Abstract
Streptococcus pneumoniae is a gram-positive bacterial pathogen that causes invasive life-threatening disease worldwide. This organism also commonly colonizes the upper respiratory epithelium in an asymptomatic fashion. To invade, this pathogen must traverse the respiratory epithelial barrier, allowing it to cause disease locally or disseminate hematogenously throughout the body. Previous work has demonstrated that S. pneumoniae choline-binding protein A, a pneumococcal surface protein, interacts specifically with the human polymeric immunoglobulin receptor, which is expressed by cells in the respiratory epithelium. Choline-binding protein A is required for efficient colonization of the nasopharynx in vivo. Additionally, a recent study showed that the R6x laboratory strain of S. pneumoniae invades a human pharyngeal cell line in a human polymeric immunoglobulin receptor-dependent manner. These findings raised the possibility that the interaction between choline-binding protein A and human polymeric immunoglobulin receptor may be a key determinant of S. pneumoniae pathogenesis. However, the strain used in prior invasion studies, R6x, is an unencapsulated, nonpathogenic strain. In the present study we determined the relative ability of strain R6x or pathogenic strains to invade a variety of human polymeric immunoglobulin receptor-expressing epithelial cell lines. The results of this work suggest that human polymeric immunoglobulin receptor-dependent enhanced invasion of epithelial cells by S. pneumoniae is a limited phenomenon that occurs in a strain-specific and cell type-specific manner.
The gram-positive bacterium Streptococcus pneumoniae is one of the most prevalent invasive human pathogens worldwide, causing such diseases as sepsis, pneumonia, and meningitis (18). Children under 5 years of age, the elderly, and immunodeficient individuals are particularly susceptible to invasive pneumococcal infection (10). S. pneumoniae is often found as a component of the flora of the nasopharynx, with up to four different serotypes simultaneously colonizing the same asymptomatic individual (1). Invasive infection results when the colonizing pneumococci gain access to the areas underlying the respiratory epithelium and disseminate (7). Thus, a better understanding of the pathophysiology of pneumococcal disease requires determination of the cellular and molecular basis for epithelial cell invasion. The fundamental events that mediate the process of invasion are poorly understood.
S. pneumoniae possesses a variety of virulence factors thought to contribute to its pathogenesis. These factors include some of the surface choline-binding proteins in the choline-rich cell wall, such as pneumococcal surface protein A, autolysin, and choline-binding protein A (CbpA) (3, 10, 17). More than 73% of S. pneumoniae isolates express CbpA, which has a predicted molecular mass of approximately 75 kDa (with some variation between isolates) (8, 20, 25). The protein exhibits a conserved C-terminal choline-binding domain and a unique N-terminal domain that is thought to mediate host cell attachment (8, 9). CbpA has been linked to colonization of the nasopharynx in rats, as a CbpA-deficient strain was 100-fold less efficient at colonizing the nasopharynx than was wild-type S. pneumoniae (20). Most recently, CbpA has been proposed to mediate invasion of human nasopharyngeal cells in vitro via binding to the human polymeric immunoglobulin receptor (hpIgR) (25). Taken together, these data provide a foundation for understanding the role of CbpA in S. pneumoniae host cell invasion.
The polymeric immunoglobulin receptor (pIgR) is a type I membrane-spanning protein produced by mucosal epithelial cells, including respiratory and intestinal epithelial cells, in humans, mice, and many other mammals. The primary function of this protein is to transport polymeric immunoglobulin A (IgA) or IgM from the internal face of the mucosal epithelium to the lumen in a process designated transcytosis (14). Transcytosis initiates on the basolateral, or bottom, face of a polarized epithelial cell, where the extracellular domain of the receptor can bind a polymeric immunoglobulin (pIg) molecule and undergo endocytosis. Once endocytosed, the pIgR-pIg complex moves to the apical, or upper, face of the cell via regulated transcytosis through a series of endosomal compartments. At the apical surface the protein is cleaved by an unknown protease, thereby releasing the extracellular domain of pIgR bound to a pIg molecule. This liberated complex is known as secretory Ig. Although the primary function of the pIgR is to transport pIg, the entire process of pIgR transcytosis can occur in the absence of pIg. pIgR molecules not bound to pIg at the apical surface of the cell are usually cleaved, in which case the extracellular domain is released as a fragment termed secretory component (SC) (16, 21, 22). Alternatively, uncleaved, apical pIgR molecules can be reinternalized and transported in a retrograde fashion to the basolateral domain of the cell; however, this process is much less efficient than basolateral-to-apical transport (4).
Recent findings demonstrated that CbpA binds the extracellular domain of hpIgR and that the unencapsulated R6x laboratory strain of S. pneumoniae invades the human nasopharyngeal cell line Detroit 562 in an hpIgR-dependent manner (8, 9, 25). These data suggested that the interaction between the hpIgR and CbpA might play a central role in the process of pneumococcal host cell invasion. We sought to expand upon these findings in two ways. First we determined if hpIgR-dependent S. pneumoniae epithelial cell invasion was applicable to a variety of epithelial cell types that express the hpIgR. Second, we assessed the ability of S. pneumoniae strains other than R6x to invade hpIgR-expressing epithelial cells. Our data demonstrate that hpIgR-mediated invasion of epithelial cells by S. pneumoniae occurs in a strain-specific and cell type-specific manner.
MATERIALS AND METHODS
Mammalian cell lines and plasmids.
MDCK cells (type II; ATCC CCL-34) were transduced to express hpIgR by subcloning the cDNA for hpIgR (kindly provided by C. S. Kaetzel) into the pBabe-puro retroviral vector, and production of transducing virus was carried out as previously described (13). Transduced cells were selected based on resistance to puromycin (1 μg/ml) and hpIgR expression. Once isolated, the cells were no longer grown in the presence of puromycin. The cell lines Detroit 562 (human pharyngeal cells; ATCC CCL-138), Calu-3 (human lung epithelium; ATCC HTB-55), MDCK (canine kidney cells; ATCC CCL-34), Caco-2 (human colon epithelium; HTB-37), HCA-7 (human colon epithelium; kindly provided by S. Kirkland), and MDCK-hpIgR (this study) were maintained in Dulbecco's modified Eagle's medium and Ham's F-12 medium (GIBCO Laboratories) supplemented with 7% fetal bovine serum, 320 μg of l-glutamine per ml, 1% (vol/vol) nonessential amino acids, 2.7 μg of amphotericin B per ml, and 45 μg of gentamicin per ml. 16HBE-14o−, a human bronchial epithelial cell line that was transfected to express hpIgR (generously provided by T. Ferkol), has been described previously and was maintained in the medium described above supplemented with 400 μg of neomycin per ml (6, 12).
Bacterial strains.
S. pneumoniae strains R36A (ATCC 12214), R6, R6x, and CbpA-deficient R6x (kindly provided by E. Tuomanen) are unencapsulated, laboratory-adapted strains derived from the capsular strain D39 (18, 20, 24). Serotyped strains designated here 14, 4, 6B, 9V, 18C, and 19F (kindly provided by K. Edwards) are previously described, invasive clinical isolates derived from blood (19). Single colony isolates of each strain were maintained at 37°C on Trypticase soy agar plates supplemented with 5% sheep blood (Becton Dickinson). Single colonies were expanded by resuspending in Todd-Hewitt broth (Becton Dickinson) supplemented with 0.5% yeast extract (Becton Dickinson) and incubating at 37°C for 6 to 8 h.
Immunoblot analysis.
To obtain cell-associated hpIgR, a confluent cell monolayer of equivalent area from each cell type indicated in Fig. 1 was washed twice with phosphate-buffered saline and then scraped into 1 ml of phosphate-buffered saline and pelleted (30 s at 13,000 × g). The pellet was resuspended in three to four pellet volumes of single detergent lysis buffer supplemented with a protease inhibitor cocktail (Sigma) and 0.1 mM phenylmethylsulfonyl fluoride (2). Liberated human SC (hSC) was obtained directly from the growth medium (30-μl sample of 1 ml of supernatant) of each tissue culture monolayer at 48 h past confluence. All polyacrylamide gel electrophoresis procedures were performed using a neutral-pH Bis-Tris electrophoresis system (NuPAGE; Invitrogen Corporation); nonreduced samples were heat denatured and run on NuPAGE 10% Bis-Tris gels using MOPS (morpholinepropanesulfonic acid) running buffer as specified by the manufacturer. All Western blot protein transfers were performed using the NuPAGE transfer system (Invitrogen Corporation) as specified by the manufacturer. Immunodetection was carried out using hSC-specific goat antiserum (Sigma) at a dilution of 1:1,000. Bound hSC-specific antiserum was detected using horseradish peroxidase-conjugated rabbit anti-goat IgG (Sigma) at a dilution of 1:1,000.
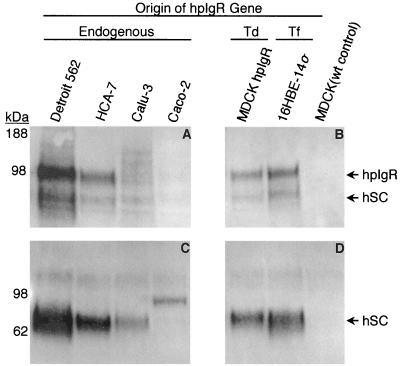
FIG. 1.
hpIgR is expressed by each of the cell lines examined for S. pneumoniae invasion. Mammalian cell lines used in invasion assays were examined for expression of hpIgR or its enzymatic cleavage product hSC in the cell-associated fraction (A and B) or the tissue culture supernatant (C and D). The cell lines examined expressed the receptor endogenously or via transfection (Tf) or retroviral transduction (Td) of hpIgR cDNA. Each cell lysate was obtained from a 9.4-cm2 sample of a confluent tissue culture cell monolayer, while the tissue culture supernatant samples contained a 30-μl aliquot of the 1-ml cell culture supernatant from each of the cell monolayers.
Protein overlay blot assay.
We used a standard method to demonstrate that the pneumococcal strains studied expressed CbpA under the growth conditions used. Pneumococcal lysates were prepared as previously described (8). Each lysate was separated by polyacrylamide gel electrophoresis and transferred to a polyvinylidene difluoride membrane as described above. After blocking the transferred proteins in Tris-buffered saline-3% (wt/vol) milk for 1 h at room temperature, the transfer membrane was washed and incubated in cell-free Detroit 562 cell tissue culture supernatant containing hSC for 1 h at room temperature. Then the membrane was washed and examined for CbpA bands with bound hSC using the hSC detection reagents described above.
Epithelial cell invasion assay.
The invasion assay was based on an endothelial cell invasion assay described previously and was carried out with the following alterations. Twelve hours prior to the invasion assay, 100% confluent epithelial cell monolayers were washed and provided with antibiotic-free cell culture growth medium (18). S. pneumoniae was grown at 37°C to mid-log phase (optical density at 590 nm of 0.3) and brought to a concentration of 8 × 107 CFU/ml in Dulbecco's modified Eagle's medium-Ham's F-12 supplemented with 1% fetal bovine serum and 1% l-glutamine. Growth medium was aspirated from epithelial cell culture monolayers and 250 μl of medium containing S. pneumoniae (2 × 107 CFU) was added, followed by a 3-h incubation at 37°C in 5% CO2. Prior to collection each cell monolayer was incubated for 1 h in medium (described above) supplemented with 20 μg of penicillin G (Sigma) per ml and 400 μg of gentamicin (Life Technologies) per ml to ensure that any extracellular bacteria were killed.
RESULTS
Expression of hpIgR by epithelial cell lines.
In order to investigate the ability of S. pneumoniae to invade hpIgR-expressing cell lines, we considered several candidate mammalian cell lines that express hpIgR. To confirm expression of hpIgR, each cell line was grown until 100% confluent and examined for endogenous expression of hpIgR via polyacrylamide gel electrophoresis followed by Western blotting and immunodetection. As shown in Fig. 1A, the cell lines Detroit 562, HCA-7, Calu-3, and Caco-2 all endogenously express a 100-kDa cell-associated protein that is detected by a polyclonal serum specific for hpIgR (15). In addition to cell lines that endogenously express hpIgR, we examined two cell lines that were modified to express hpIgR. 16HBE-14o− cells expressing hpIgR by transfection have been previously described (6). MDCK cells exhibiting stable expression of hpIgR (MDCK-hpIgR) were produced by retroviral transduction of a cDNA encoding hpIgR. Each of these cell lines was examined for expression of cell-associated hpIgR as described above. In each case receptor expression was observed, while the same protein was absent from a cell lysate prepared using the parental MDCK cell line (Fig. 1B). We observed apparent differences in expression levels of hpIgR among the cell types examined. Although our data are not quantitative, generally Detroit 562 cells appeared to exhibit the highest level of hpIgR expression, while Calu-3 and Caco-2 cells consistently had very low levels of cell-associated hpIgR.
A signature feature of functional hpIgR expression is conversion of the complete, cell-associated form of the protein to the enzymatically released form termed hSC (15). We examined the growth medium of each of the hpIgR-expressing cell lines for the presence of hSC. The presence of hSC in the cell growth medium confirmed the identity of the 100-kDa protein detected in the cell lysate fraction as hpIgR and indicated that the protein is processed normally rather than being expressed as only a cell-associated form. Also, examination of the cell culture medium may allow for detection of hpIgR expression in cell lines that express the protein at low levels, such as the Calu-3 and Caco-2 cell lines. SC is not appreciably degraded in vitro and thus concentrates in the cell culture medium over a period of time. In contrast to the minor level of cell-associated hSC, we observed hSC as the dominant form of the receptor in the supernatant of each cell line examined (Fig. 1C and D), with the exception of Caco-2 cells. The Caco-2 cell supernatant did, however, contain an immunoreactive protein of approximately 100 kDa. This suggests that Caco-2 cells may preferentially produce an uncleaved, secreted form of the receptor, which may account for the lesser degree of cell-associated hpIgR observed in Fig. 1A. For comparative purposes, this cell line was included in assays to examine for hpIgR-mediated invasion of S. pneumoniae. The diversity held within this collection of cell lines provided the means to determine whether hpIgR is sufficient to mediate enhanced invasion of S. pneumoniae.
Expression of CbpA by S. pneumoniae.
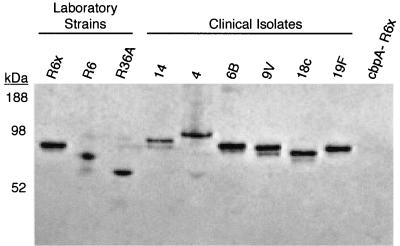
CbpA is expressed by most, but not all, strains of S. pneumoniae (5, 8, 25). The expression of this surface protein occurs in a phase-dependent manner, with greater expression being associated with the transparent phase of growth that is linked with nasopharyngeal colonization (11). Each of the strains examined in this study was analyzed for the presence of CbpA by overlay protein blot assay to ensure that our culturing methods allowed for its expression. Each S. pneumoniae strain was grown to mid-log phase, lysed, separated by polyacrylamide gel electrophoresis, and immobilized on a polyvinylidene difluoride membrane as described in Materials and Methods. Detection of immobilized CbpA was achieved by using hSC-rich tissue culture medium from Detroit 562 cell culture supernatants. CbpA-hSC binding has been shown to have a dissociation constant of 9.3 × 10−9 M and allows for a specific method of CbpA detection (8). Following hSC binding to CbpA, bound hSC was detected using an hSC-specific antiserum and visualized by chemiluminescence. As Fig. 2 illustrates, each strain of S. pneumoniae used in this study expresses CbpA, while the lysate from a CbpA-deficient R6x strain did not bind hSC. The predicted molecular mass of CbpA is 75 kDa, but previous work has demonstrated its migration by sodium dodecyl sulfate-polyacrylamide gel electrophoresis to vary from as high as 112 kDa to just below 55 kDa. Such strain-to-strain variation is also observed in Fig. 2 (5, 8, 20). This confirms the expression of CbpA by each strain under the conditions used in the bacterial invasion assays and verifies that the form of CbpA produced by each binds hSC.
FIG. 2.
The hpIgR binding protein CbpA is expressed by each S. pneumoniae strain or clinical isolate examined for hpIgR-enhanced invasion of epithelial cells. A single colony of each isolate was grown to mid-log phase. Cell lysates were prepared for each isolate and examined for CbpA expression by polyacrylamide gel electrophoresis followed by overlay protein blot analysis using hSC.
S. pneumoniae invasion is cell type specific.
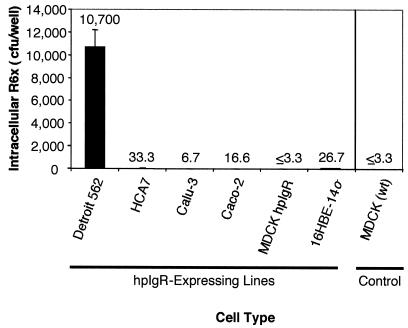
Previous work demonstrated that extensive invasion of Detroit 562 or hpIgR-expressing MDCK cells by the S. pneumoniae strain R6x occurs in an hpIgR-dependent manner (25). We sought to determine whether strain R6x would invade other hpIgR-expressing mammalian cell lines to a similar degree. To examine this, each of the hpIgR-expressing cell lines described above was used to conduct epithelial invasion assays with strain R6x. We found that strain R6x did not invade any of the other hpIgR-expressing cell lines in the manner observed for Detroit 562 cells (Fig. 3). Invasion assays of Detroit 562 cells yielded an average of 10,700 intracellular CFU per well examined. In contrast, assays using the other mammalian cell lines showed an average of 17 intracellular CFU per well, translating into a 618-fold difference between Detroit 562 and other cell types. These data indicate that the ability of S. pneumoniae to mediate significant invasion of hpIgR-expressing epithelial cells is cell type specific and that hpIgR is not sufficient to mediate invasion.
FIG. 3.
hpIgR-enhanced invasion of epithelial cells by S. pneumoniae strain R6x is cell type specific. Confluent tissue culture cell monolayers for each cell type specified were used to conduct epithelial cell invasion assays as described in Materials and Methods. Numeric values for each sample represent the average numbers of intracellular CFU of R6x detected for triplicate wells of each sample; error bars indicate standard deviations.
S. pneumoniae invasion is strain specific.
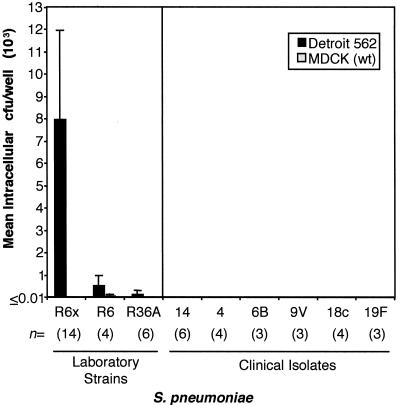
Previous work examining hpIgR-enhanced invasion of epithelial cells focused on the invasive ability of laboratory strain R6x. However, numerous pathogenic and nonpathogenic strains of S. pneumoniae exist. Therefore, we were interested in the ability of strains other than R6x to invade epithelial cells expressing hpIgR. To examine the invasive abilities of both clinical isolates and laboratory-adapted strains of S. pneumoniae, we conducted epithelial cell invasion assays using Detroit 562 cells, the most permissive cell line for invasion as demonstrated by strain R6x invasion (Fig. 3), and a variety of CbpA-expressing strains of S. pneumoniae (Fig. 2). As Fig. 4 shows, only strain R6x demonstrated substantial invasion of Detroit 562 cells. The parental strain of R6x, strain R6, and its parental strain, R36A, minimally invaded Detroit cells with 552 or 138 CFU of intracellular pneumococci, respectively, detected per well. This level of invasion was more than 14- and 57-fold less, respectively, than the 7,967 CFU per well achieved by R6x (24). The molecular basis for the observed differences in invasive capacity is not clear. In addition, epithelial cell invasion of invasive clinical isolates derived from blood cultures, serotypes 14, 4, 6B, 9V, 18C, and 19F, was below the detectable level of our assay (3.33 CFU/well) in all instances. These data suggest that hpIgR-dependent invasion of epithelial cells by S. pneumoniae is strain specific.
FIG. 4.
S. pneumoniae invasion of Detroit 562 cells is pneumococcal strain specific. Multiple strains of S. pneumoniae were examined for the ability to invade confluent Detroit 562 tissue culture cell monolayers via the epithelial cell invasion assay described in Materials and Methods. The numeric value n for each strain or isolate represents the number of experimental replicates examined. Values shown in bars are mean intracellular CFU/well (103); error bars indicate standard deviations.
DISCUSSION
The mechanism of pneumococcal invasion and penetration of the respiratory epithelium is an important biomedical question, the answer to which will provide insight into S. pneumoniae pathogenesis. The conclusion of our work is that hpIgR-enhanced invasion of epithelial cells is cell type specific and pneumococcal strain specific. Enhanced invasiveness of S. pneumoniae strain R6x was only applicable to Detroit 562 cells, one of several hpIgR-expressing epithelial cell lines examined (Fig. 3). The Detroit 562 cell line generally possesses a high degree of cell-associated hpIgR (Fig. 1) and is the only cell type we examined that was readily invaded in vitro by pneumococci. Therefore, we considered whether robust pneumococcal invasion of epithelial cells requires high levels of hpIgR expression. High levels of hpIgR expression may be necessary for recycling of the receptor, which is the proposed mechanism of pIgR-mediated pneumococcal invasion (25). However, strain R6x was the only one of nine CbpA-expressing strains examined that displayed significant invasion of Detroit 562 cells (Fig. 4), suggesting that high levels of hpIgR expression alone are not sufficient to allow for pneumococcal epithelial cell invasion. Together, these data indicate that enhanced pneumococcal invasion of hpIgR-expressing epithelial cells is limited to the combination of strain R6x and Detroit 562 cells.
Previous reports have provided data in support of pIgR-facilitated S. pneumoniae host cell invasion, while other reports have provided data to the contrary. Initially, it was shown that CbpA-deficient pneumococcal strains were 100-fold less efficient at colonizing the nasopharynx of infant rats (20). Shortly thereafter, CbpA was shown to bind hSC or human IgA containing hSC (secretory IgA), with a dissociation constant of 9.3 × 10−9 M (8). Next it was reported that a highly conserved hexapeptide motif within CbpA was responsible for mediating binding to hSC (9). It was subsequently determined that CbpA and hpIgR are required for R6x invasion of, and translocation across, Detroit 562 human nasopharyngeal cells (25). Additionally, p62yes knockout mice, defective in pIgR trafficking, and pIgR knockout mice were less susceptible to pneumococcal sepsis and nasal colonization, respectively (25). Together, these data provided the basis for proposing a central role for pIgR in S. pneumoniae epithelial cell invasion. Other data contradicted this model, however. Foremost is the fact that CbpA does not interact with SC derived from animals, specifically those from rat, mouse, rabbit, guinea pig, hamster, horse, dog, or cow (9; J.-P. Vaerman, personal communication). Additionally, in vitro tests demonstrated that neither rabbit pIgR nor murine pIgR expressed by MDCK cells enhances pneumococcal invasion (25; data not shown). These details, along with those presented in this study, directly contradict the supportive data referred to above and make the case for pIgR-mediated invasion of virulent pneumococci much less compelling.
The mechanism underlying enhanced invasion of Detroit 562 cells by strain R6x is not clear. Others have reported that encapsulated strains of pneumococci adhere to and invade cells less efficiently than unencapsulated strains, consistent with the data in Fig. 4 (18, 23). Therefore, we considered whether the relative ability of pneumococci to adhere to epithelial cells may influence invasion. We found that a variety of encapsulated pneumococci do not adhere to Detroit 562 or hpIgR-expressing MDCK cells as well as the unencapsulated strains R6x and R36A (data not shown). However, the adherence of strains R6x and R36A was similar for Detroit 562 cells and hpIgR-expressing MDCK cells (data not shown), while they differed greatly in efficiency of epithelial cell invasion. This observation suggests that the increased adherence of strain R6x to epithelial cells is not the sole factor determining its superior ability to invade Detroit 562 cells.
The ability of pIgR to mediate the initial stages of pneumococcal infection in humans would be surprising. The physiologic function of the hpIgR is to transport polymeric IgA and IgM molecules to the mucosal surface of the body's epithelium and to increase the stability of these molecules after secretion into the respiratory mucosa. In doing so, the extracellular portion of the protein is cleaved and released into the mucosa. Therefore, the secretions covering the mucosal lining of the respiratory epithelium are saturated with free hSC and secretory IgA (16). Because each of these processed forms of hpIgR can bind to CbpA, it seems plausible that these forms might inhibit pneumococcal invasion by binding CbpA of invading pneumococci before attachment to the host epithelial lining. Such activity has been observed in vitro and would be analogous to that of a CbpA-specific antibody preventing attachment of pneumococci to host epithelial cells (25).
Gaining an understanding of the importance of the role hpIgR plays in pneumococcal invasion will require more in-depth examination. Strain R6x invasion of Detroit 562 cells does occur in an hpIgR-dependent manner, as it is decreased 10-fold in the presence of hpIgR-specific antisera (25; data not shown). However, the significance of this finding is unclear, as the enhanced invasion mediated by hpIgR appears to be restricted to a nasopharyngeal carcinoma cell line derived from a metastatic pleural lesion interacting with an unencapsulated, nonpathogenic laboratory strain of S. pneumoniae studied in vitro. While making use of such reagents is practical for initial exploratory studies, future studies should incorporate clinically derived pneumococcal isolates and ex vivo cell lines derived from human respiratory epithelia in order to examine invasion in a more physiologically relevant manner.
Acknowledgments
This work was supported by the NIH/NIAID Respiratory Pathogens Research Unit grant NO1 AI-65298; the Viruses, Nucleic Acids and Cancer Training Grant program T32 CA09385 (S.C.B.); and NIH/NIAID grant R21 DE-014039.
We thank Jean-Pierre Vaerman and David Briles for helpful discussions, Christopher Aiken for assistance with retroviral transduction of MDCK cells, and Robert Coffey for assistance with obtaining human colon cell lines.
Editor: A. D. O'Brien
REFERENCES
- 1.Austrian, R. 1986. Some aspects of the pneumococcal carrier state. J. Antimicrob. Chemother. 18(Suppl. A):35-45. [DOI] [PubMed] [Google Scholar]
- 2.Ausubel, F. M. 1994. Current protocols in molecular biology. John Wiley & Sons, New York, N.Y.
- 3.Berry, A. M., A. D. Ogunniyi, D. C. Miller, and J. C. Paton. 1999. Comparative virulence of Streptococcus pneumoniae strains with insertion-duplication, point, and deletion mutations in the pneumolysin gene. Infect. Immun. 67:981-985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Breitfeld, P. P., J. M. Harris, and K. E. Mostov. 1989. Postendocytotic sorting of the ligand for the polymeric immunoglobulin receptor in Madin-Darby canine kidney cells. J. Cell Biol. 109:475-486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brooks-Walter, A., D. E. Briles, and S. K. Hollingshead. 1999. The pspC gene of Streptococcus pneumoniae encodes a polymorphic protein, PspC, which elicits cross-reactive antibodies to PspA and provides immunity to pneumococcal bacteremia. Infect. Immun. 67:6533-6542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ferkol, T., E. Eckman, S. Swaidani, C. Silski, and P. Davis. 2000. Transport of bifunctional proteins across respiratory epithelial cells via the polymeric immunoglobulin receptor. Am. J. Respir. Crit. Care Med. 161:944-951. [DOI] [PubMed] [Google Scholar]
- 7.Gorbach, S. L., N. R. Blacklow, and J. G. Bartlett. 1997. Infectious diseases, 2nd ed. Saunders, Philadelphia, Pa.
- 8.Hammerschmidt, S., S. R. Talay, P. Brandtzaeg, and G. S. Chhatwal. 1997. SpsA, a novel pneumococcal surface protein with specific binding to secretory immunoglobulin A and secretory component. Mol. Microbiol. 25:1113-1124. [DOI] [PubMed] [Google Scholar]
- 9.Hammerschmidt, S., M. P. Tillig, S. Wolff, J. P. Vaerman, and G. S. Chhatwal. 2000. Species-specific binding of human secretory component to SpsA protein of Streptococcus pneumoniae via a hexapeptide motif. Mol. Microbiol. 36:726-736. [DOI] [PubMed] [Google Scholar]
- 10.Jedrzejas, M. J. 2001. Pneumococcal virulence factors: structure and function. Microbiol. Mol. Biol Rev. 65:187-207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim, J. O., and J. N. Weiser. 1998. Association of intrastrain phase variation in quantity of capsular polysaccharide and teichoic acid with the virulence of Streptococcus pneumoniae. J. Infect. Dis. 177:368-377. [DOI] [PubMed] [Google Scholar]
- 12.Marsh, K. A., G. W. Stamp, and S. C. Kirkland. 1993. Isolation and characterization of multiple cell types from a single human colonic carcinoma: tumourigenicity of these cell types in a xenograft system. J. Pathol. 170:441-450. [DOI] [PubMed] [Google Scholar]
- 13.Morgenstern, J. P., and H. Land. 1990. Advanced mammalian gene transfer: high titre retroviral vectors with multiple drug selection markers and a complementary helper-free packaging cell line. Nucleic Acids Res. 18:3587-3596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mostov, K. E. 1994. Transepithelial transport of immunoglobulins. Annu. Rev. Immunol. 12:63-84. [DOI] [PubMed] [Google Scholar]
- 15.Mostov, K. E., and G. Blobel. 1982. A transmembrane precursor of secretory component. The receptor for transcellular transport of polymeric immunoglobulins. J. Biol. Chem. 257:11816-11821. [PubMed] [Google Scholar]
- 16.Ogra, P. L. 1998. Mucosal immunology, 2nd ed. Academic Press, San Diego, Calif.
- 17.Paton, J. C., A. M. Berry, and R. A. Lock. 1997. Molecular analysis of putative pneumococcal virulence proteins. Microb. Drug Resist. 3:1-10. [DOI] [PubMed] [Google Scholar]
- 18.Ring, A., J. N. Weiser, and E. I. Tuomanen. 1998. Pneumococcal trafficking across the blood-brain barrier. Molecular analysis of a novel bidirectional pathway. J. Clin. Investig. 102:347-360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Robinson, D. A., K. M. Edwards, K. B. Waites, D. E. Briles, M. J. Crain, and S. K. Hollingshead. 2001. Clones of Streptococcus pneumoniae isolated from nasopharyngeal carriage and invasive disease in young children in central Tennessee. J. Infect. Dis. 183:1501-1507. [DOI] [PubMed] [Google Scholar]
- 20.Rosenow, C., P. Ryan, J. N. Weiser, S. Johnson, P. Fontan, A. Ortqvist, and H. R. Masure. 1997. Contribution of novel choline-binding proteins to adherence, colonization and immunogenicity of Streptococcus pneumoniae. Mol. Microbiol. 25:819-829. [DOI] [PubMed] [Google Scholar]
- 21.Song, W., M. Bomsel, J. Casanova, J. P. Vaerman, and K. Mostov. 1994. Stimulation of transcytosis of the polymeric immunoglobulin receptor by dimeric IgA. Proc. Natl. Acad. Sci. USA 91:163-166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Song, W., J. P. Vaerman, and K. E. Mostov. 1995. Dimeric and tetrameric IgA are transcytosed equally by the polymeric Ig receptor. J. Immunol. 155:715-721. [PubMed] [Google Scholar]
- 23.Talbot, U. M., A. W. Paton, and J. C. Paton. 1996. Uptake of Streptococcus pneumoniae by respiratory epithelial cells. Infect. Immun. 64:3772-3777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tiraby, J. G., and M. S. Fox. 1973. Marker discrimination in transformation and mutation of pneumococcus. Proc. Natl. Acad. Sci. USA 70:3541-3545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang, J. R., K. E. Mostov, M. E. Lamm, M. Nanno, S. Shimida, M. Ohwaki, and E. Tuomanen. 2000. The polymeric immunoglobulin receptor translocates pneumococci across human nasopharyngeal epithelial cells. Cell 102:827-837. [DOI] [PubMed] [Google Scholar]