential cytokine production during acute or chronic infection. Immunology 72:508-513. [PMC free article] [PubMed] [Google Scholar]
- 16.Else, K. J., D. Wakelin, D. L. Wassom, and K. H. Hauda. 1990. The influence of genes mapping within the major histocompatibility complex on resistance to Trichuris muris infections in mice. Parasitology 101:61-67. [DOI] [PubMed] [Google Scholar]
- 17.Else, K. J., G. M. Entwistle, and R. K. Grencis. 1993. Correlations between worm burden and markers of Th1 and Th2 cell subset induction in an inbred strain of mouse infected with Trichuris muris. Parasitol. Immunol. 15:595-600. [DOI] [PubMed] [Google Scholar]
- 18.Engwerda, C. R., B. S. Handwerger, and B. S. Fox. 1994. Aged T cells are hyporesponsive to costimulation mediated by CD28. J. Immunol. 152:3740-3747. [PubMed] [Google Scholar]
- 19.Ernst, D. N., W. O. Weigle, D. J. Noonan, D. N. McQuitty, and M. V. Hobbs. 1993. The age-associated increase in IFN-gamma synthesis by mouse CD8+ T cells correlates with shifts in the frequencies of cell subsets defined by membrane CD44, CD45RB, 3G11, and MEL-14 expression. J. Immunol. 151:575-587. [PubMed] [Google Scholar]
- 20.Finkelman, F. D., E. J. Pearce, J. F. Urban, Jr., and A. Sher. 1991. Regulation and biological function of helminth-induced cytokine responses. Immunol. Today 12:A62-A66. [DOI] [PubMed] [Google Scholar]
- 21.Flo, J., and E. Massouh. 1997. Age-related changes of naive and memory CD4 rat lymphocyte subsets in mucosal and systemic lymphoid organs. Dev. Comp. Immunol. 21:443-453. [DOI] [PubMed] [Google Scholar]
- 22.Fujihashi, K., T. Koga, and J. R. McGhee. 2000. Mucosal vaccination and immune responses in the elderly. Vaccine 18:1675-1680. [DOI] [PubMed] [Google Scholar]
- 23.Gause, W. C., S. J. Chen, R. J. Greenwald, M. J. Halvorson, P. Lu, X. D. Zhou, S. C. Morris, K. P. Lee, C. H. June, F. D. Finkelman, J. F. Urban, and R. Abe. 1997. CD28 dependence of T cell differentiation to IL-4 production varies with the particular type 2 immune response. J. Immunol. 158:4082-4087. [PubMed] [Google Scholar]
- 24.Grencis, R. K. 1993. Cytokine-mediated regulation of intestinal helminth infections: the Trichuris muris model. Ann. Trop. Med. Parasitol. 87:643-647. [DOI] [PubMed] [Google Scholar]
- 25.Han, S. N., D. Wu, W. K. Ha, A. Beharka, D. E. Smith, B. S. Bender, and S. N. Meydani. 2000. Vitamin E supplementation increases T helper 1 cytokine production in old mice infected with influenza virus. Immunology 100:487-493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Harris, N. L., R. J. Peach, F. Ronchese. 1999. CTLA4-Ig inhibits optimal T helper 2 cell development but not protective immunity or memory response to Nippostrongylus brasiliensis. Eur. J. Immunol. 29:311-316. [DOI] [PubMed] [Google Scholar]
- 27.Hobbs, M. V., W. O. Weigle, D. J. Noonan, B. E. Torbett, R. J. McEvilly, R. J. Koch, G. J. Cardenas, and D. N. Ernst. 1993. Patterns of cytokine gene expression by CD4+ T cells from young and old mice. J. Immunol. 150:3602-3614. [PubMed] [Google Scholar]
- 28.Kawanishi, H., and J. Kiely. 1989. Immune-related alterations in aged gut-associated lymphoid tissues in mice. Dig. Dis. Sci. 34:175-184. [DOI] [PubMed] [Google Scholar]
- 29.King, C. L., R. J. Stupi, N. Craighead, C. H. June, and G. Thyphronitis. 1995. CD28 activation promotes Th2 subset differentiation by human CD4+ cells. Eur. J. Immunol. 25:587-595. [DOI] [PubMed] [Google Scholar]
- 30.King, C. L., J. Xianli, C. H. June, R. Abe, and K. P. Lee. 1996. CD28-deficient mice generate an impaired Th2 response to Schistosoma mansoni infection. Eur. J. Immunol. 26:2448-2455. [DOI] [PubMed] [Google Scholar]
- 31.Kirschmann, D. A., and D. M. Murasko. 1992. Splenic and inguinal lymph node T cells of aged mice respond differently to polyclonal and antigen-specific stimuli. Cell. Immunol. 139:426-437. [DOI] [PubMed] [Google Scholar]
- 32.Koyama, K., T. Hosokawa, and A. Aoike. 1990. Aging effect on the immune functions of murine gut-associated lymphoid tissues. Dev. Comp. Immunol. 14:465-473. [DOI] [PubMed] [Google Scholar]
- 33.Kubo, M., and B. Cinader. 1990. Polymorphism of age-related changes in interleukin (IL) production: differential changes of T helper subpopulations, synthesizing IL-2, IL-3 and IL-4. Eur. J. Immunol. 20:1289-1296. [DOI] [PubMed] [Google Scholar]
- 34.Kurashima, C., and M. Utsuyama. 1997. Age-related changes of cytokine production by murine helper T cell subpopulations. Pathobiology 65:155-162. [DOI] [PubMed] [Google Scholar]
- 35.Lee, J. J., M. P. McGarry, S. C. Farmer, K. L. Denzler, K. A. Larson, P. E. Carrigan, I. E. Brenneise, M. A. Horton, A. Haczku, E. W. Gelfand, et al. 1997. Interleukin-5 expression in the lung epithelium of transgenic mice leads to pulmonary changes pathognomonic of asthma. J. Exp. Med. 185:2143-2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Linton, P., and M. L. Thoman. 2001. T cell senescence. Front. Biosci. 6:D248-D261. [DOI] [PubMed] [Google Scholar]
- 37.Lio, D., C. R. Balistreri, G. Candore, C. D'Anna, G. Di Lorenzo, F. Gervasi, F. Listi, L. Scola, and C. Caruso. 2000. In vitro treatment with interleukin-2 normalizes type-1 cytokine production by lymphocytes from elderly. Immunopharmacol. Immunotoxicol. 22:195-203. [DOI] [PubMed] [Google Scholar]
- 38.Lowry, O. H., N. J. Rosebrough, A. L. Farr, and R. J. Randall. 1951. Protein measurement with the folin phenol reagent. J. Biol. Chem. 193:265.. [PubMed] [Google Scholar]
- 39.Markey, A. C., L. J. Churchill, and D. M. MacDonald. 1989. Human cutaneous mast cells; a study of fixative and staining reactions in normal skin. Br. J. Dermatol. 120:625-631. [DOI] [PubMed] [Google Scholar]
- 40.Negoro, S., H. Hara, S. Miyata, O. Saiki, T. Tanaka, K. Yoshizaki, T. Igarashi, and S. Kishimoto. 1986. Mechanisms of age-related decline in antigen-specific T cell proliferative response: IL-2 receptor expression and recombinant IL-2 induced proliferative response of purified Tac-positive T cells. Mech. Ageing Dev. 36:223-241. [DOI] [PubMed] [Google Scholar]
- 41.Pawelec, G., W. Wagner, M. Adibzadeh, and A. Engel. 1999. T cell immunosenescence in vitro and in vivo. Exp. Gerontol. 34:419-429. [DOI] [PubMed] [Google Scholar]
- 42.Rink, L., I. Cakman, and H. Kirchner. 1998. Altered cytokine production in the elderly. Mech. Ageing Dev. 102:199-209. [DOI] [PubMed] [Google Scholar]
- 43.Sakata-Kaneko, S., Y. Wakatsuki, Y. Matsunaga, T. Usui, and T. Kita. 2000. Altered Th1/Th2 commitment in human CD4+ T cells with ageing. Clin. Exp. Immunol. 120:267-273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schwab, R., C. A. Walters, and M. E. Weksler. 1989. Host defence mechanisms and aging. Semin. Oncol. 16:20-27. [PubMed] [Google Scholar]
- 45.Scollay, R. G., E. C. Butcher, and I. L. Weissman. 1980. Thymus cell migration. Quantitative aspects of cellular traffic from the thymus to the periphery in mice. Eur. J. Immunol. 10:210-218. [DOI] [PubMed] [Google Scholar]
- 46.Shahinian, A., K. Pfeffer, K. P. Lee, T. M. Kundig, K. Kishihara, A. Wakeham, K. Kawai, P. S. Ohashi, C. B. Thompson, and T. W. Mak. 1993. Differential T cell costimulatory requirements in CD28-deficient mice. Science 261:609-612. [DOI] [PubMed] [Google Scholar]
- 47.Simon, A. K., E. Seipelt, and J. Sieper. 1994. Divergent T-cell cytokine patterns in inflammatory arthritis. Proc. Natl. Acad. Sci. USA 91:8562-8566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Smith, P., D. W. Dunne, and P. G. Fallon. 2001. Defective in vivo induction of functional type 2 cytokine responses in aged mice. Eur. J. Immunol. 31:1495-1502. [DOI] [PubMed] [Google Scholar]
- 49.Temann, U. A., B. G. Prasad, M. W. C. Basbaum, S. B. Ho, R. A. Flavell, and J. A. Rankin. 1997. A novel role for murine IL-4 in vivo: induction of MUC5AC gene expression and mucin hypersecretion. Am. J. Respir. Cell Mol. Biol. 16:471-478. [DOI] [PubMed] [Google Scholar]
- 50.Utsuyama, M., K. Hirokawa, C. Kurashima, M. Fukayama, T. Inamatsu, K. Suzuki, W. Hashimoto, and K. Sato. 1992. Differential age-change in the numbers of CD4+CD45RA+ and CD4+CD29+ T cell subsets in human peripheral blood. Mech. Ageing Dev. 63:57-68. [DOI] [PubMed] [Google Scholar]
- 51.Wakelin, D. 1967. Acquired immunity to Trichuris muris in the albino laboratory mouse. Parasitology 57:515-524. [DOI] [PubMed] [Google Scholar]
- 52.Weng, N. P., B. L. Levine, C. H. June, and R. J. Hodes. 1995. Human naive and memory T lymphocytes differ in telomeric length and replicative potential. Proc. Natl. Acad. Sci. USA 92:11091-11094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Weng, N. P., L. D. Palmer, B. L. Levine, H. C. Lane, C. H. June, and R. J. Hodes. 1997. Tales of tails: regulation of telomere length and telomerase activity during lymphocyte development, differentiation, activation, and aging. Immunol. Rev. 160:43-54. [DOI] [PubMed] [Google Scholar]
- 54.Weston, S. A., and C. R. Parish. 1990. New fluorescent dyes for lymphocyte migration studies. Analysis by flow cytometry and fluorescence microscopy. J. Immunol. Methods 133:87-97. [DOI] [PubMed] [Google Scholar]
- 55.Zhao, K. S., Y. F. Wang, R. Gueret, and M. E. Weksler. 1995. Dysregulation of the humoral immune response in old mice. Int. Immunol. 7:929-934. [DOI] [PubMed] [Google Scholar]