Abstract
Pathogenic Yersinia spp. uncouple an array of signal transduction pathways in macrophages to disrupt their response to infection. This compels the macrophage to undergo apoptosis. Our study shows that macrophages that had acquired tolerance to Yersinia infection by preexposure to lipopolysaccharide were considerably protected against Y. enterocolitica-induced apoptosis. The desensitization of macrophages by lipopolysaccharide, which is thought to be a self-protective, adaptive response to sustained bacterial stimulation, may represent an immune mechanism that aids in overcoming Yersinia-mediated apoptosis and infection.
Pathogenic Yersinia spp. deploy a panel of strategies to disarm macrophages and to disrupt their response to infection. For this purpose, yersiniae engage a type III protein secretion system that is highly conserved in the three species that are pathogenic for rodents and humans (9). Yersinia pestis is the causative agent of plague, and Yersinia enterocolitica and Yersinia pseudotuberculosis cause gastrointestinal syndromes, lymphadenitis, and septicemia. The Yersinia type III protein secretion system is activated upon host cell contact and specifically mediates the polarized translocation of Yersinia effector proteins (Yersinia outer proteins [Yops]) inside eukaryotic cells (9). At least six effector Yops are known to be injected (YopE, YopH, YopM, YopT, YopO/YpkA, and YopP/YopJ). In the host cell, the Yops act on different cellular levels to neutralize a sequence of programmed phagocyte effector functions. By interference with the actin cytoskeleton dynamics, Yersinia blocks its phagocytosis and prevents its killing by the phagocytic oxidative burst. These effects are largely mediated by YopE and YopH (2, 6, 9). YopE displays a GTPase-activating protein activity and mediates inactivation of Rho-GTPases, which critically regulate actin cytoskeleton rearrangements (6). YopH is a tyrosine phosphatase and dephosphorylates host cell proteins, such as p130Cas and the focal adhesion kinase, leading to disruption of peripheral focal adhesion complexes (6). Besides these immediate effects on the phagocyte, Yersinia inhibits production of the proinflammatory cytokine tumor necrosis factor alpha (TNF-α) and triggers macrophage apoptosis (2, 9, 24). Both effects are conducted by YopP (Y. enterocolitica) or by its homologue, YopJ (Y. pseudotuberculosis and Y. pestis) (2, 9, 24). YopP/YopJ engages multiple cellular binding partners. It interacts with members of the mitogen-activated protein kinase (MAPK) kinase (MKK) superfamily, which leads to disruption of downstream MAPK activities (25). In addition, YopP/YopJ targets the NF-κB-activating IκB kinase-β (IKK-β), which results in inhibition of NF-κB activation (25). Since the NF-κB and MAPK signaling cascades synergistically control production of the macrophage TNF-α in response to bacterial infection (12), subversion of these pathways by YopP/YopJ accounts for the cytokine suppressive effect of Yersinia (2, 9, 24)
The modulation of NF-κB signaling by YopP/YopJ also plays crucial role in the mechanism of apoptosis induction. As a global regulator of the inflammatory and stress response, NF-κB functions to up-regulate the synthesis of antiapoptotic proteins, such as inhibitors of apoptosis proteins (IAP) and Bcl-2 family members (1, 16). These proteins counteract proapoptotic signals elicited by diverse extracellular stimuli. Accordingly, NF-κB activation provides protection against apoptosis otherwise induced under these conditions. The antiapoptotic function of NF-κB is essential for self-defense and survival of macrophages when encountered with bacteria or lipopolysaccharide (LPS) (3, 17, 27). Suppression of NF-κB activation by using specific inhibitors sensitizes macrophages to undergo apoptosis upon LPS treatment (17, 27). In Yersinia infection, the initiation of LPS-responsive signaling cooperates with the NF-κB-inhibitory action of YopP/YopJ to mediate macrophage apoptosis (28). This indicates that Yersinia spp. exploit proapoptotic LPS signaling to efficiently trigger macrophage apoptosis under conditions in which NF-κB activation is suppressed by YopP/YopJ.
In this study, we addressed the question of whether cellular hyporesponsivness in LPS-tolerized macrophages influences apoptosis due to Y. enterocolitica infection. LPS as major constituent of the outer membrane of gram-negative bacteria is a potent inducer of multiple proinflammatory cytokines in monocytes and macrophages, such as TNF-α, interleukin-1 (IL-1), and IL-6 (12). Although these cytokines are indispensable for the efficient control of growth and dissemination of bacteria, an overshooting inflammatory response is potentially autodestructive for the compromised host. Repeated exposure to LPS therefore confers the status of LPS hyporesponsiveness to macrophages, which is also known as endotoxin tolerance (8, 18). In this status, macrophages do not respond to subsequent LPS stimulation by adequate activation of cellular signaling and cytokine production (8, 18). This mechanism protects the cells and the organism from developing damage caused by hyperactivation of macrophages with persisting bacteria and LPS. The development of endotoxin tolerance therefore represents a means of macrophage adaptation to bacterial infection. This tolerance phenomenon is conferred not only by exposure to LPS, but also by other bacterial components: i.e., bacterial lipopeptides and lipoteichoic acids (19, 30).
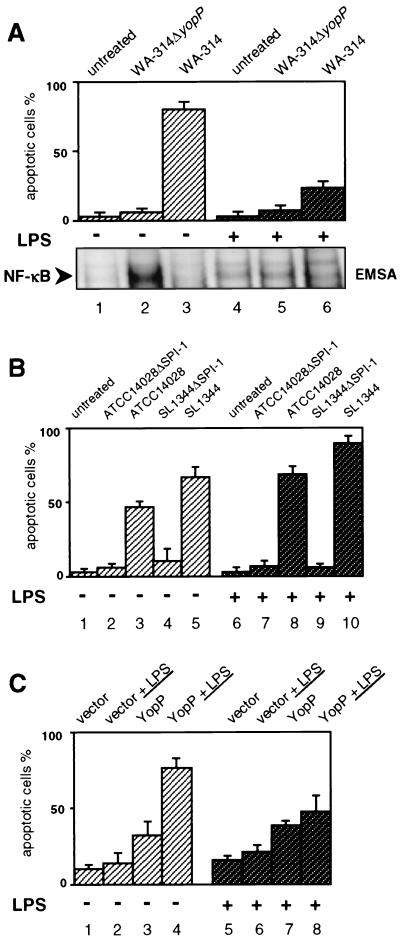
To induce macrophage anergy to LPS signaling by LPS desensitization, we exposed the murine J774A.1 macrophage cell line to LPS (10 μg/ml) for 16 h. The cells were routinely cultured in RPMI 1640 cell growth medium supplemented with 10% heat-inactivated fetal calf serum and 5 mM l-glutamine (29). Infections were performed with the Y. enterocolitica serogroup O8 wild-type strain WA-314 (7, 13) and the corresponding yopP-knockout mutant WA-314ΔyopP (28). The bacterial cultures were first grown overnight at 26°C and then were diluted 1:20 in fresh Luria-Bertani broth and grown for another 2 h at 37°C. After washing, the bacteria were resuspended in phosphate-buffered saline (PBS) and used for infection at a ratio of 30 bacteria per cell. The bacteria were killed after 90 min by addition of gentamicin (100 μg/ml), and onset of apoptosis in the infected cells was assayed after a final incubation time of 5 h (29). The apoptotic cells were specifically labeled with fluorescein-conjugated annexin V (Boehringer-Mannheim, Mannhein, Germany), which binds with high affinity to phosphatidylserine exposed on the outer leaflet of apoptotic cells. This confers green fluorescence to cells undergoing apoptosis. The simultaneous application of the DNA stain propidium iodide (Sigma, St. Louis, Mo.) allowed the discrimination of apoptotic from necrotic cells. The rate of apoptosis was determined by analyzing the cells in a fluorescence microscope (29). As depicted in Fig. 1A, the wild-type strain WA-314 efficiently mediated apoptosis in J774A.1 cells that were not pretreated with LPS (Fig. 1A, lane 3), whereas the YopP-negative mutant did not elicit cell death (lane 2). Interestingly, wild-type yersiniae were considerably less efficient at triggering apoptosis in LPS-pretreated macrophages (79% ± 4% apoptosis versus 22% ± 3% apoptosis in non-pretreated cells; compare lane 6 with lane 3, Fig. 1A). This indicates that prechallenge of macrophages with LPS is able to provide a substantial degree of protection against Y. enterocolitica WA-314-induced cell death. A similar effect was mediated by Yersinia bacterial culture supernatant: pretreatment of the cells with the supernatant from an overnight culture (37°C) of Y. enterocolitica WA-314 diluted in cell culture medium reduced Y. enterocolitica-induced apoptosis by 50 to 70%. Thus, protection from apoptosis is not restricted to Escherichia coli LPS, but can also be conferred by Yersinia-derived bacterial components.
FIG. 1.
LPS-desensitized J774A.1 macrophages respond with reduced apoptosis to Y. enterocolitica infection and YopP-LPS treatment. (A) Apoptosis and NF-κB activation in Y. enterocolitica-infected macrophages. J774A.1 cells were not stimulated (lanes 1 to 3) or were exposed to LPS (10 μg/ml) for 16 h (lanes 4 to 6) and then either left untreated (lanes 1 and 4) or infected with the YopP-negative mutant WA-314ΔyopP (lanes 2 and 5) or the wild-type strain WA-314 (lanes 3 and 6). Apoptosis was assayed 5 h after onset of infection by staining cells with annexin V and analyzing apoptotic cells by fluorescence microscopy (upper panel). The nuclear translocation of NF-κB wasdetermined 90 min after onset of infection by EMSA (lower panel). Only sections of the autoradiogram containing the NF-κB-DNA complexes are shown. The EMSA shows data from one experiment representative of four performed. (B) Apoptosis in S. enterica serovar Typhimurium-infected macrophages. J774A.1 cells were not stimulated (lanes 1 to 5) or were exposed to LPS for 16 h (lanes 6 to 10) and then either left untreated (lanes 1 and 6) or infected with S. enterica serovar Typhimurium strains impaired in secretion of SPI-1-encoded virulence proteins (ATCC 14028ΔSPI-1, lanes 2 and 7; SL1344ΔSPI-1, lanes 4 and 9) or the respective wild-type strains (ATCC 14028, lanes 3 and 8; SL1344, lanes 5 and 10). Apoptosis was assayed 5 h after onset of infection by analyzing annexin V-positive, apoptotic cells by fluorescence microscopy. (C) LPS-enhanced cell death in YopP-transfected macrophages. J774A.1 cells were not stimulated (lanes 1 to 4) or were exposed to LPS for 16 h (lanes 5 to 8) and then cotransfected with β-galactosidase reporter vector and either empty expression vector (vector) or YopP expression vector (YopP). Transfected cells were left untreated (lanes 1, 3, 5, and 7) or were exposed to LPS for an additional 8 h (lanes 2, 4, 6, and 7). Cells were stained with X-Gal, and single transfected cells were analyzed for an apoptotic morphology. Quantitative results are expressed as the mean percentages of apoptotic cells ± standard deviations from three independent experiments.
We investigated whether the LPS protective effect specifically occurs in the J774A.1 macrophage cell line. Elicited mouse peritoneal macrophages were obtained from male C3H/HeN mice 3 days after intraperitoneal inoculation of 1 ml of 10% Proteose Peptone broth (27). Peritoneal exudate cells were washed and then cultured at 37°C in cell growth medium, and nonadherent cells were removed by repeated washing. Seventy to 90% of remanent macrophages that were not pretreated with LPS underwent apoptosis after infection with the Y. enterocolitica wild-type strain WA-314 within 5 h, whereas mediation of cell death in LPS-exposed cells was markedly reduced (20 to 40% apoptosis). The yopP-negative mutant WA-ΔyopP did not confer cell death under both conditions (<10% apoptosis). Thus, suppression of apoptosis through LPS pretreatment is not a unique characteristic in a macrophage cell line, but also occurs in primary macrophages.
The prestimulation of J774A.1 cells with TNF-α (200 ng/ml) or gamma interferon (100 U/ml) for 16 h instead of LPS could not rescue the cells from Y. enterocolitica WA-314-mediated cell death. Under both conditions, 70 to 80% of the infected J774A.1 cells became apoptotic within 5 h, similar to non-pretreated cells. This indicates that priming of macrophages to bacterial infection by cytokine pretreatment, which is known to enhance macrophage antibacterial activities, does not restrict the ability of Y. enterocolitica to trigger apoptosis.
Various intracellular signaling pathways that are activated in non-pretreated macrophages by bacterial infection remain silent in endotoxin-tolerized macrophages (11, 18, 22, 30). These include pathways of NF-κB and of MAPKs. We analyzed the impact of Yersinia strains on NF-κB activation under LPS-pretreated and non-pretreated conditions. Translocation of NF-κB into the nucleus, reflecting activation of the NF-κB pathway, was determined by electrophoretic mobility shift assay (EMSA) from nuclear extracts of infected J774A.1 cells as described previously (27, 28). In brief, cells were washed after 90 min of infection with ice-cold PBS and lysed with hypotonic buffer on ice (5 mM HEPES [pH 7.9], 1.5 mM MgCl2, 10 mM KCl, 0.5 mM dithiothreitol [DTT], protease inhibitors). Proteins were extracted from the nuclei by resuspension of the nuclear pellets in ice-cold extraction buffer (20 mM HEPES [pH 7.9], 25% glycerol, 1 M NaCl, 1.5 mM MgCl2, 0.5 mM EDTA, 0.5 mM DTT, protease inhibitors). Seven micrograms of the extracted nuclear proteins was incubated with 2 to 5 ng of a radiolabeled oligonucleotide probe that encompasses the consensus binding site for NF-κB dimeric complexes (5′-AGT TGA GGG GAC TTT CCC AGG C-3′; Santa Cruz Biotechnology). The DNA-binding reactions were performed in the presence of a mixture containing 25 mM HEPES (pH 7.9), 0.5 mM EDTA, 0.5 mM DTT, and 5% glycerol for 30 min on ice. The DNA-protein complexes were subsequently separated by 5% polyacrylamide gel electrophoresis (PAGE) and analyzed by autoradiography. Figure 1A (lower panel) shows that the strong apoptotic response induced by wild-type WA-314 yersiniae in J774A.1 cells not pretreated with LPS correlated with suppression of NF-κB activation after onset of infection (Fig. 1A, lane 3). In contrast, the YopP mutant WA-314ΔyopP triggered substantial NF-κB activation, which preceded cellular survival (lane 2). In LPS-pretreated macrophages, neither the wild-type strain nor the YopP mutant elicited nuclear translocation of NF-κB (lanes 5 and 6), and the NF-κB activation profiles were similar to that of noninfected cells (lane 4). Because the NF-κB pathway was not activated in response to both strains, pretreatment with LPS apparently rendered the cells unresponsive to subsequent NF-κB activation by Y. enterocolitica. The cells obviously acquired a state of cellular refractoriness to bacterial infection. In this status, macrophages apparently better withstand apoptosis due to Y. enterocolitica WA-314 infection. The survival of LPS-desensitized macrophages does not seem to depend on the antiapoptotic activities of NF-κB, because impaired NF-κB activation after infection with the YopP mutant was not associated with reduced cellular viability (Fig. 1A, lane 5). This suggests uncoupling or concomitant downregulation of death-inducing and -preventing signals in LPS-tolerized cells.
To analyze whether resistance of LPS-desensitized macrophages to apoptosis is unique to Y. enterocolitica infection or is a more general phenomenon, we investigated the impact of LPS pretreatment on apoptosis induced by Salmonella enterica serovar Typhimurium. Salmonella triggers apoptosis in macrophages by inducing the caspase cascade through caspase-1 activation (15). This mechanism involves SipB, a protein that is secreted and translocated by the type III protein secretion machinery encoded by Salmonella pathogenicity island 1 (SPI-1). The S. enterica serovar Typhimurium strains used in this study were kindly provided by M. Hensel (Institute for Clinical Microbiology, Immunology, and Hygiene, University of Erlangen, Erlangen, Germany). We analyzed two S. enterica serovar Typhimurium wild-type strains (ATCC 14028 and SL1344) and their respective mutants (ATCC 14028-MvP215 and SL1344-EE659), which are impaired in secretion of virulence proteins of SPI-1 due to transposon mutation of prgK (4, 10). S. enterica serovar Typhimurium strains were cultured until they entered the early stationary growth phase, a stage by which Salmonella acquires the capability to induce apoptosis (21). The two S enterica serovar Typhimurium wild-type strains efficiently induced macrophage apoptosis (Fig. 1B, lanes 3 and 5), whereas the respective SPI-1 mutants did not affect cellular survival (lanes 2 and 4). Interestingly, LPS pretreatment appeared to enhance the onset of apoptosis in cells infected with the Salmonella wild-type strains (lanes 8 and 10), which is in opposition to the results obtained with Y. enterocolitica WA-314. This suggests that macrophage hyporesponsiveness to bacteria due to LPS preexposure plays a specific protective role against the apoptotic response triggered by Yersinia.
In our previous study, we used an experimental approach that allowed the characterization of the contribution of LPS stimulation to Yersinia-mediated apoptosis (28). In these experiments, the activation of LPS-responsive signaling processes by LPS stimulation significantly enhanced apoptosis in J774A.1 macrophages that were transfected with a eukaryotic expression plasmid encoding yopP from Y. enterocolitica WA-314 (28). In this plasmid, yopP is constitutively expressed under control of the cytomegalovirus promoter [plasmid pcDNA3.1(−); Invitrogen, Carlsbad, Calif.]. Since LPS-responsive signaling pathways appear to remain silent in LPS-pretreated J774A.1 macrophages, we wondered whether LPS prestimulation also influences J774A.1 cell death upon YopP transfection and additional LPS challenge. Accordingly, J774A.1 cells were left untreated or were treated with LPS (10 μg/ml) for 16 h and subsequently transfected with either 0.66 μg of YopP expression plasmid or 0.66 μg of empty vector control by using the ExGen500 transfection reagent (Fermentas, Hanover, Md.) as described previously (28). For identification of the transfected cells, the cells were cotransfected with 0.33 of a β-galactosidase-encoding reporter vector (Promega, Madison, Wis.). Staining with X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyransoside) enables the detection of β-galactosidase expression by conferring a blue color to the transfected cells (28). After transfection, the cells were left untreated or additionally stimulated with LPS and assayed for cell death microscopically 8 h later. Apoptotic death in YopP-transfected cells is characterized by a typical condensed, misshapen cellular appearance (28). The number of β-galactosidase-expressing apoptotic cells was determined in relation to the total number of transfected cells (Fig. 1C). In correlation with our previous study, transfection of J774A.1 cells with YopP led to a moderate degree of death in transfected cells that were not pretreated with LPS (Fig. 1C, lane 3). Additional stimulation with LPS remarkably enhanced the apoptotic effect (lane 4). Also, LPS-pretreated macrophages underwent substantial apoptosis upon YopP transfection (lane 7). However, additional LPS stimulation subsequently to transfection could not considerably increase cell death (lane 8), and the total number of dead cells remained reduced compared to the non-pretreated macrophages (compare lanes 4 and 8). This indicates that the cytotoxic signals, which mediate cell death in YopP-transfected macrophages upon LPS stimulation, are latent or reduced in LPS-tolerized macrophages.
In fact, the development of tolerance to bacterial infection depends on the orchestrated action of multiple factors (8, 18). It involves alterations of very early steps of signal transduction that are activated by primary exposure to conserved bacterial components (11, 22, 30). Such components, like LPS or lipoteichoic acids, activate members of the transmembrane Toll-like receptor (TLR) family, whose intracellular signals are relayed by molecules of the IL-1 receptor pathway (1), including the adapter protein myeloid differentiation factor 88 (MyD88) and IL-1 receptor-associated kinases (IRAK). The induction of cross-tolerance by differential activation of TLRs as well as by IL-1 stimulation suggests modulation of signal transduction via common signaling intermediates, such as MyD88 or IRAK, during development of the tolerance phenomenon (19, 22, 30). Accordingly, the induction of endotoxin tolerance has been shown to cause downregulation of surface TLR4 expression (23), as well as to decrease the content of IRAK and its association with MyD88 (20). Some of these receptor and early postreceptor signaling molecules can also play a role in the mediation of apoptosis. The induction of MyD88 through TLR2 activates the Fas-associated death domain (FADD) protein/caspase-8 cytotoxic pathway, which leads to apoptotic cell death (3). Similarly, IRAK molecules can be involved in signaling apoptosis (5). From these data, inhibition of proximal signal transduction in the state of LPS-mediated refractoriness appears to suppress the submission of proapoptotic signals, which confers protection against Yersinia-induced apoptosis. However, in Western immunoblotting experiments, the protein expression patterns of MyD88, IRAK2, and FADD were similar in LPS-pretreated and non-pretreated J774A.1 cells (data not shown). It may therefore be speculated that resistance to Yersinia-mediated apoptosis resulted from a modified interplay, but not from reduced synthesis, of these potentially proapoptotic signaling molecules. Alternatively, the priming of macrophages with LPS triggers the upregulation of antiapoptotic factors, which efficiently protect against cell death upon secondary microbial stimulation without additional NF-κB activation.
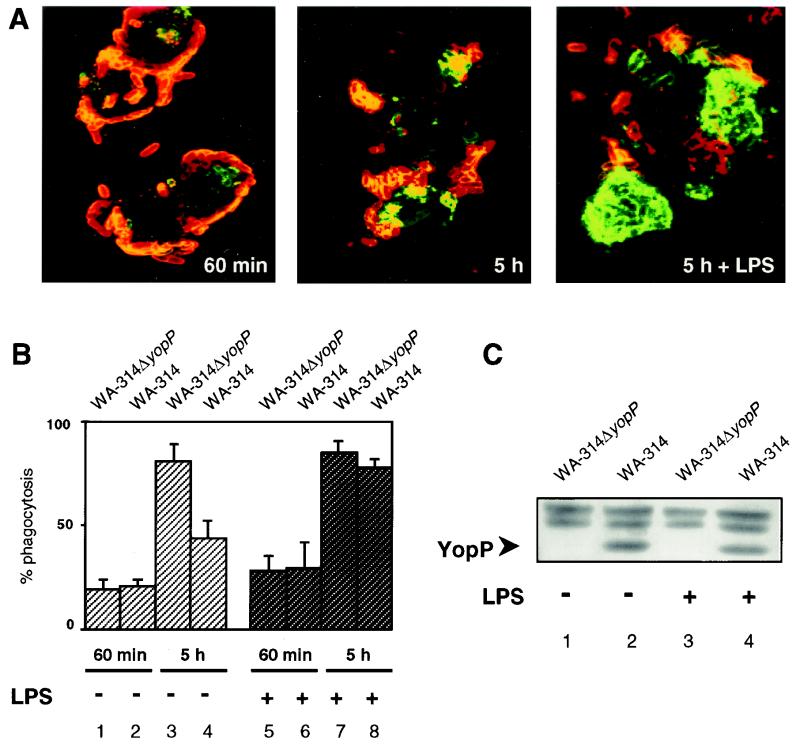
To determine whether better survival of LPS-tolerized macrophages cells could be advantageous for the host antibacterial activities, we investigated the phagocytic capacities of the infected cells. Resistance of yersiniae to phagocytosis is predominantly conferred by YopH and YopE (2, 6, 9). Non-pretreated and LPS-pretreated J774A.1 macrophages were infected with wild-type yersiniae (strain WA-314) or the YopP-negative mutant (strain WA-314ΔyopP), and the location of the bacteria was analyzed by a differential immunofluorescent staining method as described previously (14). Briefly, extracellular bacteria were first marked with anti-YadA monoclonal and secondary antimouse tetramethyl rhodamine isothiocyanate (TRITC)-labeled antibodies (26). After fixation and permeabilization of the cell membrane with methanol, intracellularly located bacteria were similarly treated with anti-YadA antibodies and visualized with secondary fluorescein isothiocyanate (FITC)-conjugated antibodies. This technique allows determination of the numbers of both cell-associated (fluorescing red and green) and ingested (fluorescing exclusively green) bacteria. The wild-type strain WA-314 and the YopP-negative mutant efficiently resisted ingestion by the macrophages, and more than 75% of the bacteria were located extracellularly at the macrophage membrane 60 min after onset of infection (Fig. 2A, left panel, and 2B, lanes 1 and 2). Although this effect was more pronounced in non-pretreated cells, LPS-prechallenged macrophages were also receptive to the antiphagocytic action of Y. enterocolitica (Fig. 2B, lanes 5 and 6). In both conditions, with and without LPS pretreatment, around 20 to 40 bacteria were associated per cell. This indicates that LPS exposure does not diminish bacterial interaction. Furthermore, wild-type Y. enterocolitica WA-314 translocated YopP in LPS-pretreated and non-pretreated macrophages, as determined by Western immunoblotting (Fig. 2C). In these experiments, J774A.1 cells were lysed after 60 min of infection on ice with lysis buffer (10 mM HEPES [pH 7.8], 10 mM KCl, 2 mM MgCl2, 0.1 mM EDTA, 1% NP-40, 1 mM DTT, phosphatase, protease inhibitors). These conditions selectively lyse the cells, but not the bacteria, and do not extract critical amounts of bacterium-associated Yops. Identical amounts of protein in the cellular lysates, quantified by a protein assay kit (Bio-Rad Laboratories, Munich, Germany), were separated by sodium dodecyl sulfate (SDS)-PAGE, electrotransferred to polyvinylidene difluoride membrane, and probed with a polyclonal rabbit anti-YopP antiserum. The antibodies were raised against the YopP N terminus by immunizing a New Zealand rabbit with a recombinant glutathione S-transferase (GST)-fused YopP fragment that encompasses amino acids 1 to 125 of YopP. The antiserum was utilized without purification for Western blot analysis. The immunoblot result rules out the possibility that reduced apoptosis in LPS-pretreated macrophages may result from blockade of YopP injection (Fig. 2C).
FIG. 2.
LPS-desensitized J774A.1 macrophages efficiently internalize nonviable yersiniae. (A) Determination of intra- and extracellularly located yersiniae. J774A.1 cells were not stimulated (left and middle panels) or were exposed to LPS (10 μg/ml) for 16 h (right) and then infected with wild-type Y. enterocolitica WA-314. The location of yersiniae was determined 60 min after onset of infection (left) or after a total incubation period of 5 h, following treatment with gentamicin 90 min after onset of infection (middle and right panels). Bacteria were stained by a double-immunofluorescence method, which allows discrimination of intra- and extracellular bacteria. Extracellular yersiniae are characterized by red fluorescence, and intracellular yersiniae are characterized by green fluorescence. (B) Quantitation of phagocytosis. J774A.1 cells were not stimulated (lanes 1 to 4) or were exposed to LPS for 16 h (lanes 5 to 8) and then infected with the YopP-negative mutant WA-314ΔyopP (lanes 1, 3, 5, and 7) or the wild-type strain WA-314 (lanes 2, 4, 6, and 8). The location of yersiniae was assayed 60 min after onset of infection (lanes 1 and 2 and 5 and 6) or after a total incubation period of 5 h, following treatment with gentamicin 90 min after onset of infection (lanes 3 and 4 and 7 and 8), by the method described above. Mean percentages of ingested versus total cell-associated bacteria ± standard deviations were determined from three independent experiments. (C) Determination of translocated YopP. J774A.1 cells were not stimulated (lanes 1 and 2) or were exposed to LPS for 16 h (lanes 3 and 4) and then infected with the YopP-negative mutant WA-314ΔyopP (lanes 1 and 3) or the wild-type strain WA-314 (lanes 2 and 4). After 60 min, cells were lysed under conditions that selectively lyse the cells. The lysates were subjected to SDS-PAGE, immunoblotted with anti-YopP antibodies, and visualized with enhanced chemiluminescence reagents. The arrow indicates translocated YopP. Nonspecific bands appearing above YopP confirm equal loading of the gel with cellular lysates.
In an additional set of experiments, yersiniae were killed by addition of gentamicin (100 μg/ml) after 90 min of infection, and phagocytosis of bacteria was determined after a total incubation time of 5 h. Interestingly, the YopP mutant, which did not trigger apoptosis, was efficiently ingested after antibiotic killing. This effect was independent of preexposure of the cells to LPS (Fig. 2B, lanes 3 and 7). This indicates that the antiphagocytic effects of Yersinia are reversible and that infected macrophages can regain the ability to phagocytose yersiniae upon antibiotic killing. In the same manner, wild-type yersiniae were remarkably phagocytosed after treatment with gentamicin. In this case, LPS-pretreated macrophages ingested considerably more bacteria than the non-pretreated cells (Fig. 2A, middle and right panels, and B, lanes 4 and 8). This difference correlated well with the onset of Y. enterocolitica-induced apoptosis: the majority of the macrophages not pretreated with LPS and displaying less efficient phagocytosis started to undergo apoptosis at that time (Fig. 1A, lane 3). In contrast, reduced apoptosis in LPS-prestimulated cells (Fig. 1A, lane 6) coincided with elevated bacterial uptake. These data suggest that macrophages that survive Yersinia infection restore their microbicidal properties after clearance of the bacteria. From this observation, the translocation of YopP appears to be an effort of Yersinia to irreversibly suppress the antibacterial action of macrophages by the induction of apoptosis. With respect to host immunity functions, the development of endotoxin tolerance is potentially advantageous to the host, because LPS desensitization does not impair macrophage phagocytic activities, but provides protection against Yersinia-triggered apoptosis. Thus, the reprogramming of macrophages during the infectious process, resulting in endotoxin tolerance, could help the compromised host overcome Yersinia-mediated apoptosis and infection. This gives a clue about how adaptation of host cells to coexisting bacteria could decrease responsiveness of the host to a bacterial virulence mechanism.
Acknowledgments
We thank Gudrun Pfaffinger for expert technical assistance and Jürgen Heesemann, Martin Aepfelbacher, and Bruno Rouot for constructive discussions. Furthermore, we thank Michael Hensel for providing us with Salmonella strains.
This work was supported by grants from the Deutsche Forschungsgemeinschaft (grant DFG Ru788/1 and -2).
Editor: B. B. Finlay
REFERENCES
- 1.Aderem, A., and R. J. Ulevitch. 2000. Toll-like receptors in the induction of the innate immune response. Nature 406:782-787. [DOI] [PubMed] [Google Scholar]
- 2.Aepfelbacher, M., R. Zumbihl, K. Ruckdeschel, C. A. Jacobi, C. Barz, and J. Heesemann. 1999. The tranquilizing injection of Yersinia proteins: a pathogen's strategy to resist host defense. Biol. Chem. 380:795-802. [DOI] [PubMed] [Google Scholar]
- 3.Aliprantis, A. O., R. B. Yang, D. S. Weiss, P. Godowski, and A. Zychlinsky. 2000. The apoptotic signaling pathway activated by Toll-like receptor-2. EMBO J. 19:3325-3336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bajaj, V., C. Hwang, and C. A. Lee. 1995. hilA is a novel ompR/toxR family member that activates the expression of Salmonella typhimurium invasion genes. Mol. Microbiol. 18:715-727. [DOI] [PubMed] [Google Scholar]
- 5.Bannerman, D. D., J. C. Tupper, R. D. Erwert, R. K. Winn, and J. M. Harlan. 2002. Divergence of bacterial lipopolysaccharide pro-apoptotic signaling downstream of IRAK-1. J. Biol. Chem. 277:8048-8053. [DOI] [PubMed] [Google Scholar]
- 6.Bliska, J. B. 2000. Yop effectors of Yersinia spp. and actin rearrangements. Trends Microbiol. 8:205-208. [DOI] [PubMed] [Google Scholar]
- 7.Carter, P. B., C. F. Varga, and E. E. Keet. 1973. New strain of Yersinia enterocolitica pathogenic for rodents. Appl. Microbiol. 26:1016-1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cavaillon, J. M. 1995. The nonspecific nature of endotoxin tolerance. Trends Microbiol. 3:320-324. [DOI] [PubMed] [Google Scholar]
- 9.Cornelis, G. R., A. Boland, A. P. Boyd, C. Geuijen, M. Iriarte, C. Neyt, M.-P. Sory, and I. Stainier. 1998. The virulence plasmid of Yersinia, an antihost genome. Microbiol. Mol. Biol. Rev. 62:1315-1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deiwick, J., T. Nikolaus, J. E. Shea, C. Gleeson, D. W. Holden, and M. Hensel. 1998. Mutations in Salmonella pathogenicity island 2 (SPI2) genes affecting transcription of SPI1 genes and resistance to antimicrobial agents. J. Bacteriol. 180:4775-4780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ferlito, M., O. G. Romanenko, S. Ashton, F. Squadrito, P. V. Halushka, and C. A. Cook. 2001. Effect of cross-tolerance between endotoxin and TNF-α or IL-1β on cellular signaling and mediator production. J. Leukoc. Biol. 70:821-829. [PubMed] [Google Scholar]
- 12.Guha, M., and N. Mackman. 2001. LPS induction of gene expression in human monocytes. Cell. Signal. 13:85-94. [DOI] [PubMed] [Google Scholar]
- 13.Heesemann, J., and R. Laufs. 1983. Construction of a mobilizable Yersinia enterocolitica virulence plasmid. J. Bacteriol. 155:761-767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heesemann, J., and R. Laufs. 1985. Double immunofluorescence microscopic technique for accurate differentiation of extracellularly and intracellularly located bacteria in cell culture. J. Clin. Microbiol. 22:168-175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hersh, D., D. M. Monack, M. R. Smith, N. Ghori, S. Falkow, and A. Zychlinsky. 1999. The Salmonella invasin SipB induces macrophage apoptosis by binding to caspase-1. Proc. Natl. Acad. Sci. USA 96:2396-2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Karin, M., and A. Lin. 2002. NF-κB at the crossroads of life and death. Nat. Immunol. 3:221-227. [DOI] [PubMed] [Google Scholar]
- 17.Kitamura, M. 1999. NF-κB-mediated self defense of macrophages faced with bacteria. Eur. J. Immunol. 29:1647-1655. [DOI] [PubMed] [Google Scholar]
- 18.Lehner, M. D., and T. Hartung. 2002. Endotoxin tolerance—mechanisms and beneficial effects in bacterial infection. Rev. Physiol. Biochem. Pharmacol. 144:95-141. [DOI] [PubMed] [Google Scholar]
- 19.Lehner, M. D., S. Morath, K. S. Michelsen, R. R. Schumann, and T. Hartung. 2001. Induction of cross-tolerance by lipopolysaccharide and highly purified lipoteichoic acid via different Toll-like receptors independent of paracrine mediators. J. Immunol. 166:5161-5167. [DOI] [PubMed] [Google Scholar]
- 20.Li, L., S. Cousart, J. Hu, and C. E. McCall. 2000. Characterization of interleukin-1 receptor-associated kinase in normal and endotoxin-tolerant cells. J. Biol. Chem. 275:23340-23345. [DOI] [PubMed] [Google Scholar]
- 21.Lundberg, U., U. Vinatzer, D. Berdnik, A. von Gabain, and M. Baccarini. 1999. Growth phase-regulated induction of Salmonella-induced macrophage apoptosis correlates with transient expression of SPI-1 genes. J. Bacteriol. 181:3433-3437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Medvedev, A. E., K. M. Kopydlowski, and S. N. Vogel. 2000. Inhibition of lipopolysaccharide-induced signal transduction in endotoxin-tolerized mouse macrophages: dysregulation of cytokine, chemokine, and toll-like receptor 2 and 4 gene expression. J. Immunol. 164:5564-5574. [DOI] [PubMed] [Google Scholar]
- 23.Nomura, F., S. Akashi, Y. Sakao, S. Sato, T. Kawai, M. Matsumoto, K. Nakanishi, M. Kimoto, K. Miyake, K. Takeda, and S. Akira. 2000. Cutting edge: endotoxin tolerance in mouse peritoneal macrophages correlates with down-regulation of surface toll-like receptor 4 expression. J. Immunol. 164:3476-3479. [DOI] [PubMed] [Google Scholar]
- 24.Orth, K. 2002. Function of the Yersinia effector YopJ. Curr. Opin. Microbiol. 5:38-43. [DOI] [PubMed] [Google Scholar]
- 25.Orth, K., L. E. Palmer, Z. Q. Bao, S. Stewart, A. E. Rudolph, J. B. Bliska, and J. E. Dixon. 1999. Inhibition of the mitogen-activated protein kinase kinase superfamily by a Yersinia effector. Science 285:1920-1923. [DOI] [PubMed] [Google Scholar]
- 26.Roggenkamp, A., H. R. Neuberger, A. Flügel, T. Schmoll, and J. Heesemann. 1995. Substitution of two histidine residues in YadA protein of Yersinia enterocolitica abrogates collagen binding, cell adherence and mouse virulence. Mol. Microbiol. 16:1207-1219. [DOI] [PubMed] [Google Scholar]
- 27.Ruckdeschel, K., S. Harb, A. Roggenkamp, M. Hornef, R. Zumbihl, S. Köhler, J. Heesemann, and B. Rouot. 1998. Yersinia enterocolitica impairs activation of transcription factor NF-κB: involvement in the induction of programmed cell death and in the suppression of the macrophage TNFα production. J. Exp. Med. 187:1069-1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ruckdeschel, K., O. Mannel, K. Richter, C. A. Jacobi, K. Trülzsch, B. Rouot, and J. Heesemann. 2001. Yersinia outer protein P of Yersinia enterocolitica simultaneously blocks the nuclear factor-kappaB pathway and exploits lipopolysaccharide signaling to trigger apoptosis in macrophages. J. Immunol. 166:1823-1831. [DOI] [PubMed] [Google Scholar]
- 29.Ruckdeschel, K., A. Roggenkamp, V. Lafont, P. Mangeat, J. Heesemann, and B. Rouot. 1997. Interaction of Yersinia enterocolitica with macrophages leads to macrophage cell death through apoptosis. Infect. Immun. 65:4813-4821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sato, S., F. Nomura, T. Kawai, O. Takeuchi, P. F. Mühlradt, K. Takeda, and S. Akira. 2000. Synergy and cross-tolerance between toll-like receptor (TLR) 2- and TLR4-mediated signaling pathways. J. Immunol. 165:7096-7101. [DOI] [PubMed] [Google Scholar]