Abstract
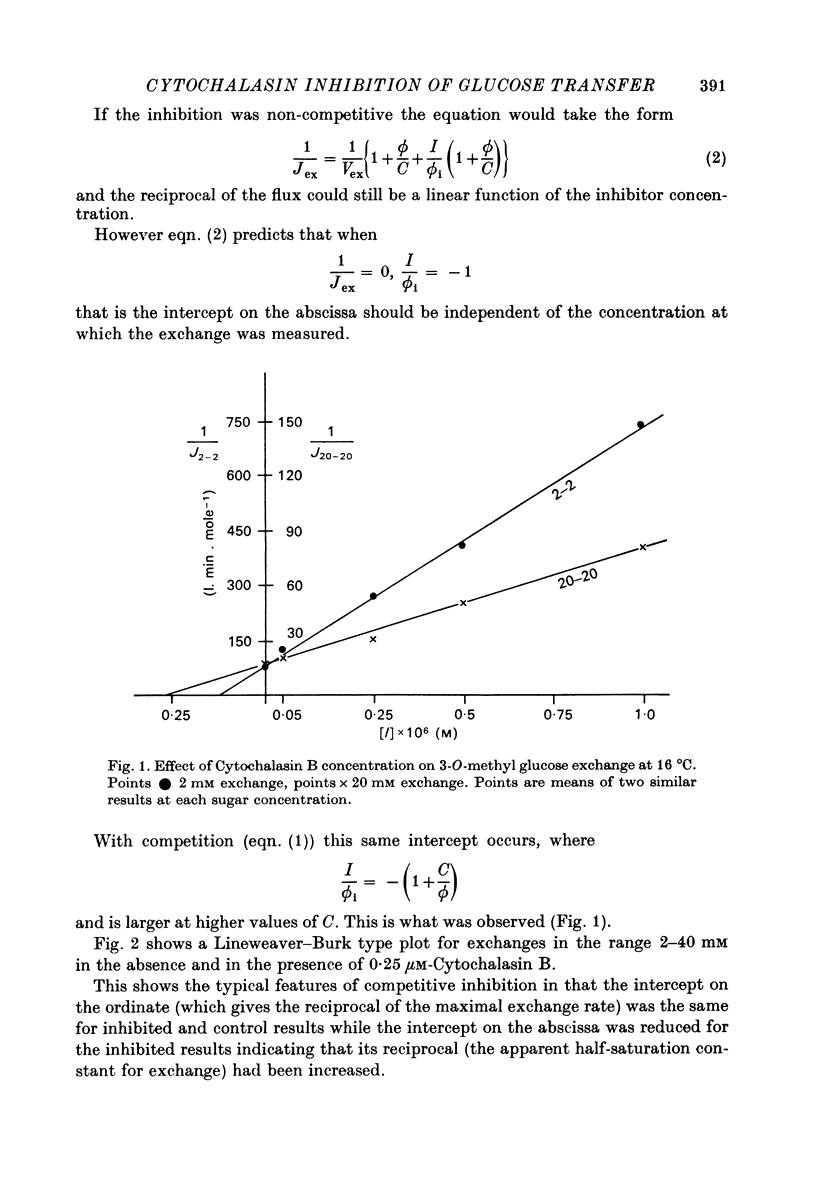
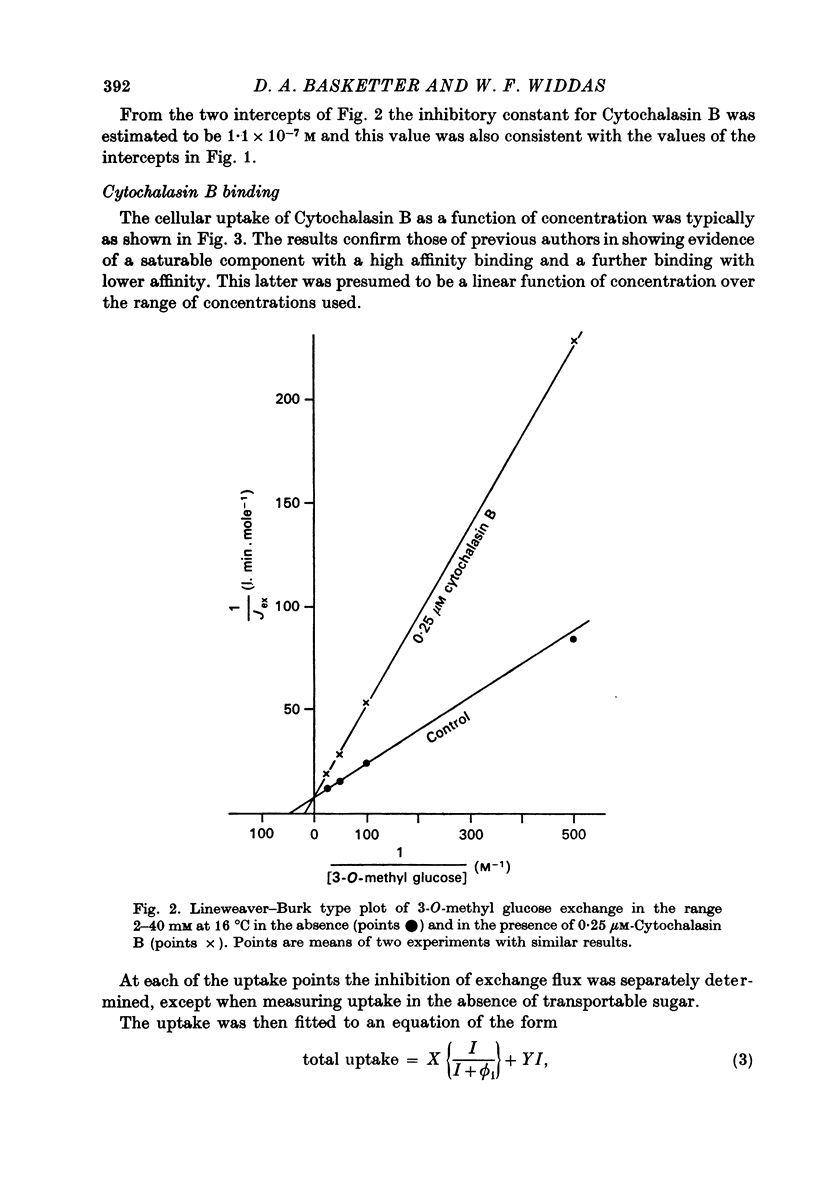
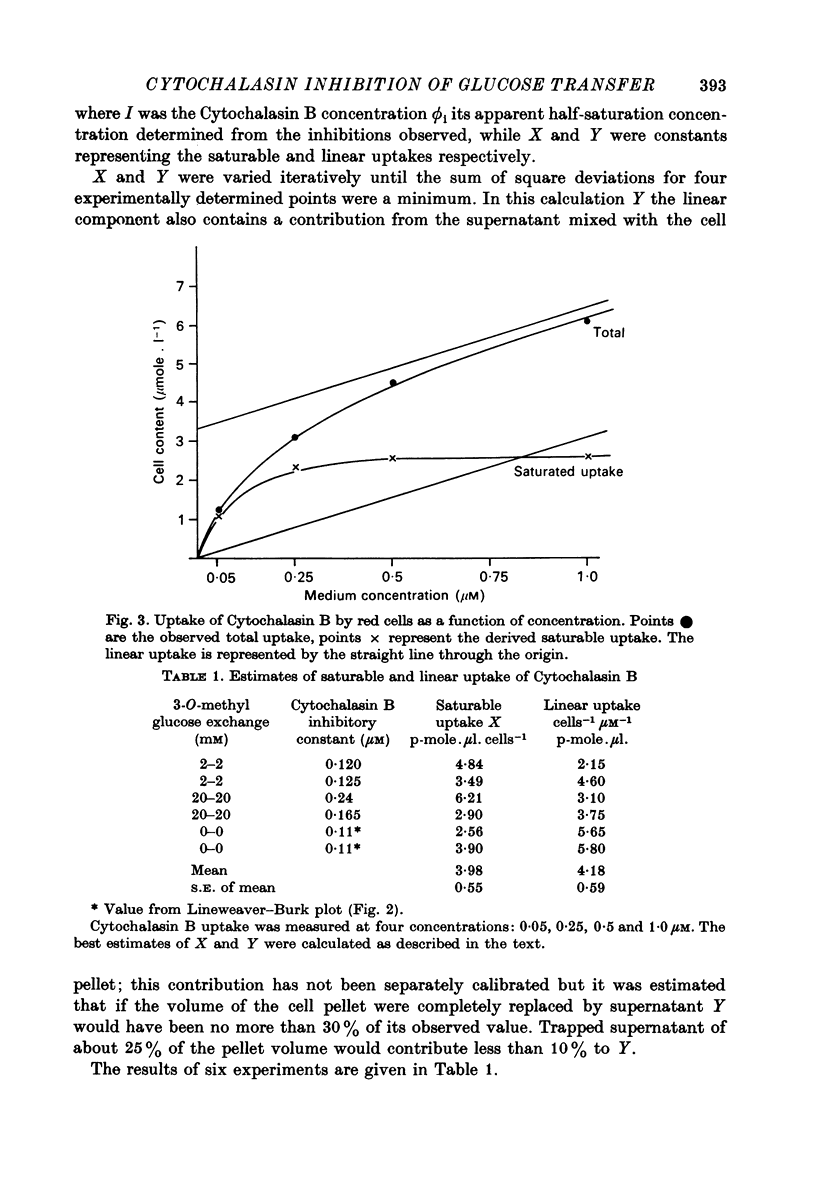
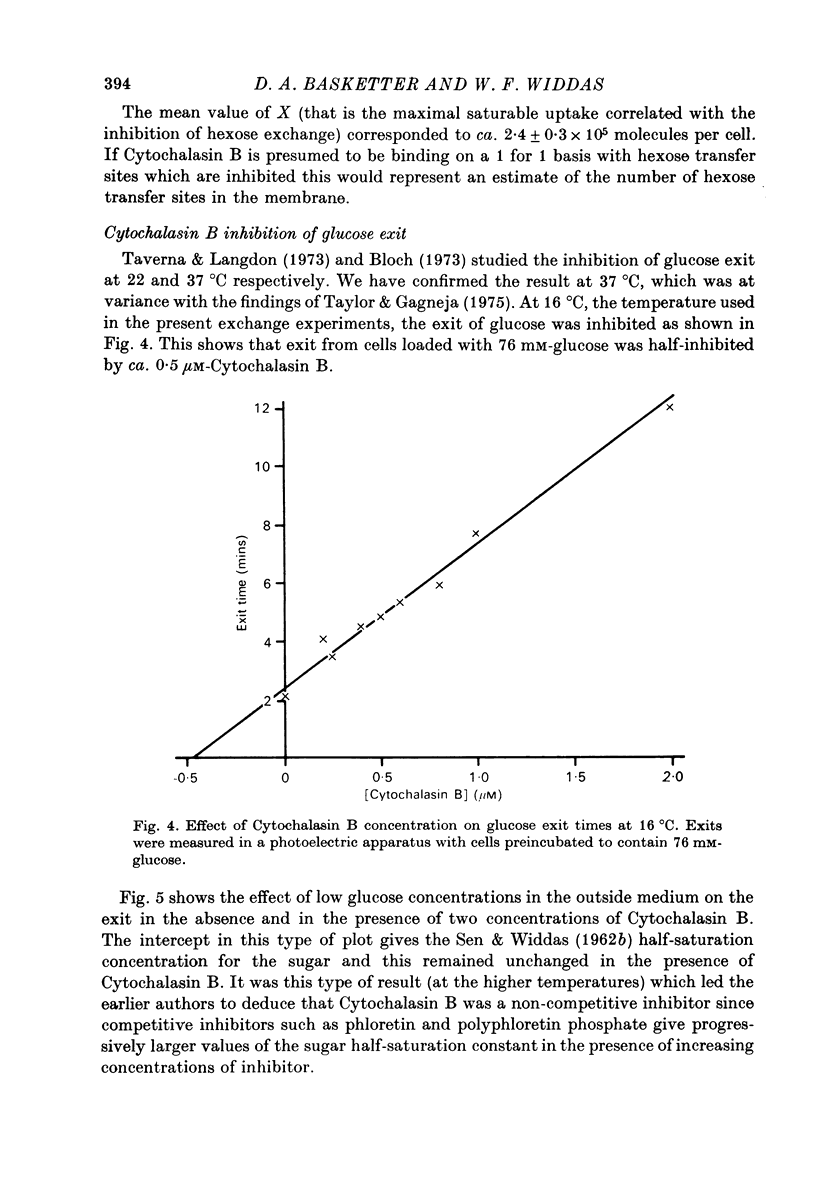
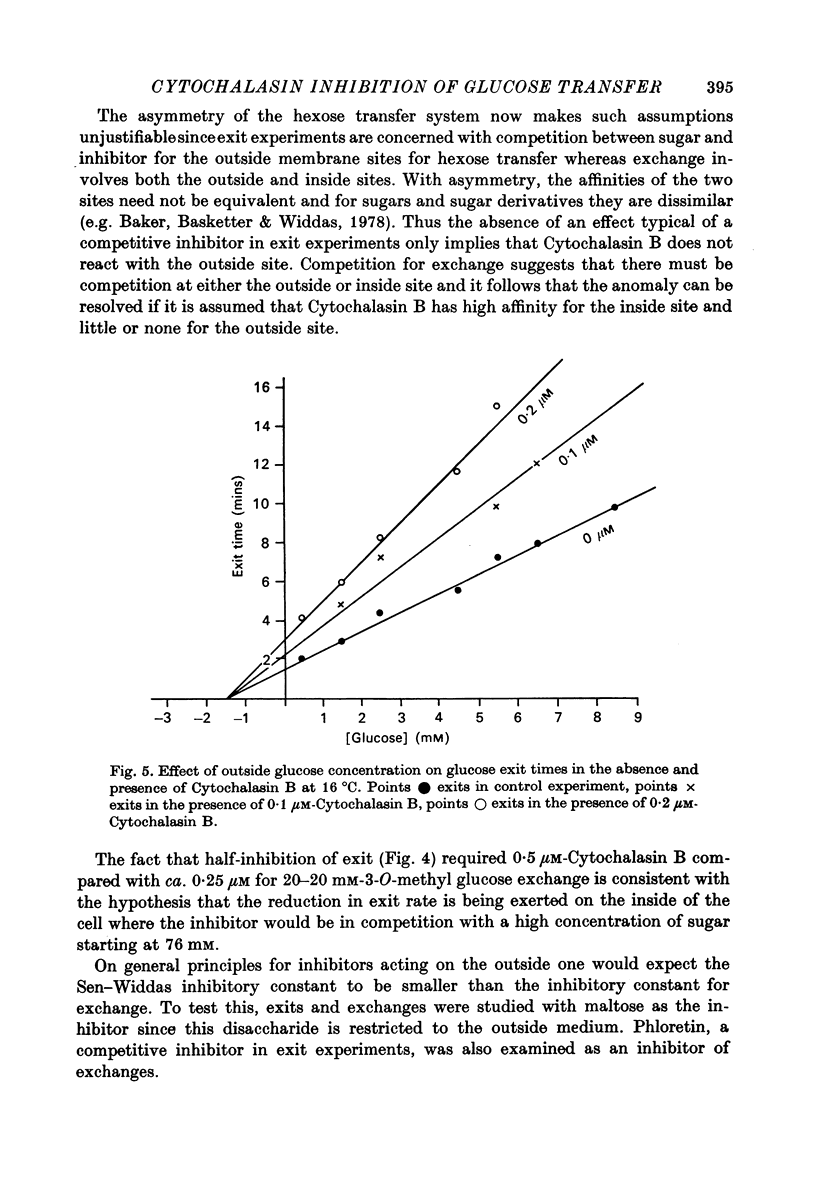
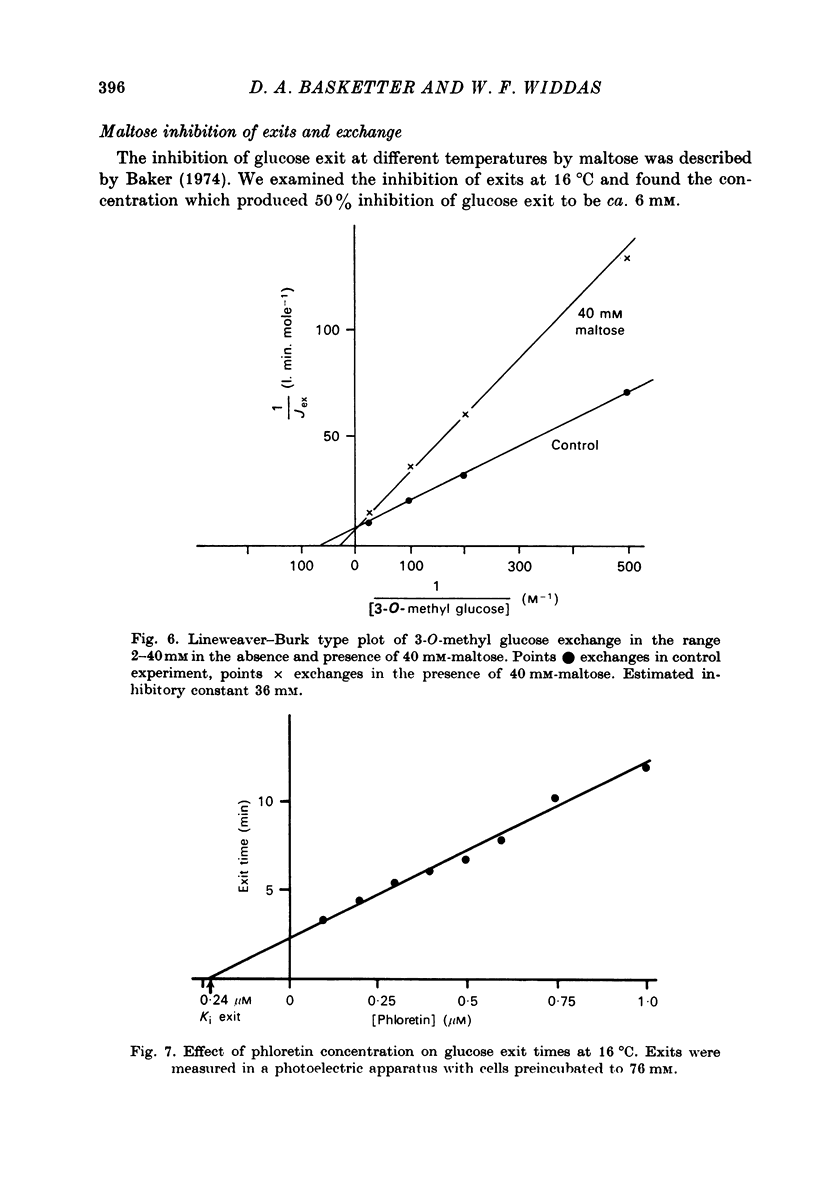
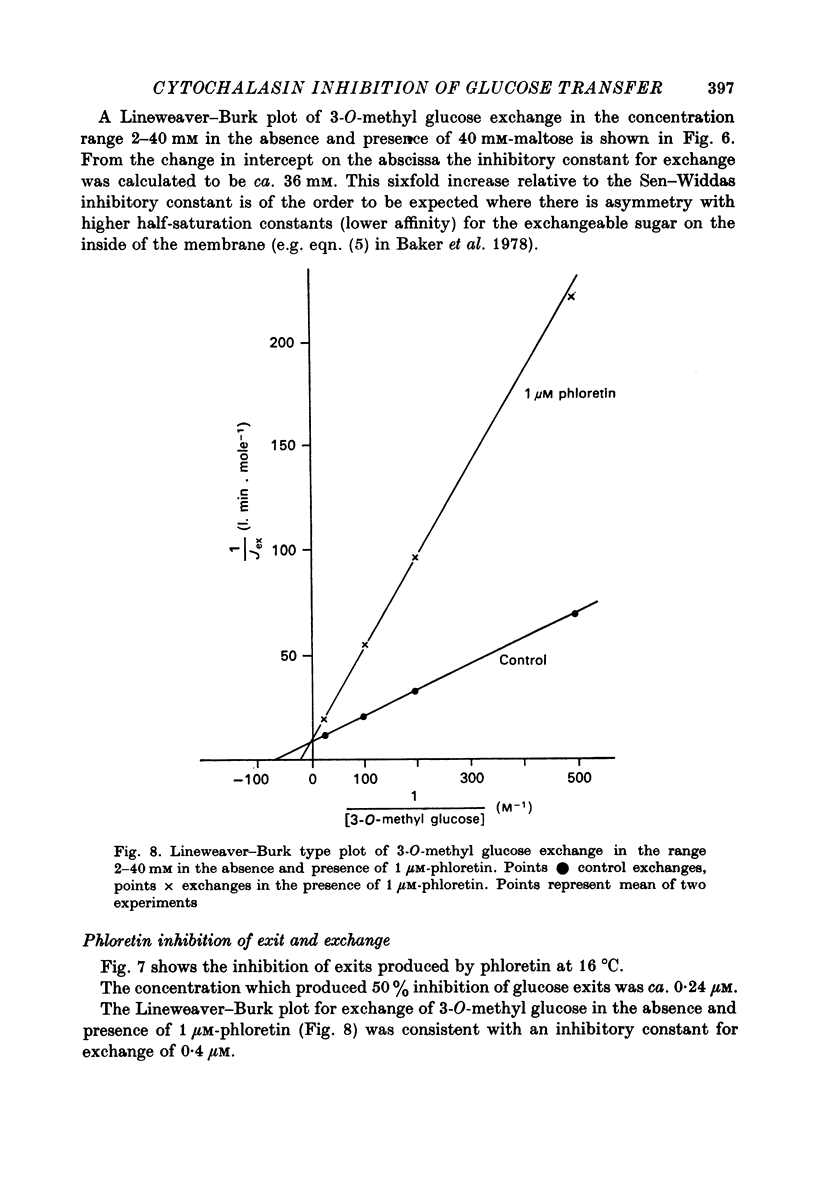
1. Cytochalasin B inhibits glucose transfer in human red cells. With glucose exit the inhibition is typically non-competitive, but hexose exchange is competitively inhibited. 2. At 16 degrees C the inhibitory constant for inhibition of 3-O-methyl glucose exchange is estimated at 1.1 X 10(-7) M while that for inhibition of glucose exit is 5.0 X 10(-7) M. 3. Uptake of labelled Cytochalasin B includes a saturable component which when correlated with the inhibition of exchange corresponds to a maximal binding of ca. 2.4 X 10(5) molecules per cell. 4. The kinetic parameters are compared with those for maltose (a competitive inhibitor acting on the outside only) and phloretin (an inhibitor acting both inside and out). 5. Kinetic evidence suggests that Cytochalasin B reacts with the inside of the hexose transfer system and that the anomalous inhibitory characteristics are due to the chemical asymmetry of the system. Independent evidence in support of this view is discussed.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker G. F., Basketter D. A., Widdas W. F. Asymmetry of the hexose transfer system in human erythrocytes. Experiments with non-transportable inhibitors. J Physiol. 1978 May;278:377–388. doi: 10.1113/jphysiol.1978.sp012310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker G. F., Widdas W. F. The asymmetry of the facilitated transfer system for hexoses in human red cells and the simple kinetics of a two component model. J Physiol. 1973 May;231(1):143–165. doi: 10.1113/jphysiol.1973.sp010225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basketter D. A., Widdas W. F. Competitive inhibition of hexose transfer in human erythrocytes by Cytochalasin B [proceedings]. J Physiol. 1977 Feb;265(1):39P–40P. [PubMed] [Google Scholar]
- Bloch R. Inhibition of glucose transport in the human erythrocyte by cytochalasin B. Biochemistry. 1973 Nov 6;12(23):4799–4801. doi: 10.1021/bi00747a036. [DOI] [PubMed] [Google Scholar]
- Geck P. Eigenschaften eines asymmetrischen Carrier-Modells für den Zuckertrnasport am menschlichen Erythrozyten. Biochim Biophys Acta. 1971 Aug 13;241(2):462–472. doi: 10.1016/0005-2736(71)90045-9. [DOI] [PubMed] [Google Scholar]
- Jennings M. L., Solomon A. K. Interaction between phloretin and the red blood cell membrane. J Gen Physiol. 1976 Apr;67(4):381–397. doi: 10.1085/jgp.67.4.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahlenberg A., Urman B., Dolansky D. Preferential uptake of D-glucose by isolated human erythrocyte membranes. Biochemistry. 1971 Aug 3;10(16):3154–3162. doi: 10.1021/bi00792a027. [DOI] [PubMed] [Google Scholar]
- Lin S., Spudich J. A. Biochemical studies on the mode of action of cytochalasin B. Cytochalasin B binding to red cell membrane in relation to glucose transport. J Biol Chem. 1974 Sep 25;249(18):5778–5783. [PubMed] [Google Scholar]
- Masiak S. J., LeFevre P. G. Failure of equilibrium dialysis to show selective monosaccharide binding by erythrocyte membranes. J Membr Biol. 1972;9(3):291–296. [PubMed] [Google Scholar]
- Masiak S. J., LeFevre P. G. Glucose transport inhibition by proteolytic degradation of the human erythrocyte membrane inner surface. Biochim Biophys Acta. 1977 Mar 1;465(2):371–377. doi: 10.1016/0005-2736(77)90086-4. [DOI] [PubMed] [Google Scholar]
- Regen D. M., Tarpley H. L. Anomalous transport kinetics and the glucose carrier hypothesis. Biochim Biophys Acta. 1974 Mar 15;339(2):218–233. doi: 10.1016/0005-2736(74)90320-4. [DOI] [PubMed] [Google Scholar]
- SEN A. K., WIDDAS W. F. Determination of the temperature and pH dependence of glucose transfer across the human erythrocyte membrane measured by glucose exit. J Physiol. 1962 Mar;160:392–403. doi: 10.1113/jphysiol.1962.sp006854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SEN A. K., WIDDAS W. F. Variations of the parameters of glucose transfer across the human erythrocyte membrane in the presence of inhibitors of transfer. J Physiol. 1962 Mar;160:404–416. doi: 10.1113/jphysiol.1962.sp006855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein W. D. The transport of sugars. Br Med Bull. 1968 May;24(2):146–149. doi: 10.1093/oxfordjournals.bmb.a070617. [DOI] [PubMed] [Google Scholar]
- Taverna R. D., Langdon R. G. Reversible association of cytochalasin B with the human erythrocyte membrane. Inhibition of glucose transport and the stoichiometry of cytochalasin binding. Biochim Biophys Acta. 1973 Oct 11;323(2):207–219. doi: 10.1016/0005-2736(73)90145-4. [DOI] [PubMed] [Google Scholar]
- Taylor N. F., Gagneja G. L. A model for the mode of action of cytochalasin B inhibition of D-glucose transport in the human erythrocyte. Can J Biochem. 1975 Oct;53(10):1078–1084. doi: 10.1139/o75-148. [DOI] [PubMed] [Google Scholar]
- VANSTEVENINCK J., WEED R. I., ROTHSTEIN A. LOCALIZATION OF ERYTHROCYTE MEMBRANE SULFHYDRYL GROUPS ESSENTIAL FOR GLUCOSE TRANSPORT. J Gen Physiol. 1965 Mar;48:617–632. doi: 10.1085/jgp.48.4.617. [DOI] [PMC free article] [PubMed] [Google Scholar]


