Abstract
1. The effect of ouabain on the noradrenaline output from peripheral adrenergic neurones has been studied using isolated guinea-pig vasa deferentia.
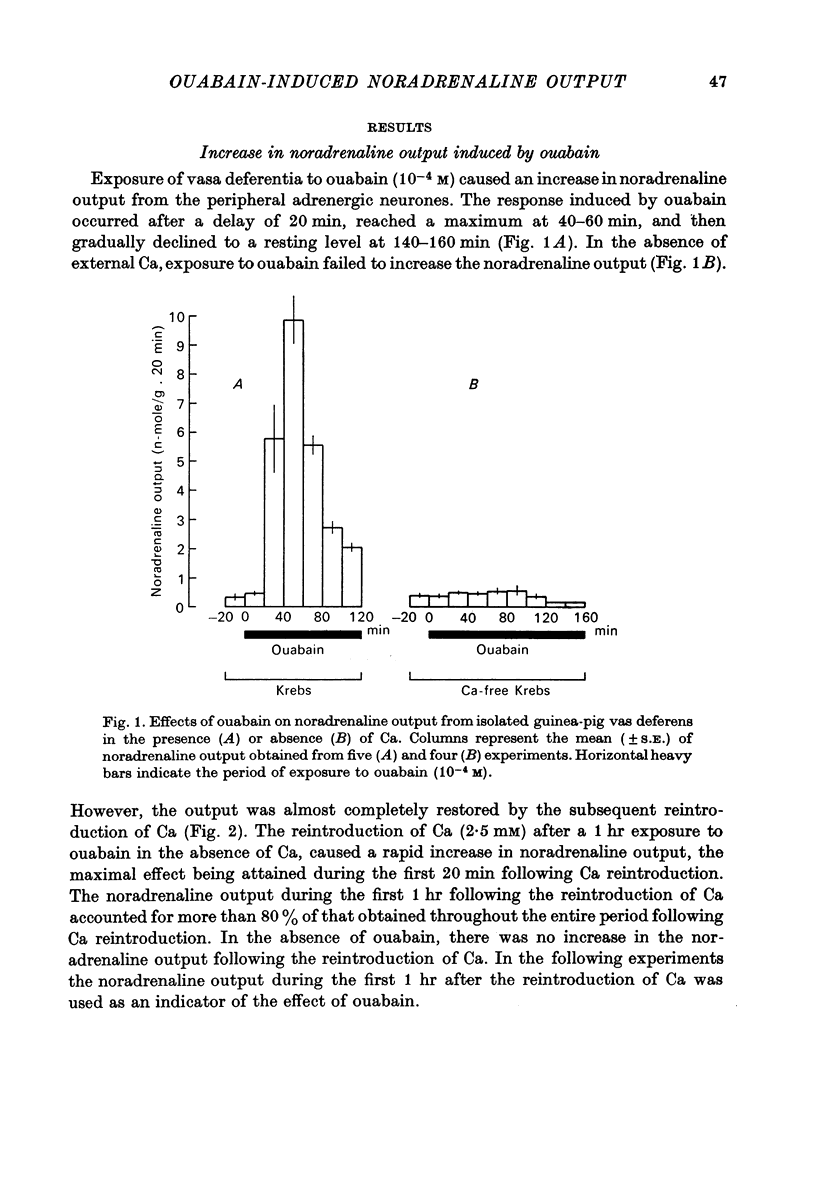
2. Exposure to ouabain (10-4 M) causes a gradual increase in the noradrenaline output. The effect occurs after a delay of 20 min and reaches a maximum during the period from 40-60 min.
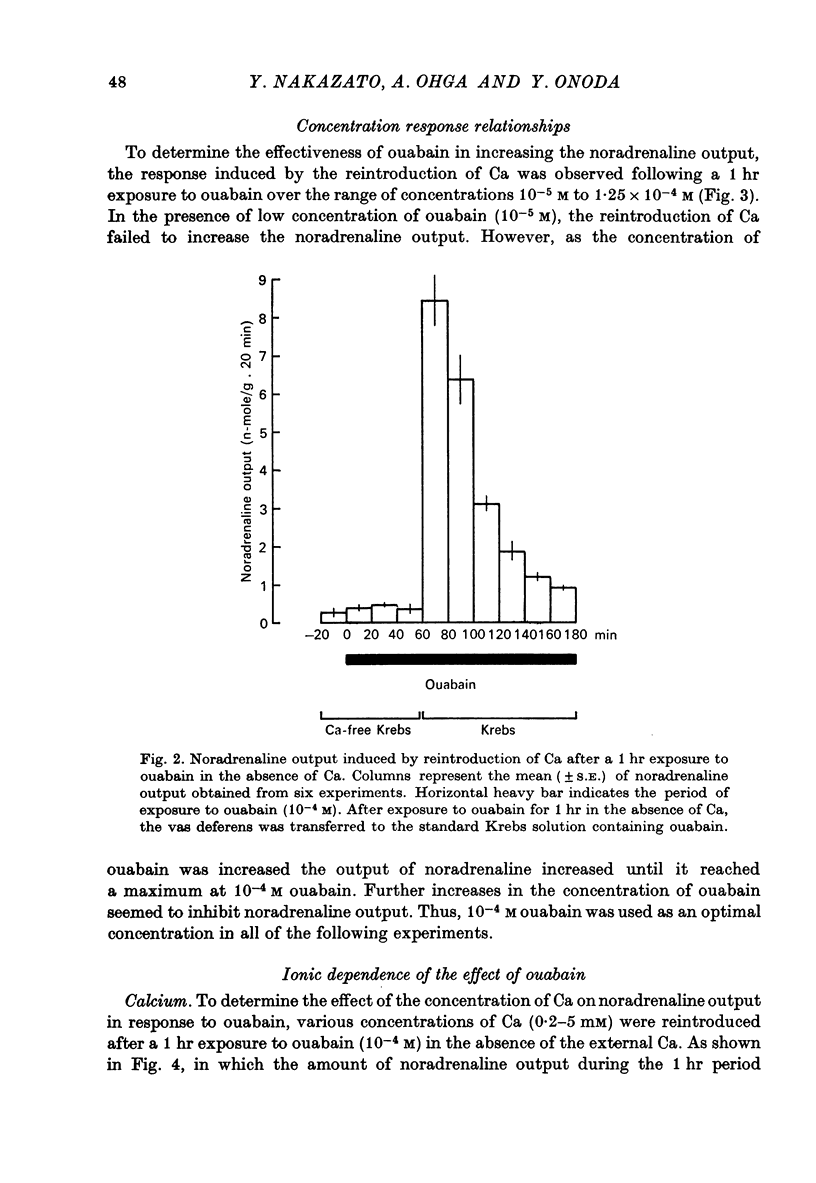
3. In the absence of external Ca, exposure to ouabain fails to produce an increase in the noradrenaline output. However, the reintroduction of Ca (2·5 mM) after a 1 hr exposure to ouabain in Ca-free media causes a rapid rise in noradrenaline output which reaches a maximum within the first 20 min.
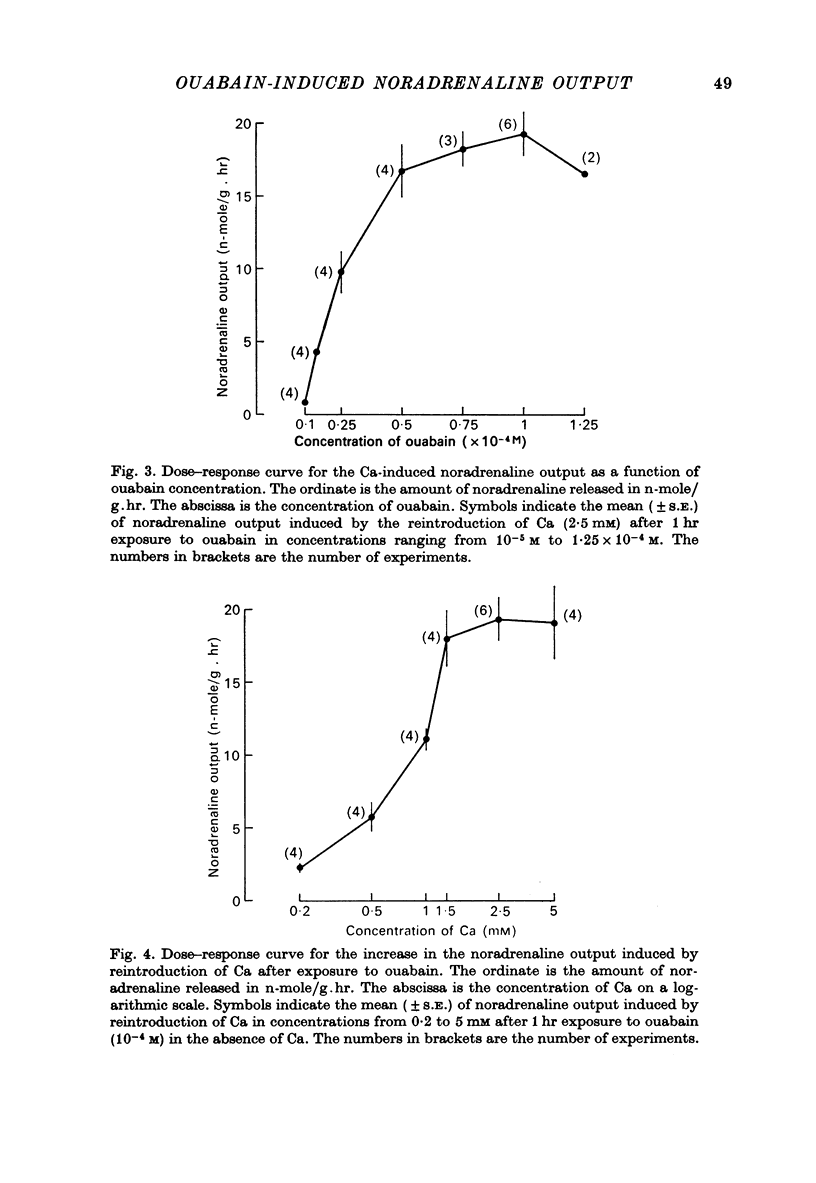
4. After a 1 hr exposure to a low concentration of ouabain (10-5 M) the reintroduction of Ca is almost ineffective in increasing the noradrenaline output. When the concentration of ouabain is increased, the reintroduction of Ca becomes effective and causes a maximum effect with 10-4 M ouabain. In the presence of a constant amount of ouabain (10-4 M) the noradrenaline output induced by the reintroduction of Ca increases over the range 0·2-2·5 mM.
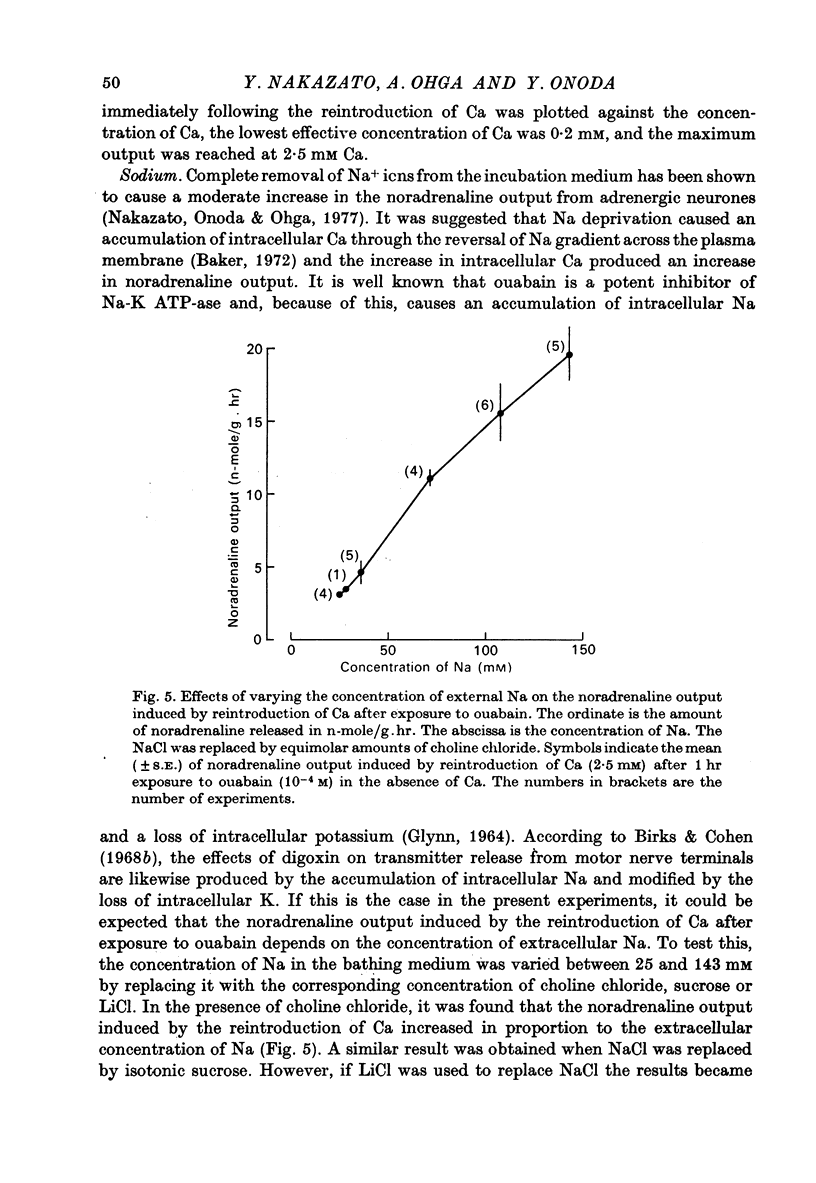
5. In the presence of ouabain (10-4 M) the Ca-induced noradrenaline output increases in a linear fashion with increasing Na concentrations from 25 to 143 mM, as long as NaCl is replaced with equimolar choline chloride or isotonic sucrose.
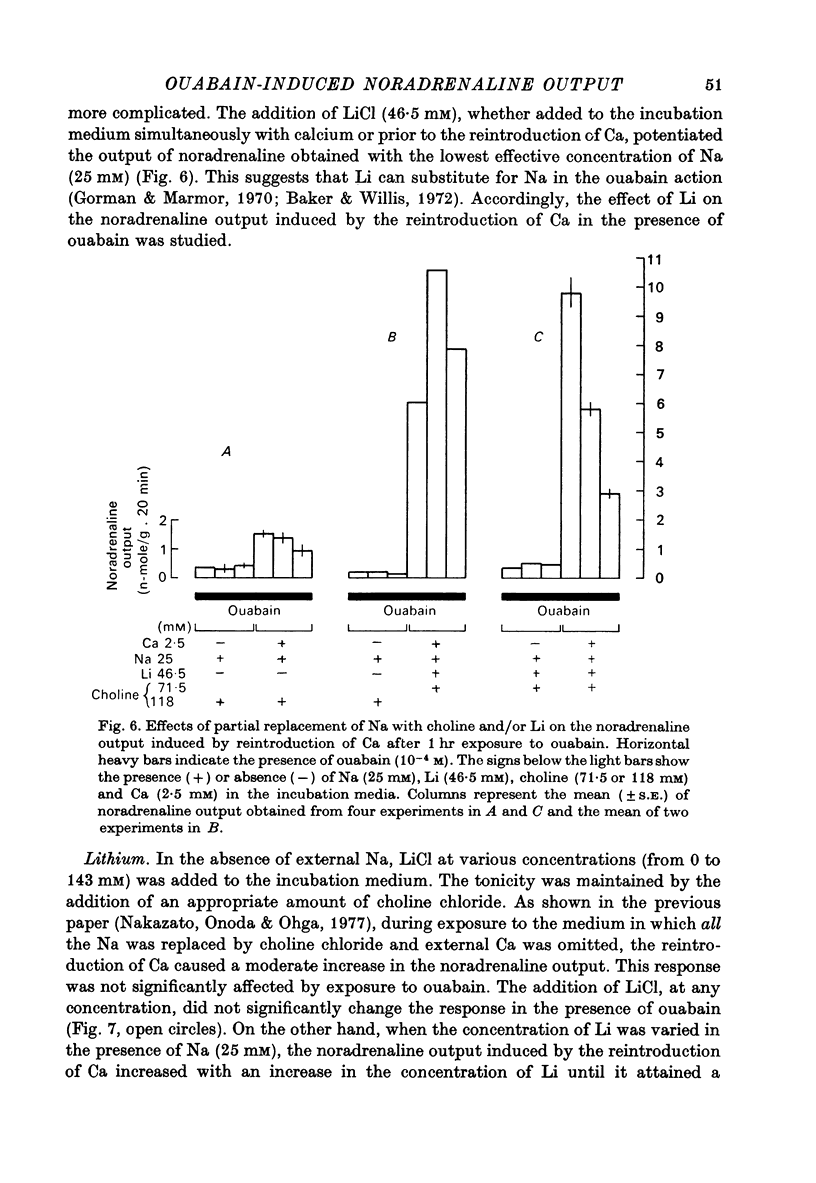
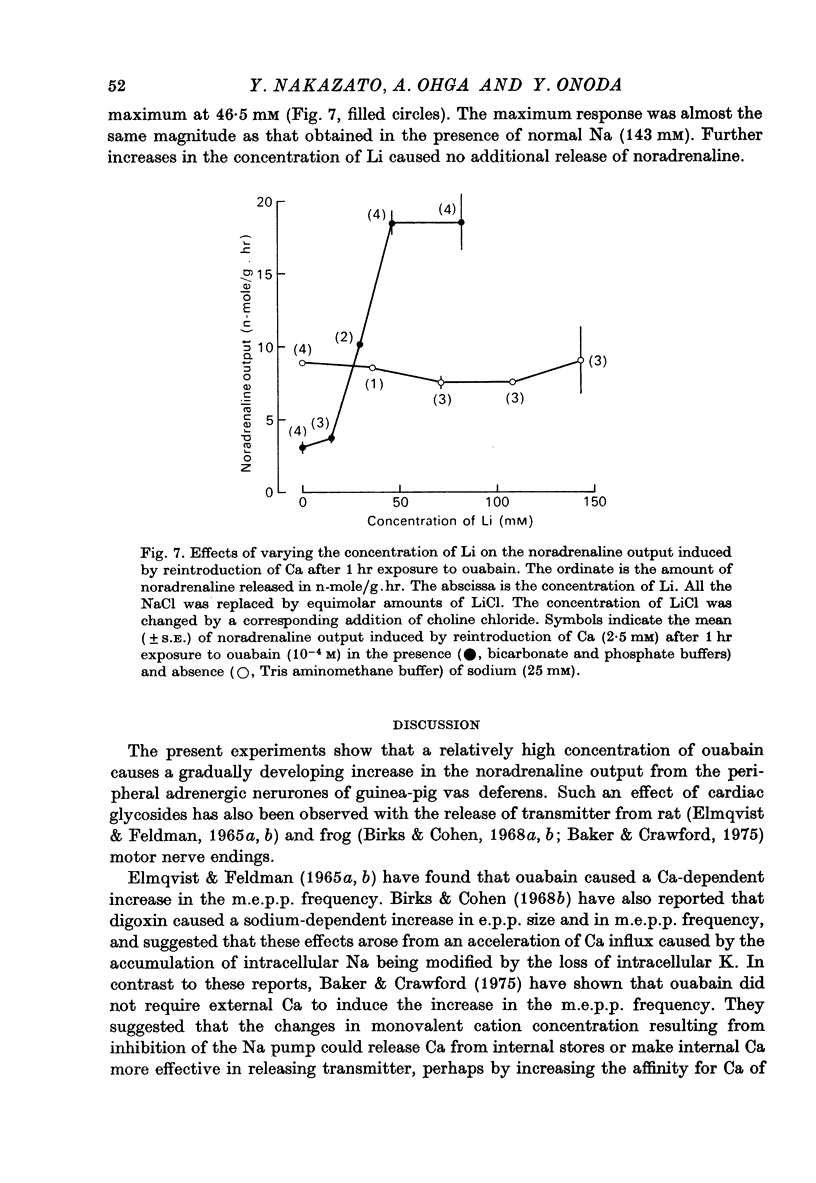
6. In the presence of the lowest effective concentration of sodium (25 mM) the noradrenaline output induced by the reintroduction of Ca after a 1 hr exposure to ouabain is potentiated by LiCl. However, in the complete absence of Na+ ions, there is no Li-dependent increase in the Ca-induced noradrenaline output.
7. It is suggested that ouabain may cause an increase in noradrenaline output by an effect on the Na-dependent Ca influx system.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANTON A. H., SAYRE D. F. A study of the factors affecting the aluminum oxide-trihydroxyindole procedure for the analysis of catecholamines. J Pharmacol Exp Ther. 1962 Dec;138:360–375. [PubMed] [Google Scholar]
- Baker P. F., Crawford A. C. A note of the mechanism by which inhibitors of the sodium pump accelerate spontaneous release of transmitter from motor nerve terminals. J Physiol. 1975 May;247(1):209–226. doi: 10.1113/jphysiol.1975.sp010928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker P. F. Transport and metabolism of calcium ions in nerve. Prog Biophys Mol Biol. 1972;24:177–223. doi: 10.1016/0079-6107(72)90007-7. [DOI] [PubMed] [Google Scholar]
- Baker P. F., Willis J. S. Inhibition of the sodium pump in squid giant axons by cardiac glycosides: dependence on extracellular ions and metabolism. J Physiol. 1972 Jul;224(2):463–475. doi: 10.1113/jphysiol.1972.sp009905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks P. The effect of ouabain on the secretion of catecholamines and on the intracellular concentration of potassium. J Physiol. 1967 Dec;193(3):631–637. doi: 10.1113/jphysiol.1967.sp008383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birks R. I., Cohen M. W. The action of sodium pump inhibitors on neuromuscular transmission. Proc R Soc Lond B Biol Sci. 1968 Jul 9;170(1021):381–399. doi: 10.1098/rspb.1968.0046. [DOI] [PubMed] [Google Scholar]
- Birks R. I., Cohen M. W. The influence of internal sodium on the behaviour of motor nerve endings. Proc R Soc Lond B Biol Sci. 1968 Jul 9;170(1021):401–421. doi: 10.1098/rspb.1968.0047. [DOI] [PubMed] [Google Scholar]
- Elmqvist D., Feldman D. S. Calcium dependence of spontaneous acetylcholine release at mammalian motor nerve terminals. J Physiol. 1965 Dec;181(3):487–497. doi: 10.1113/jphysiol.1965.sp007777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmqvist D., Feldman D. S. Effects of sodium pump inhibitors on spontaneous acetylcholine release at the neuromuscular junction. J Physiol. 1965 Dec;181(3):498–505. doi: 10.1113/jphysiol.1965.sp007778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRANKENHAEUSER B., HODGKIN A. L. The action of calcium on the electrical properties of squid axons. J Physiol. 1957 Jul 11;137(2):218–244. doi: 10.1113/jphysiol.1957.sp005808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GLYNN I. M. THE ACTION OF CARDIAC GLYCOSIDES ON ION MOVEMENTS. Pharmacol Rev. 1964 Dec;16:381–407. [PubMed] [Google Scholar]
- Gorman A. L., Marmor M. F. Temperature dependence of the sodium-potassium permeability ratio of a molluscan neurone. J Physiol. 1970 Nov;210(4):919–931. doi: 10.1113/jphysiol.1970.sp009249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., KATZ B. The effect of sodium ions on the electrical activity of giant axon of the squid. J Physiol. 1949 Mar 1;108(1):37–77. doi: 10.1113/jphysiol.1949.sp004310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUXLEY A. F., STAMPFLI R. Effect of potassium and sodium on resting and action potentials of single myelinated nerve fibers. J Physiol. 1951 Feb;112(3-4):496–508. doi: 10.1113/jphysiol.1951.sp004546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakazato Y., Onoda Y., Ohga A. Role of calcium in the release of noradrenaline induced by sodium deprivation from the guinea-pig vas deferens. Pflugers Arch. 1977 Nov 25;372(1):63–67. doi: 10.1007/BF00582207. [DOI] [PubMed] [Google Scholar]
- Paton W. D., Vizi E. S., Zar M. A. The mechanism of acetylcholine release from parasympathetic nerves. J Physiol. 1971 Jul;215(3):819–848. doi: 10.1113/jphysiol.1971.sp009500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vizi E. S. Stimulation, by inhibition of (Na + -K + -Mg 2+ )-activated ATP-ase, of acetylcholine release in cortical slices from rat brain. J Physiol. 1972 Oct;226(1):95–117. doi: 10.1113/jphysiol.1972.sp009975. [DOI] [PMC free article] [PubMed] [Google Scholar]


