Abstract
The core oligosaccharides of Campylobacter jejuni lipopolysaccharides (LPS) display molecular mimicry with gangliosides. Cross-reactive anti-LPS-antiganglioside antibodies have been implicated to show a crucial role in the pathogenesis of the Guillain-Barré and Miller Fisher syndrome. The specificity of the antiganglioside response is thought to depend on the oligosaccharide structure of the ganglioside mimic. To test this hypothesis and to investigate the potential of LPS from Campylobacter strains from enteritis patients to induce an antiganglioside response, we immunized rabbits with purified LPS from eight Campylobacter jejuni reference strains with biochemically well-defined distinct ganglioside mimics and determined the presence of antiganglioside antibodies. All rabbits produced immunoglobulin G (IgM) and IgG anti-LPS antibodies, and the specificity of the cross-reactive antiganglioside response indeed corresponded with the biochemically defined mimic. Most rabbits also had antibody reactivity against additional gangliosides, and there were slight differences in the fine specificity of the antibody response between rabbits that had been immunized with LPS from the same Campylobacter strain. High anti-LPS and antiganglioside titers persisted over a 10-month period. In conclusion, the structure of the LPS only partly determines the antiganglioside specificity. Other strain-specific as well as host-related factors influence the induction and fine-specificity of the cross-reactive anti-LPS-antiganglioside response.
The core oligosaccharide fraction of Campylobacter jejuni lipopolysaccharides (LPS) displays mimicry with mammalian gangliosides (20). An antibody response against these ganglioside-like structures leads to antibodies that cross-react with gangliosides. The Guillain-Barré syndrome (GBS) and Miller Fisher syndrome (MFS) are frequently preceded by an infection with C. jejuni, and GBS and MFS patients have cross-reactive antibodies against LPS and gangliosides (16, 36). These antibodies have been implicated to play a role in the pathogenesis of both neurological diseases (30, 33). In contrast to patients with GBS and MFS, patients with an uncomplicated C. jejuni enteritis do have a low-titer antibody response against LPS, but they do not have a cross-reactive antiglycolipid response (10, 15).
The specificity of the antiganglioside antibodies differs between GBS and MFS patients. Campylobacter-related GBS patients have antiganglioside antibodies that react with GM1, GM1b, GalNAc-GD1a, GD1b, and GD1a (4, 13, 18, 37). In MFS patients, serum antibodies against GQ1b occur in up to 90% of the cases (11, 34). The differences in antiganglioside specificity are probably caused by differences in the LPS structure of the Campylobacter strain that triggered the neurological disease. Inhibition studies demonstrated that anti-GM1 reactivity could be decreased after incubation with LPS from a GBS-related Campylobacter strain but after incubation with LPS not from an MFS-related strain. Conversely, serum anti-GQ1b reactivity could only be inhibited by incubation with LPS from an MFS-related strain (16, 23). In previous studies we investigated the antiganglioside antibody response after immunization of rabbits with purified LPS from GBS- and MFS-related strains (1, 2). The exact biochemical structure of the LPS of these strains is not known, but the rabbits had an antiganglioside response comparable to the antiganglioside response in the patients from which the strains were cultured (1). Using biochemical methods, LPS from a GBS-related C. jejuni strain was shown to contain a GM1-mimic, whereas LPS from a MFS-related strain had a GD3-mimic (29, 38). With serological methods, mimics of GQ1b were detected in C. jejuni strains from MFS patients (14, 40). Mimics of GM1, GM2, GM3, GD1a, and GD3 were also demonstrated in several Campylobacter reference strains of the Penner serotyping system, which was derived from patients with uncomplicated enteritis (21).
In the present study, we immunized rabbits with purified LPS from Campylobacter reference strains of which the core oligosaccharide structure has been biochemically defined. To investigate whether the structure of a molecular mimic is the only determinant of the specificity of cross-reactive antibodies, we determined the specificity of the antiganglioside antibody response.
MATERIALS AND METHODS
Campylobacter strains.
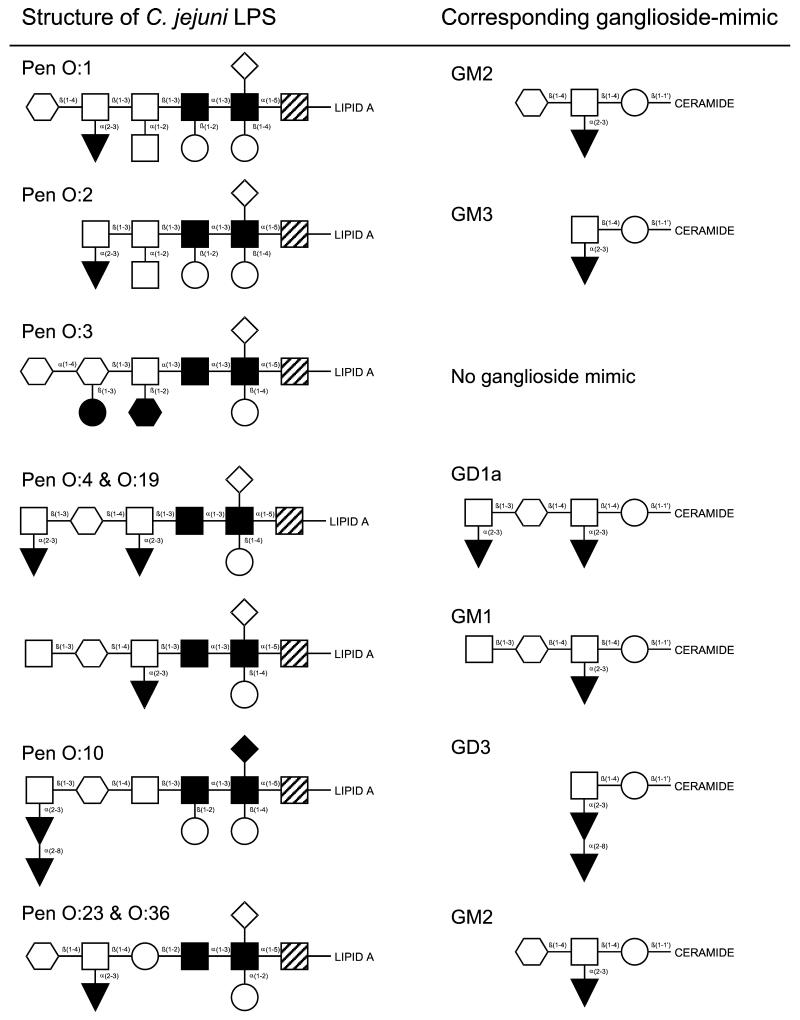
Penner serotyping reference strains for serotypes O:1 (CCUG 10935, GM2-mimic) (9), O:2 (CCUG 10936, GM3-mimic) (8), O:3 (CCUG 10937, no ganglioside mimic) (6), O:4 (CCUG 10938, GM1- and GD1a-mimic) (5, 9), O:10 (CCUG 10943, GD3-mimic) (22), O:19 (CCUG 10950, GM1- and GD1a-mimic) (7), O:23 (CCUG 10954, GM2-mimic) (9), and O:36 (CCUG 10966, GM2-mimic) (9) were used. An overview of the LPS structure and corresponding ganglioside mimics is given in Fig. 1. Purification of LPS and confirmation of the presence of ganglioside-like epitopes was confirmed as described previously (3).
FIG. 1.
Structures of various C. jejuni LPS used for immunization of rabbits and corresponding ganglioside mimics. Symbols: , N-acetylgalactosamine; □, galactose; ▾, sialic acid; ○, glucose; ⋄, (phosphoryl)ethanolamine; , N-acetylglucosamine; ▪, heptose; ┘, 2-keto-3-deoxy octulosonic acid (KDO); •, dideoxyglucose; ♦, 2-aminoethyl phosphate.
Immunization protocol.
New Zealand White rabbits (2.0 to 2.5 kg) were immunized with 400 μg of LPS in complete Freund adjuvant (Difco Laboratories, Detroit, Mich.) as described before (2). Two animals were immunized with each LPS. Booster injections with the same amount of LPS in incomplete Freund adjuvant (Difco) were given at days 14, 28, 42, and 56. Blood was collected from the ear vein prior to each immunization. Follow-up serum samples were taken every 2 months until 10 months after the first immunization. The feces of all animals was cultured for C. jejuni prior to immunization and at day 56. This experiment was approved by the animal ethics committee that serves the Erasmus University Medical Centre in Rotterdam.
Serology.
Antibody reactivity against asialo-GM1 (GA1), GM1, GM2, GM3, GD1a, GD3, and GQ1b was detected with enzyme-linked immunosorbent assay and confirmed with thin-layer chromatography (TLC) as described before (2). The titer was defined as the highest serum dilution with an optical density of >0.1, corrected for binding to uncoated wells. Anti-LPS reactivity was detected with enzyme-linked immunosorbent assay and Western blot by using diluted serum samples (2).
To assess the cross-reactivity of anti-LPS antibodies with glycolipids, serum samples from immunized rabbits were incubated with C. jejuni LPS conjugated to octyl-Sepharose CL4B beads as described previously (2).
RESULTS
All rabbits responded to the immunization with the production of immunoglobulin M (IgM) and IgG anti-LPS antibodies. In general, the titer of anti-LPS antibodies was highest against the homologous LPS. Most rabbits also had serum antibody reactivity against other LPS, but the pattern of additional reactivity could not be explained by shared structures between LPS from different serostrains. One rabbit that was immunized with O:4 LPS had an adverse reaction after the second booster injection and was sacrificed at day 42. The stool cultures for C. jejuni were all negative, indicating that the antibodies had been induced by the immunization and not by a concurrent infection with C. jejuni. Data on antiglycolipid antibody responses determined in serum taken at day 56 are summarized in Table 1.
TABLE 1.
Serum antiglycolipid antibody titers in rabbits immunized with C. jejuni LPSa
| C. jejuni strain and serotype | IgM titer |
IgG titer |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| GA1 | GM1 | GM2 | GM3 | GD1a | GD3 | GQ1b | GA1 | GM1 | GM2 | GM3 | GD1a | GD3 | GQ1b | |
| Pen O:1 | 200 | 200 | 100 | - | - | - | - | 100 | 800 | 25,600 | - | - | - | - |
| Pen O:1 | 200 | 200 | 100 | - | - | - | - | - | 400 | 200 | - | - | - | - |
| Pen O:2 | 400 | 100 | - | 100 | - | - | - | 400 | - | - | 100 | - | - | - |
| Pen O:2 | 200 | 100 | - | - | - | - | - | 100 | - | - | 1,600 | 1,600 | - | - |
| Pen O:3 | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| Pen O:3 | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| Pen O:4 | 100 | - | - | - | 200 | - | - | 100 | - | - | - | 100 | - | - |
| Pen O:10 | 400 | - | - | - | - | - | - | >51,200 | - | - | - | - | - | - |
| Pen O:10 | 200 | 100 | - | - | - | - | - | 51,200 | - | - | - | - | - | - |
| Pen O:19 | 800 | 800 | - | - | - | - | - | >51,200 | 25,600 | 100 | 100 | - | - | - |
| Pen O:19 | 400 | 800 | - | - | - | - | - | - | 3,200 | 100 | - | 1,600 | - | - |
| Pen O:23 | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| Pen O:23 | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| Pen O:36 | 100 | - | - | - | - | - | - | - | - | 800 | - | - | - | - |
| Pen O:36 | - | 200 | 200 | - | 800 | - | - | - | - | 800 | - | 400 | 400 | - |
Serum samples were obtained at day 56. -, <100. Antibody specificity as deduced from the corresponding ganglioside mimic is in boldface.
Immunization with LPS from the O:1, O:2, O:4, O:19, and O:36 serostrains resulted in an antiglycolipid response with a specificity that could be predicted from the structure of the ganglioside mimic in the LPS (Table 1). Rabbits that were immunized with O:1 and O:36 had a strong anti-GM2 antibody response. LPS from the O:19 serostrain induced IgM and IgG anti-GM1 antibodies. Rabbits that were immunized with O:2 LPS had an anti-GM3 response, and one of the O:4-immunized animals had an IgM and IgG response against GD1a, although the titers were low (Table 1). Immunization with LPS from the O:3 serostrain, which does not bear any ganglioside mimics, only resulted in a strong anti-LPS response, with no reactivity against any of the purified glycolipids.
In addition to this expected pattern of antiganglioside response, the specificity of the antibodies was broader than could be expected from the biochemical characterization of the LPS. The O:1-immunized animals both had additional anti-GM1 reacivity, and one of the rabbits that was immunized with O:2 LPS had an additional strong response against GD1a. The two animals that had received injections with O:19 LPS differed in the broadening of their antiglycolipid specificity. One of the rabbits had, in addition to its anti-GM1 reactivity, a very strong response against GA1. The other rabbit had IgG antibodies against GD1a. Most patterns of broadening can be explained by sharing of similar di- or trisaccharides by the different gangliosides (see Fig. 1).
There are two exceptions to the general pattern of antiglycolipid reactivity. Animals that were immunized with O:10 LPS did not mount a strong antibody response against GQ1b or GD3 except for a low-titer IgM response during the first 6 weeks of the immunization procedure. In contrast, these animals produced high-titer IgG GA1 antibodies already 14 days after the first immunization. The other exception is the lack of antiganglioside reactivity after immunization with O:23 LPS that contains a GM2-like structure (Fig. 1 and Table 1). Despite an IgM and IgG anti-LPS response, we could not detect antibody reactivity against any of the purified glycolipids.
Despite the presence of a shared GM2-mimic in O:1, O:23, and O:36 LPS, antisera raised against the three different LPS preparations contained the highest anti-LPS titers against their homologous LPS (data not shown). Furthermore, on Western blot, purified anti-GM2 antibodies from an O:36-immunized animal only reacted with O:36 LPS and not with O:1 or O:23 (data not shown).
To determine whether the induced antiglycolipid antibodies are indeed cross-reactive with LPS, we performed inhibition experiments with LPS-coated Sepharose beads. Incubation of antisera with beads coated with homologous LPS resulted in an inhibition of antiglycolipid reactivity. The antiglycolipid antibodies induced by O:1, O:2, O:4, O:19, and O:36 were all shown to be cross-reactive with their homologous LPS. The anti-GA1 reactivity in O:10 antiserum could not be decreased after incubation with O:10 LPS-coated beads.
Antibody titers were followed until 10 months after the first immunization. Animals with IgG titers of >800 had persistent antiglycolipid reactivity during this period of follow-up. Lower titers of IgG antibodies and most of the IgM reactivity had decreased below the detection level. During the whole observation period, none of the animals developed neurological signs such as limb weakness or ataxia.
DISCUSSION
In accordance with the molecular mimicry hypothesis, immunization of rabbits with Campylobacter LPS mimicking different glycolipids leads to an antiglycolipid response with a specificity that corresponds with the structure of the LPS. Our studies confirm and extend earlier reports with LPS from Campylobacter strains isolated from GBS, MFS, and uncomplicated enteritis patients (1, 12, 28, 35).
In most rabbits, the antiglycolipid response was not restricted to the biochemically defined mimic. There are several explanations for this broadened specificity, including the reactivity against GA1. First, the purified LPS fraction we have used to immunized the rabbits may have contained LPS molecules displaying microheterogeneity in the ganglioside-mimicking core oligosaccharide. This may be due to incomplete biosynthesis of the LPS, loss of carbohydrate residues during the extraction and purification procedures or phase variation during culture (7, 19). All of these mechanisms would lead to the expression of multiple LPS types with multiple ganglioside mimics. For the O:1 serostrain, which bears a GM2-mimic, it has been decribed that due to phase variation in LPS biosynthesis genes, isolated colonies revert to a cholera toxin-binding phenotype (19). Cholera toxin does not bind to GM2 but has a high affinity for GM1, indicating that bulk quantities of LPS from the O:1 serostrain may not only contain GM2-mimics but also GM1-mimics (27). Serological studies with monoclonal antiganglioside antibodies to detect ganglioside mimics in Campylobacter LPS have indicated that other serostrains, such as O:4, also express multiple ganglioside mimics (39). Alternatively, the broadened specificity of the antiglycolipid response may depend on the immune response of the rabbit. Animals that were immunized with highly purified glycolipids also demonstrate a slightly broadened antiglycolipid antibody specificity (27).
LPS from the O:10 serostrain did not induce high-titer anti-GQ1b or anti-GD3 antibodies despite the reported GD3-mimic (22). Instead, the O:10-immunized animals had a strong anti-GA1 response. Purified O:10 LPS reacted with a monoclonal anti-GQ1b/GD3 antibody (data not shown), confirming the presence of a GD3-mimic in the preparation we used for the immunizations. In addition, the LPS also reacted with sera from GBS patients containing anti-GM1 and anti-GA1 reactivity (data not shown). This indicates that O:10 LPS may also contain incompletely sialylated carbohydrate structures, mimicking asialo-gangliosides such as GA1 (22). However, depletion studies with O:10 LPS coated beads did not show inhibition of anti-GA1 reactivity by O:10 LPS. Therefore, we cannot exclude that the anti-GA1 antibodies in O:10-immunized rabbits have been induced by a mechanism that is different from molecular mimicry. However, polyclonal B-cell activation by Campylobacter LPS seems unlikely because all immunized rabbits would have had high-titer anti-GA1 antibodies.
We confirmed the results from previous studies that immunization with O:23 LPS did not induce an anti-GM2 antibody response, despite the biochemically defined presence of a GM2-mimic (28). Earlier studies demonstrated the lack of binding of monoclonal anti-GM2 antibodies with O:23 LPS in Western blot or TLC, indicating the absence of a GM2-mimic (28, 39). Another explanation may be that differences in the density of GM2-mimics and the adjuvant properties of the lipid A portion and/or O-chain influence the induction of antiglycolipid antibodies and the presentation of the GM2-mimics in the Western blot between the O:1, O:23, and O:36 serostrains (28, 33).
None of the rabbits showed any neurological signs during the 10-month follow-up period. Although this argues against a pathogenic role for antiganglioside antibodies there are several factors that might explain the lack of clinical symptoms. Several reports indicate that a putative pathogenic role of antiglycolipid antibodies is complement mediated (25, 32). Ritter et al. showed that the IgG anti-GM2 antibodies induced with the current immunization protocol had only a low level of complement-dependent cytotoxicity (28). Another factor might be the presence of an intact blood-nerve barrier, preventing access of antiglycolipid antibodies to their presumed site of action. In addition, in contrast to a mucosal infection with the whole Campylobacter organism, systemic immunization with purified LPS might not generate adequate T-cell responses that may be needed to induce a full-spectrum immune-mediated attack on the nerve (26).
We did not find a difference in the antibody response of rabbits to LPS derived from Campylobacter strains from GBS patients compared to enteritis patients without neurological symptoms (1). This indicates that there are no intrinsic differences between the LPS obtained from both groups of Campylobacter strains. The differential response to glycolipids in GBS and MFS patients compared to uncomplicated enteritis patients therefore probably depends on other factors. In the gut of patients with uncomplicated enteritis, the density of ganglioside mimics may be lower or absent, thereby not invoking a response. Alternatively, host-dependent factors such as polymorphisms in immune-response genes, may determine the induction of a cross-reactive anti-LPS-glycolipid response and concurrent neurological symptoms (17, 24, 31).
In conclusion, this study demonstrates that the LPS structure is a major determinant of the specificity of a cross-reactive anti-LPS-glycolipid response. However, the equivalent capacity of LPS from enteritis strains and GBS or MFS strains to induce cross-reactive antibodies indicates that the sole presence of molecular mimicry between C. jejuni LPS and peripheral nerve glycolipids is not sufficient to induce an antiglycolipid response in humans with GBS or MFS.
Editor: R. N. Moore
REFERENCES
- 1.Ang, C. W., M. A. De Klerk, H. P. Endtz, B. C. Jacobs, J. D. Laman, F. G. A. Van der Meché, and P. A. Van Doorn. 2001. Guillain-Barré syndrome- and Miller-Fisher syndrome-associated Campylobacter jejuni lipopolysaccharides induce anti-GM1 and anti-GQ1b antibodies in rabbits. Infect. Immun. 69:2462-2469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ang, C. W., H. P. Endtz, B. C. Jacobs, J. D. Laman, M. A. De Klerk, F. G. A. Van der Meché, and P. A. Van Doorn. 2000. Campylobacter jejuni lipopolysaccharides from Guillain-Barré syndrome patients induce IgG anti-GM1 antibodies in rabbits. J. Neuroimmunol. 104:133-138. [DOI] [PubMed] [Google Scholar]
- 3.Ang, C. W., J. D. Laman, H. J. Willison, E. R. Wagner, H. P. Endtz, M. A. De Klerk, A. P. Tio-Gillen, N. Van den Braak, B. C. Jacobs, and P. A. Van Doorn. 2002. Structure of Campylobacter jejuni lipopolysaccharides determines antiganglioside specificity and clinical features of Guillain-Barré and Miller Fisher patients. Infect. Immun. 70:1202-1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ang, C. W., N. Yuki, B. C. Jacobs, M. Koga, P. A. Van Doorn, P. I. M. Schmitz, and F. G. A. Van Der Meché. 1999. Rapidly progressive, predominantly motor Guillain-Barré syndrome with anti-GalNAc-GD1a antibodies. Neurology 53:2122-2127. [DOI] [PubMed] [Google Scholar]
- 5.Aspinall, G. O., S. Fujimoto, A. G. McDonald, H. Pang, L. A. Kurjanczyk, and J. L. Penner. 1994. Lipopolysaccharides from Campylobacter jejuni associated with Guillain-Barré syndrome patients mimic human gangliosides in structure. Infect. Immun. 62:2122-2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aspinall, G. O., C. M. Lynch, H. Pang, R. T. Shaver, and A. P. Moran. 1995. Chemical structures of the core region of Campylobacter jejuni O:3 lipopolysaccharide and an associated polysaccharide. Eur. J. Biochem. 231:570-578. [PubMed] [Google Scholar]
- 7.Aspinall, G. O., A. G. McDonald, H. Pang, L. A. Kurjanczyk, and J. L. Penner. 1994. Lipopolysaccharides of Campylobacter jejuni serotype O:19: structures of core oligosaccharide regions from the serostrain and two bacterial isolates from patients with the Guillain-Barré syndrome. Biochemistry 33:241-249. [DOI] [PubMed] [Google Scholar]
- 8.Aspinall, G. O., A. G. McDonald, T. S. Raju, H. Pang, L. A. Kurjanczyk, J. L. Penner, and A. P. Moran. 1993. Chemical structure of the core region of Campylobacter jejuni serotype O:2 lipopolysaccharide. Eur. J. Biochem. 213:1029-1037. [DOI] [PubMed] [Google Scholar]
- 9.Aspinall, G. O., A. G. McDonald, T. S. Raju, H. Pang, A. P. Moran, and J. L. Penner. 1993. Chemical structures of the core regions of Campylobacter jejuni serotypes O:1, O:4, O:23, and O:36 lipopolysaccharides. Eur. J. Biochem. 213:1017-1027. [DOI] [PubMed] [Google Scholar]
- 10.Blaser, M. J., and G. I. Perez-Perez. 1992. Humoral immune response to lipopolysaccharide antigens of Campylobacter jejuni, p. 230-235. In I. Nachamkin, M. J. Blaser, and L. S. Tompkins (ed.), Campylobacter jejuni: current status and future trends. American Society for Microbiology, Washington, D.C.
- 11.Chiba, A., S. Kusunoki, T. Shimizu, and I. Kanazawa. 1992. Serum IgG antibody to ganglioside GQ1b is a possible marker of Miller-Fisher syndrome. Ann. Neurol. 31:677-679. [DOI] [PubMed] [Google Scholar]
- 12.Goodyear, C. S., G. M. O'Hanlon, J. J. Plomp, E. R. Wagner, I. Morrison, J. Veitch, L. Cochrane, R. W. Bullens, P. C. Molenaar, J. Conner, and H. J. Willison. 1999. Monoclonal antibodies raised against Guillain-Barré syndrome-associated Campylobacter jejuni lipopolysaccharides react with neuronal gangliosides and paralyze muscle-nerve preparations. J. Clin. Investig. 104:697-708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ho, T. W., H. J. Willison, I. Nachamkin, C. Y. Li, J. Veitch, H. Ung, G. R. Wang, R. C. Liu, D. R. Cornblath, A. K. Asbury, J. W. Griffin, and G. M. McKhann. 1999. Anti-GD1a antibody is associated with axonal but not demyelinating forms of Guillain-Barré syndrome. Ann. Neurol. 45:168-173. [DOI] [PubMed] [Google Scholar]
- 14.Jacobs, B. C., H. P. Endtz, F. G. A. Van der Meché, M. P. Hazenberg, H. A. M. Achtereekte, and P. A. Van Doorn. 1995. Serum anti-GQ1b IgG antibodies recognize surface epitopes on Campylobacter jejuni from patients with Miller-Fisher syndrome. Ann. Neurol. 37:260-264. [DOI] [PubMed] [Google Scholar]
- 15.Jacobs, B. C., H. P. Endtz, F. G. A. Van der Meché, M. P. Hazenberg, M. A. De Klerk, and P. A. Van Doorn. 1997. Humoral immune response against Campylobacter jejuni lipopolysaccharides in Guillain-Barré and Miller Fisher syndrome. J. Neuroimmunol. 79:62-68. [DOI] [PubMed] [Google Scholar]
- 16.Jacobs, B. C., M. P. Hazenberg, P. A. Van Doorn, H. P. Endtz, and F. G. A. Van der Meché. 1997. Cross-reactive antibodies against gangliosides and Campylobacter jejuni lipopolysaccharides in patients with Guillain-Barré or Miller Fisher syndrome. J. Infect. Dis. 175:729-733. [DOI] [PubMed] [Google Scholar]
- 17.Jeremias, J., P. Giraldo, S. Durrant, A. Ribeiro-Filho, and S. S. Witkin. 1999. Relationship between Ureaplasma urealyticum vaginal colonization and polymorphism in the interleukin-1 receptor antagonist gene. J. Infect. Dis. 180:912-914. [DOI] [PubMed] [Google Scholar]
- 18.Kusunoki, S., A. Chiba, K. Kon, S. Ando, K. Arisawa, A. Tate, and I. Kanazawa. 1994. N-Acetylgalactosaminyl GD1a is a target molecule for serum antibody in Guillain-Barré syndrome. Ann. Neurol. 35:570-576. [DOI] [PubMed] [Google Scholar]
- 19.Linton, D., M. Gilbert, P. G. Hitchen, A. Dell, H. R. Morris, W. W. Wakarchuk, N. A. Gregson, and B. W. Wren. 2000. Phase variation of a β-1,3-galactosyltransferase involved in generation of the ganglioside GM1-like lipo-oligosaccharide of Campylobacter jejuni. Mol. Microbiol. 37:501-514. [DOI] [PubMed] [Google Scholar]
- 20.Moran, A. P. 1997. Structure and conserved characteristics of Campylobacter jejuni lipopolysaccharides. J. Infect. Dis. 176:S115-121. [DOI] [PubMed] [Google Scholar]
- 21.Moran, A. P., M. M. Prendergast, and B. J. Appelmelk. 1996. Molecular mimicry of host structures by bacterial lipopolysaccharides and its contribution to disease. FEMS Immunol. Med. Microbiol. 16:105-115. [DOI] [PubMed] [Google Scholar]
- 22.Nam Shin, J. E., S. Ackloo, A. S. Mainkar, M. A. Monteiro, H. Pang, J. L. Penner, and G. O. Aspinall. 1998. Lipo-oligosaccharides of Campylobacter jejuni serotype O:10. Structures of core oligosaccharide regions from a bacterial isolate from a patient with the Miller-Fisher syndrome patient and from the serotype reference strain. Carbohydr. Res. 305:223-232. [DOI] [PubMed] [Google Scholar]
- 23.Neisser, A., H. Bernheimer, T. Berger, A. P. Moran, and B. Schwerer. 1997. Serum antibodies against gangliosides and Campylobacter jejuni lipopolysaccharides in Miller Fisher syndrome. Infect. Immun. 65:4038-4042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pandey, J. P., and M. J. Blaser. 1986. Heterozygosity at the Km locus associated with humoral immunity to Campylobacter jejuni. Exp. Clin. Immunogenet. 3:49-53. [PubMed] [Google Scholar]
- 25.Plomp, J. J., P. C. Molenaar, G. M. O' Hanlon, B. C. Jacobs, J. Veitch, M. R. Daha, P. A. Van Doorn, F. G. A. Van der Meché, A. Vincent, B. P. Morgan, and H. J. Willison. 1999. Miller Fisher anti-GQ1b antibodies: alpha-latrotoxin-like effects on motor endplates. Ann. Neurol. 45:189-199. [DOI] [PubMed] [Google Scholar]
- 26.Pollard, J. D., K. W. Westland, G. K. Harvey, S. Jung, J. Bonner, J. M. Spies, K. V. Toyka, and H. P. Hartung. 1995. Activated T cells of nonneural specificity open the blood-nerve barrier to circulating antibody. Ann. Neurol. 37:467-475. [DOI] [PubMed] [Google Scholar]
- 27.Prendergast, M. M., A. J. Lastovica, and A. P. Moran. 1998. Lipopolysaccharides from Campylobacter jejuni O:41 strains associated with Guillain-Barré syndrome exhibit mimicry of GM1 ganglioside. Infect. Immun. 66:3649-3655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ritter, G., S. R. Fortunato, L. Cohen, Y. Noguchi, E. M. Bernard, E. Stockert, and L. J. Old. 1996. Induction of antibodies reactive with GM2 ganglioside after immunization with lipopolysaccharides from Campylobacter jejuni. Int. J. Cancer 66:184-190. [DOI] [PubMed] [Google Scholar]
- 29.Salloway, S., L. A. Mermel, M. Seamans, G. O. Aspinall, J. E. Nam Shin, L. A. Kurjanczyk, and J. L. Penner. 1996. Miller-Fisher syndrome associated with Campylobacter jejuni bearing lipopolysaccharide molecules that mimic human ganglioside GD3. Infect. Immun. 64:2945-2949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Van der Meché, F. G. A., and P. A. Van Doorn. 1995. Guillain-Barré syndrome and chronic inflammatory demyelinating polyneuropathy: immune mechanisms and update on current therapies. Ann. Neurol. 37:S14-S31. [DOI] [PubMed] [Google Scholar]
- 31.Wassenaar, T. M., M. Engelskirchen, S. Park, and A. Lastovica. 1997. Differential uptake and killing potential of Campylobacter jejuni by human peripheral monocytes/macrophages. Med. Microbiol. Immunol. 186:139-144. [DOI] [PubMed] [Google Scholar]
- 32.Weber, F., R. Rudel, P. Aulkemeyer, and H. Brinkmeier. 2000. Anti-GM1 antibodies can block neuronal voltage-gated sodium channels. Muscle Nerve 23:1414-1420. [DOI] [PubMed] [Google Scholar]
- 33.Willison, H. J., G. O'Hanlon, G. Paterson, O. L. C. P., J. Veitch, G. Wilson, M. Roberts, T. Tang, and A. Vincent. 1997. Mechanisms of action of anti-GM1 and anti-GQ1b ganglioside antibodies in Guillain-Barré syndrome. J. Infect. Dis. 176:S144-S149. [DOI] [PubMed] [Google Scholar]
- 34.Willison, H. J., and G. M. O'Hanlon. 1999. The immunopathogenesis of Miller Fisher syndrome. J. Neuroimmunol. 100:3-12. [DOI] [PubMed] [Google Scholar]
- 35.Wirguin, I., C. Briani, L. Suturkova-Milosevic, T. Fisher, P. Della-Latta, P. Chalif, and N. Latov. 1997. Induction of anti-GM1 ganglioside antibodies by Campylobacter jejuni lipopolysaccharides. J. Neuroimmunol. 78:138-142. [DOI] [PubMed] [Google Scholar]
- 36.Yuki, N. 1997. Molecular mimicry between gangliosides and lipopolysaccharides of Campylobacter jejuni isolated from patients with Guillain-Barré syndrome and Miller Fisher syndrome. J. Infect. Dis. 176:S150-153. [DOI] [PubMed] [Google Scholar]
- 37.Yuki, N., T. Taki, and S. Handa. 1996. Antibody to GalNAc-GD1a and GalNAc-GM1b in Guillain-Barré syndrome subsequent to Campylobacter jejuni enteritis. J. Neuroimmunol. 71:155-161. [DOI] [PubMed] [Google Scholar]
- 38.Yuki, N., T. Taki, F. Inagaki, T. Kasama, M. Takahashi, K. Saito, S. Handa, and T. Miyatake. 1993. A bacterium lipopolysaccharide that elicits Guillain-Barré syndrome has a GM1 ganglioside-like structure. J. Exp. Med. 178:1771-1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yuki, N., T. Taki, M. Takahashi, K. Saito, T. Tai, T. Miyatake, and S. Handa. 1994. Penner's serotype 4 of Campylobacter jejuni has a lipopolysaccharide that bears a GM1 ganglioside epitope as well as one that bears a GD1 a epitope. Infect. Immun. 62:2101-2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yuki, N., T. Taki, M. Takahashi, K. Saito, H. Yoshino, T. Tai, S. Handa, and T. Miyatake. 1994. Molecular mimicry between GQ1b ganglioside and lipopolysaccharides of Campylobacter jejuni isolated from patients with Fisher's syndrome. Ann. Neurol. 36:791-793. [DOI] [PubMed] [Google Scholar]