Abstract
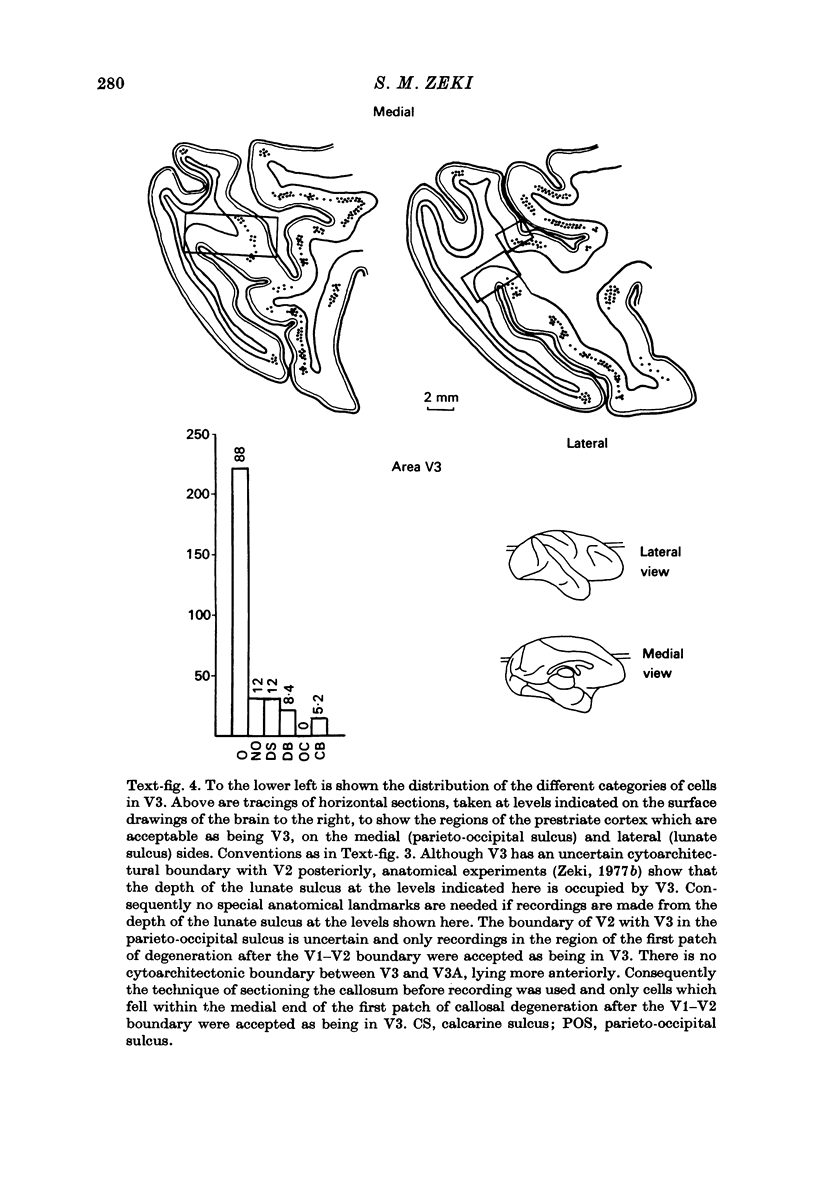
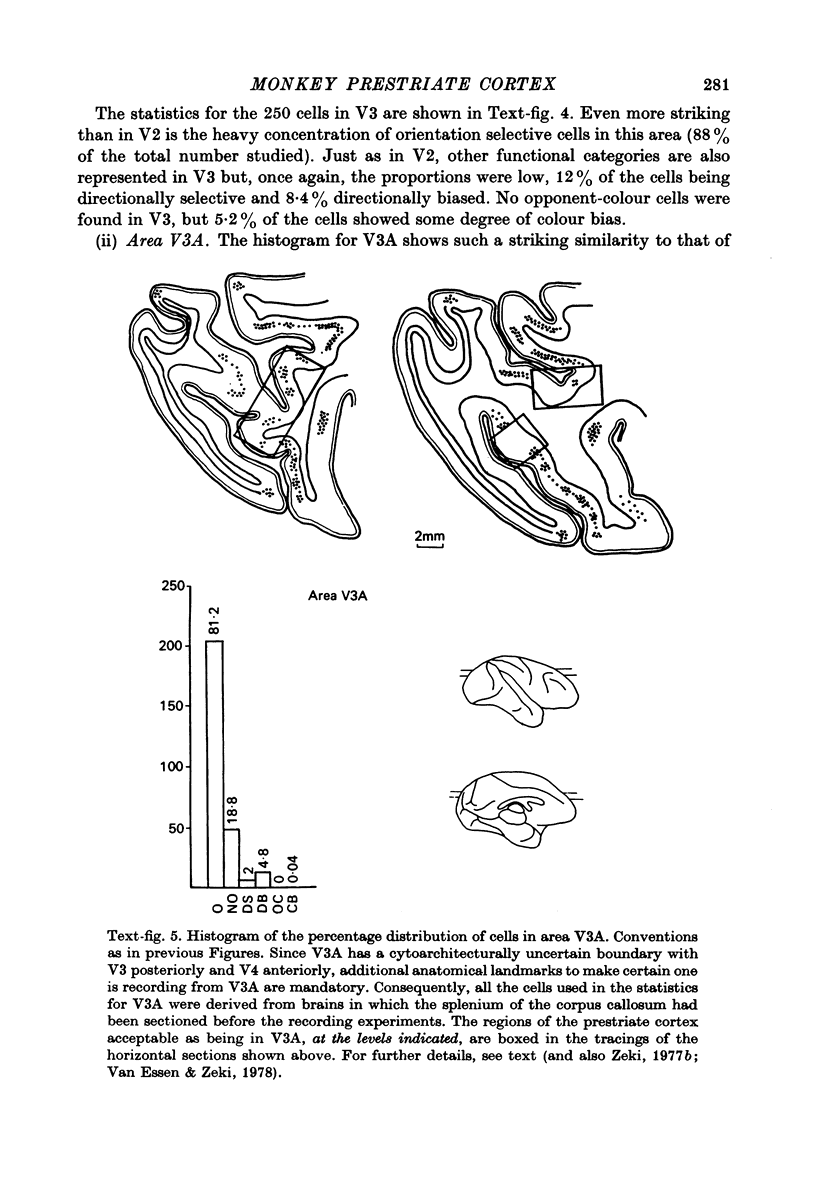
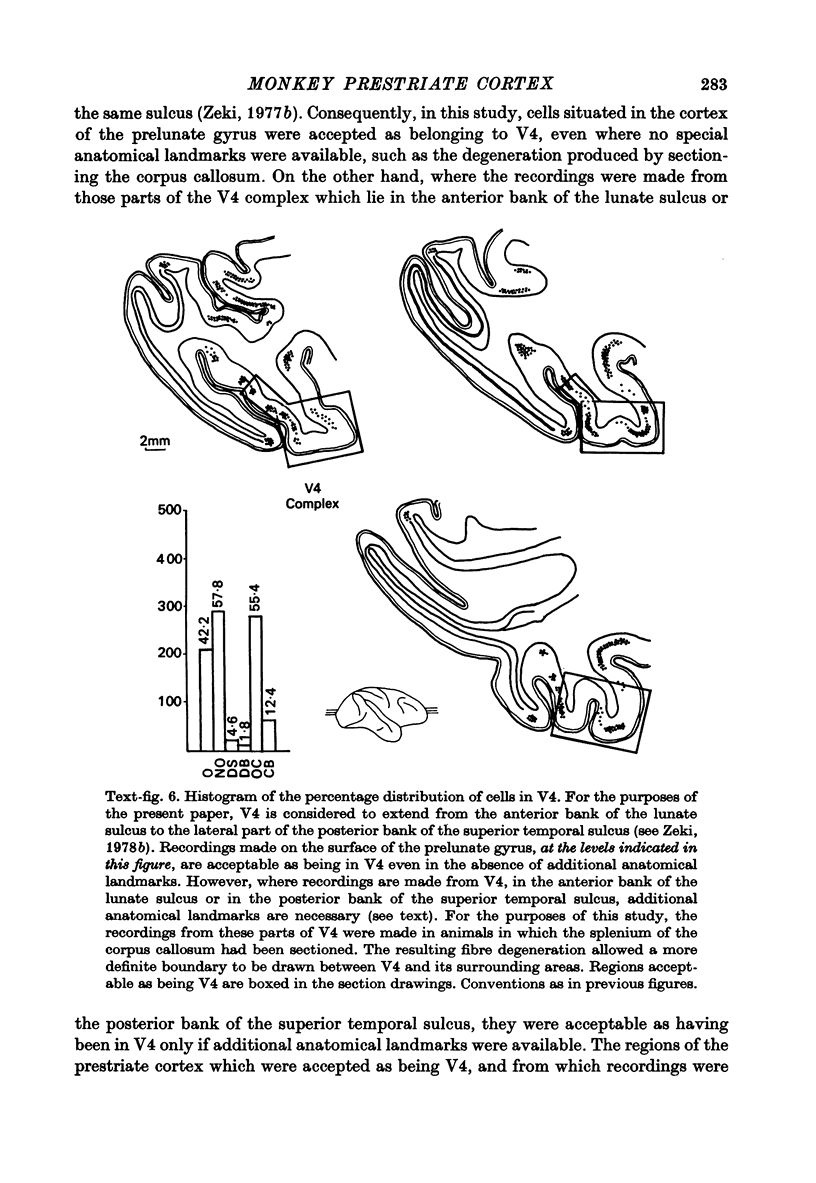
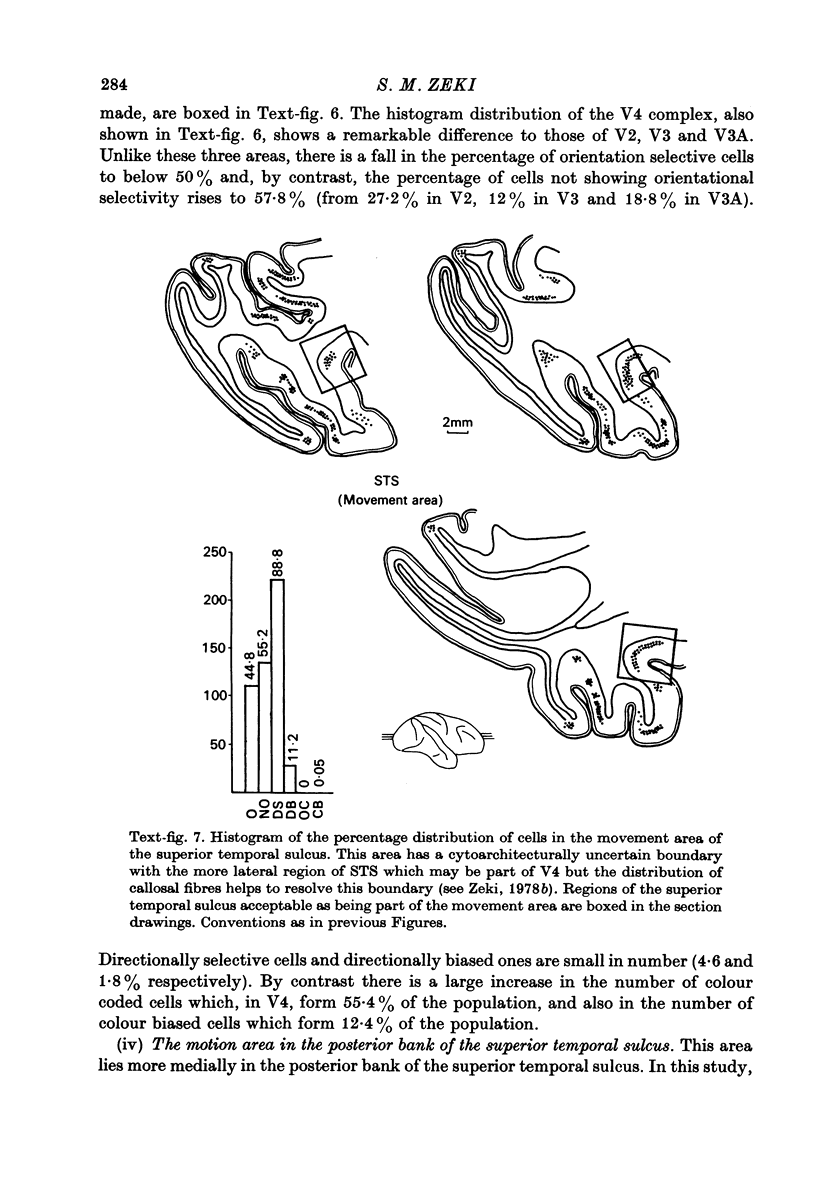
1. Recordings were made from single neurones, or small clusters of cells, in five prestriate visual areas of rhesus monkey cortex. The cells were studied for their binocularity, as well as for their orientational, motion and colour preferences. In all, 1500 cells were studied, 250 cells for each of the areas V2, V3, V3A and the motion area of the posterior bank of the superior temporal sulcus, and 500 cells for V4. All the cells referred to in this study can be placed in one prestriate area or another unambiguously. 2. The great majority of cells in all areas were binocularly driven, without monocular preferences. Within each area, there were cells that either preferred binocular stimulation markedly, or were responsive to binocular stimulation only. The ocular interaction histograms for all areas are remarkably similar when tested at a fixed disparity. 3. Over 70% of the cells in areas V2, V3 and V3A were selective for orientation. The receptive fields of cells were larger in V3 and V3A than in V2. By contrast, less than 50% of the cells in V4 and the motion area of the superior temporal sulcus were orientation selective. 4. Directionally selective cells were found in all areas. But they were present in small numbers (less than 15%) in areas V2, V3, V3A and V4. By contrast, 90% of the cells in the motion area of the superior temporal sulcus were directionally selective. 5. 8% of the cells in V2 had opponent colour properties. Cells with such properties were not found in V3, V3A or in the motion area of the superior temporal sulcus. By contrast, 54% of the cells in the V4 complex had opponent colour properties. 6. It is argued that despite its uniformity in cytoarchitectural appearance and in ocular interaction patterns, there is a functional division of labour within the prestriate cortex. Evidence for this is seen not only in the different concentrations of functional cell types in distinct areas of the prestriate cortex, but also in the differential anatomical and callosal connexions of each area.
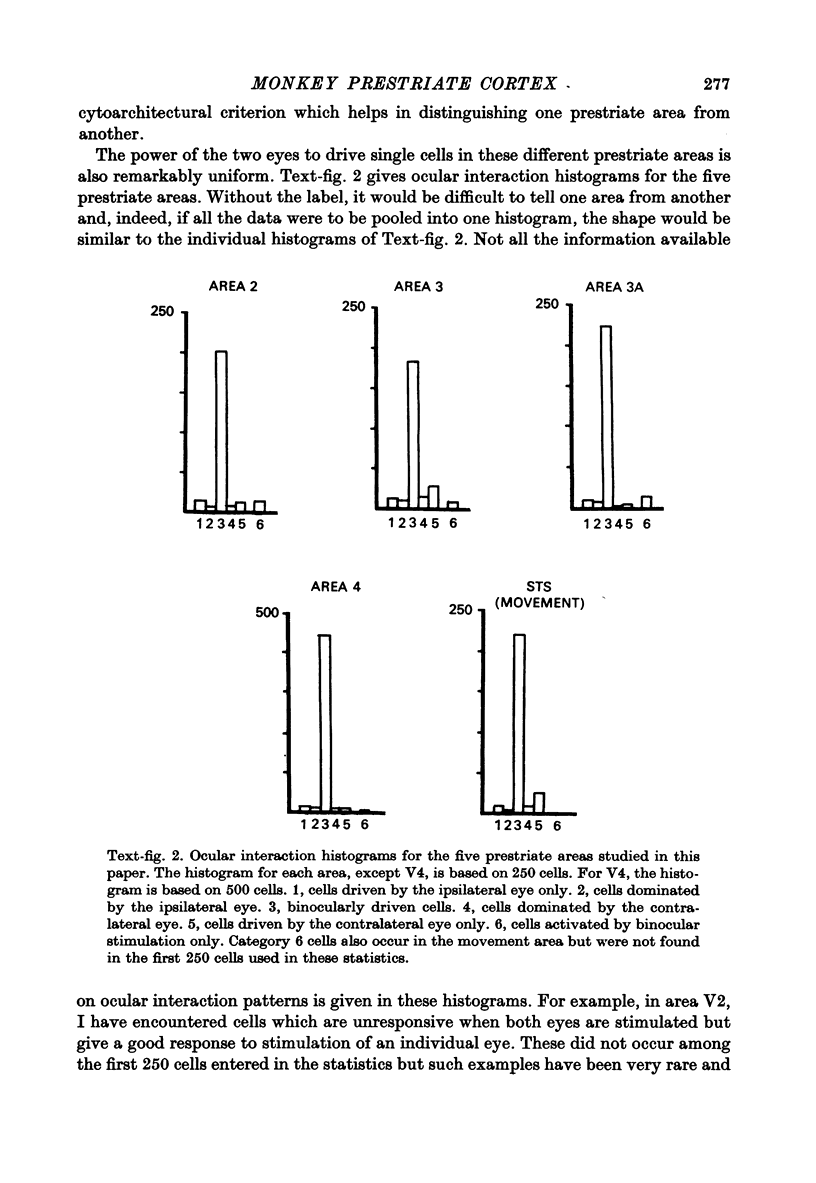
Full text
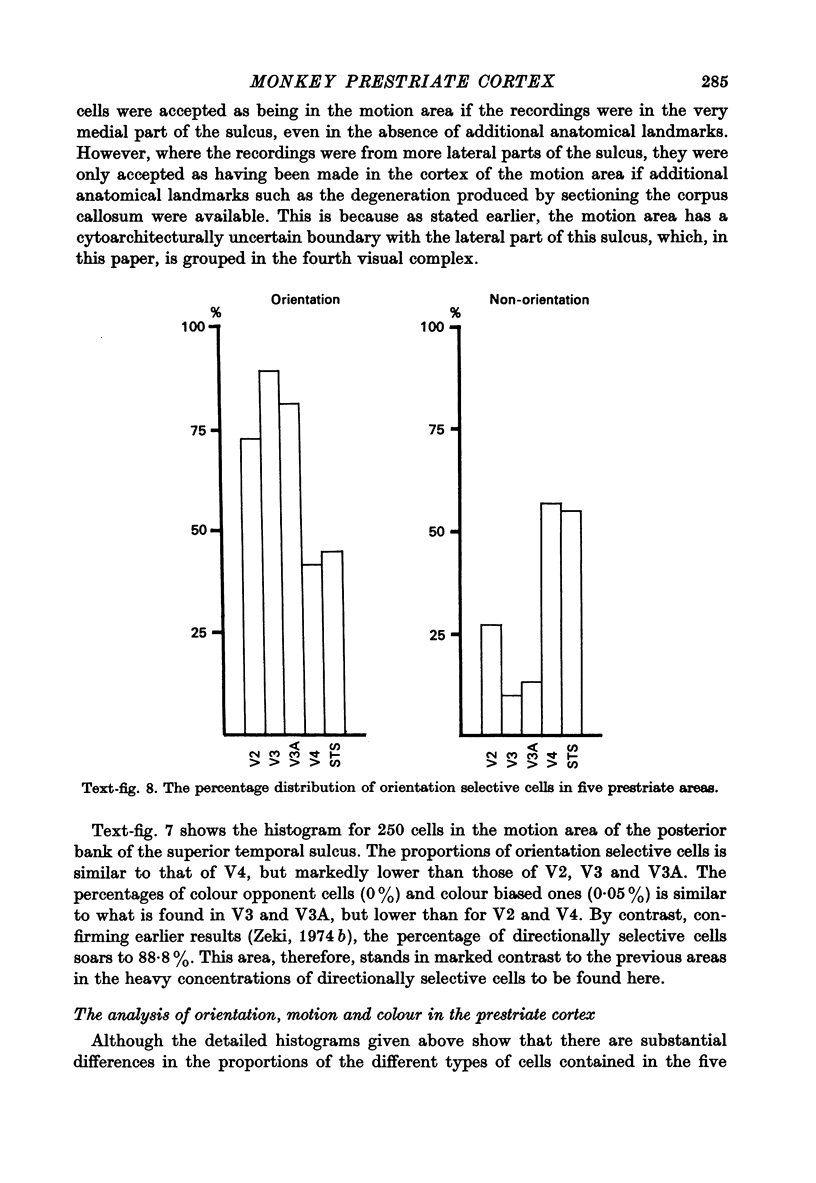
PDF


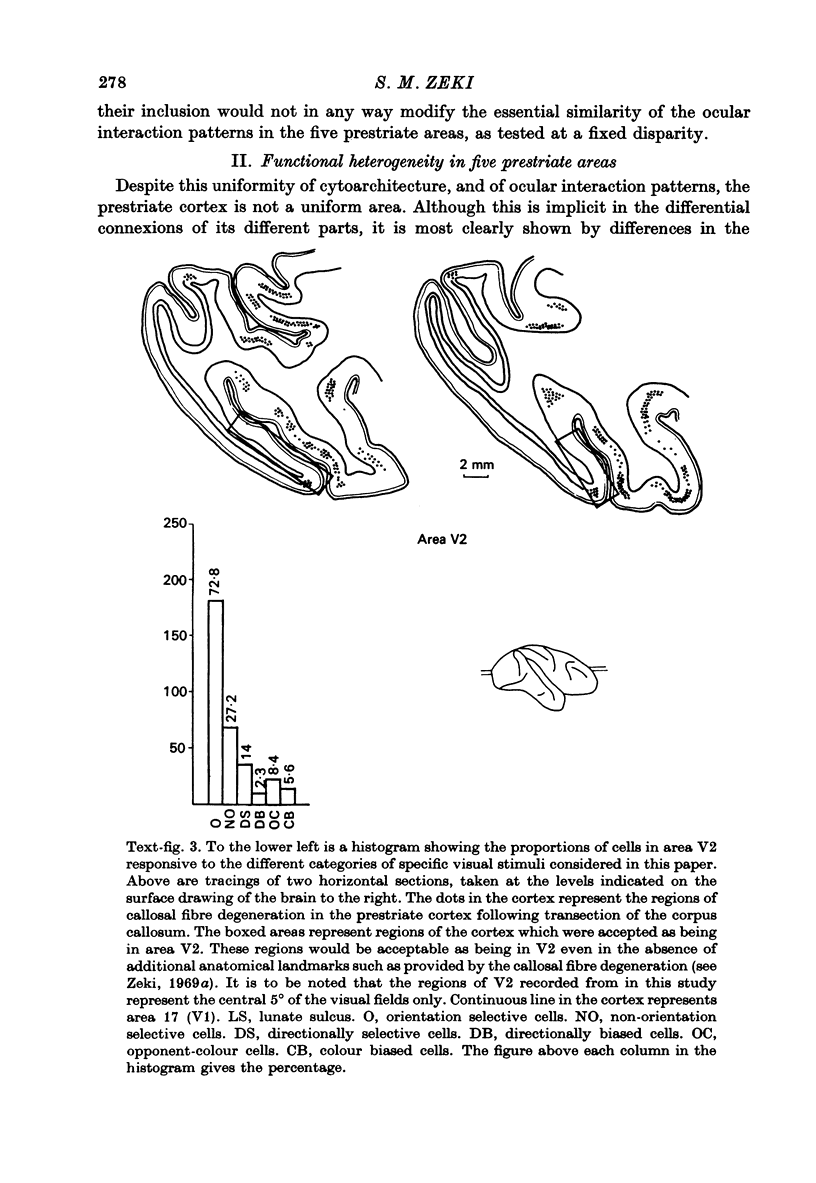


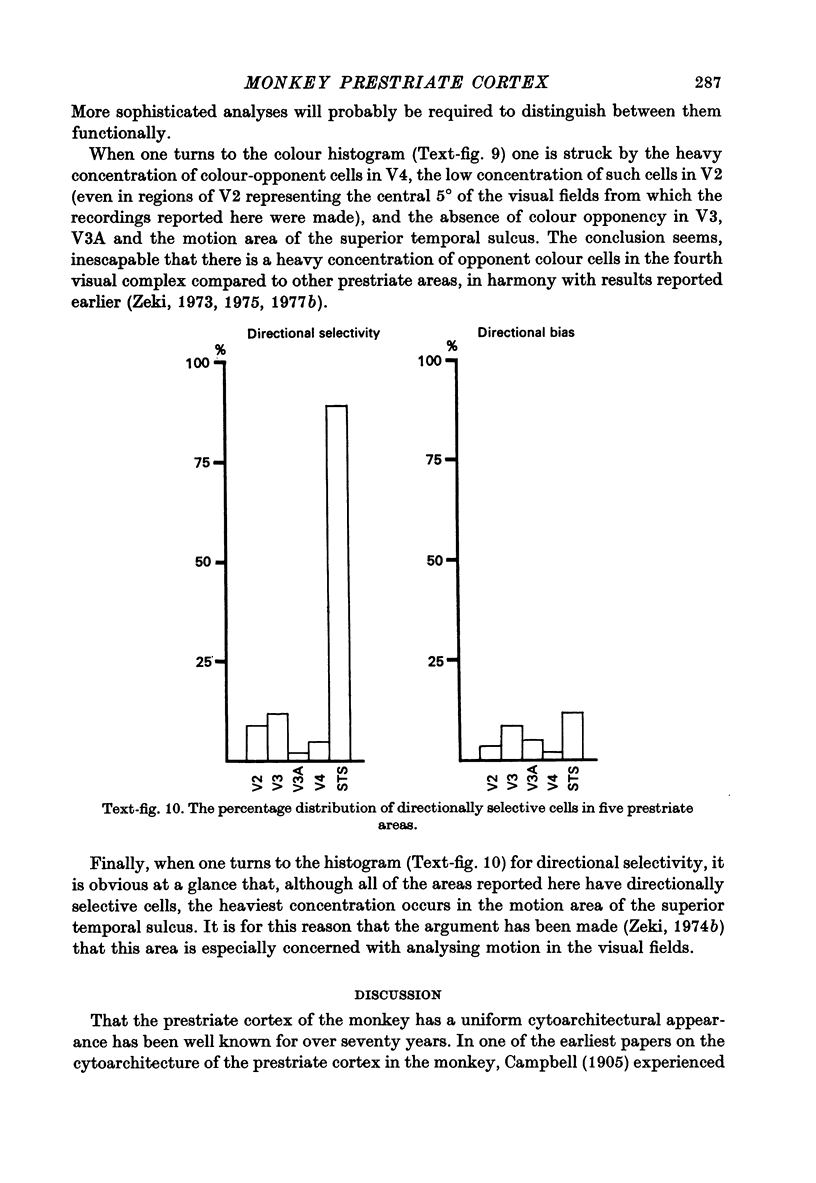



Images in this article
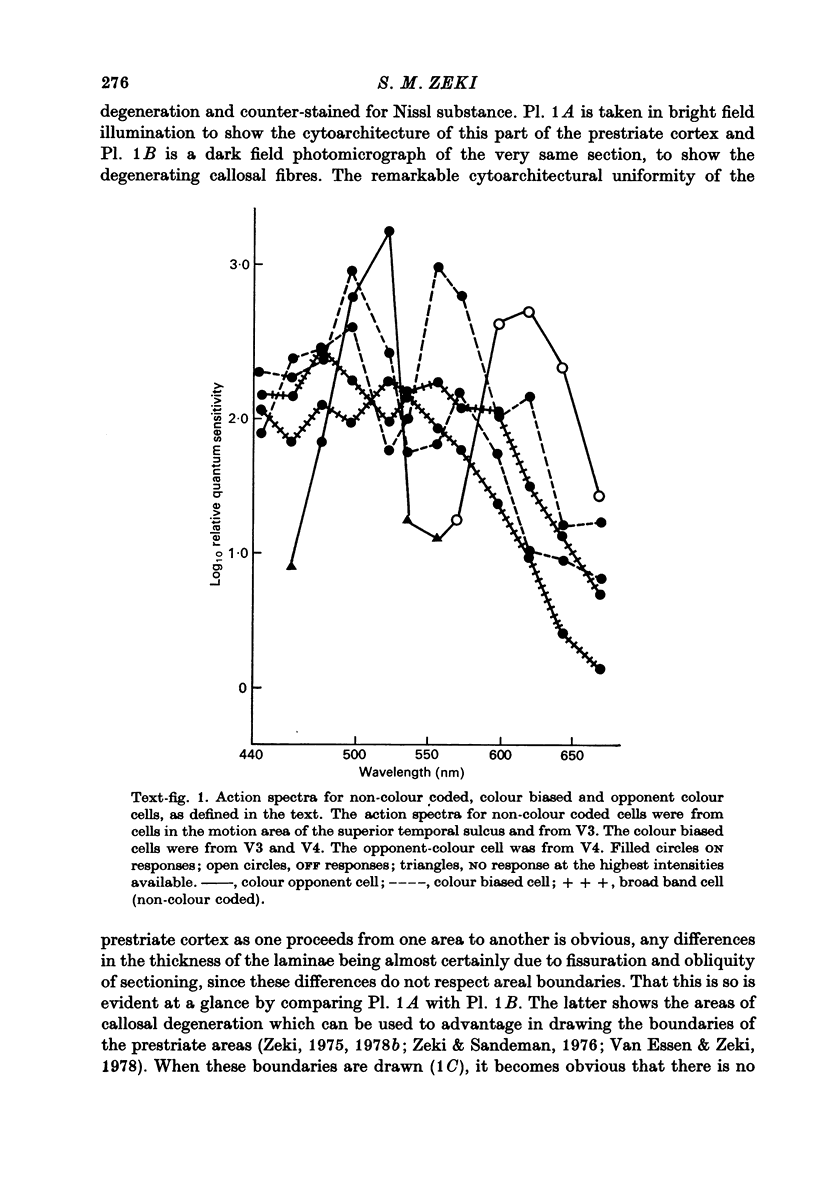
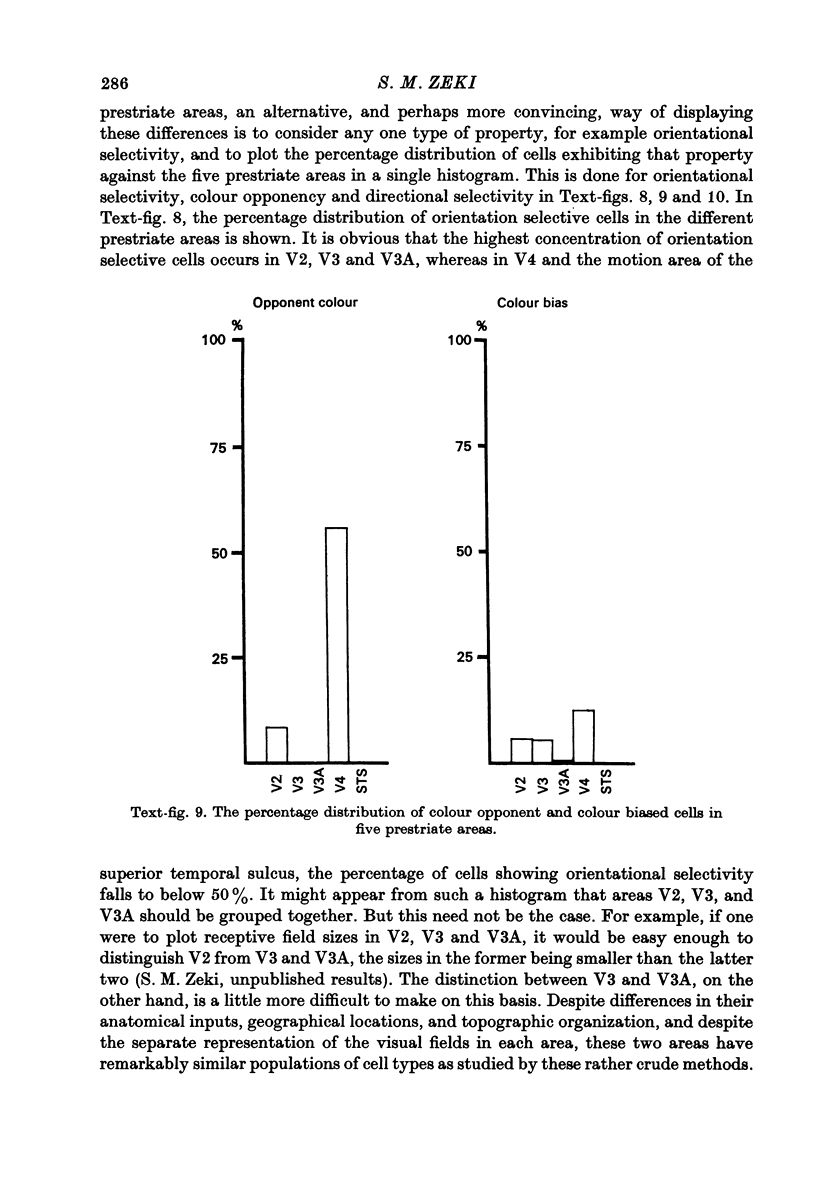
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cragg B. G. The topography of the afferent projections in the circumstriate visual cortex of the monkey studied by the Nauta method. Vision Res. 1969 Jul;9(7):733–747. doi: 10.1016/0042-6989(69)90011-x. [DOI] [PubMed] [Google Scholar]
- Essen D. C., Zeki S. M. The topographic organization of rhesus monkey prestriate cortex. J Physiol. 1978 Apr;277:193–226. doi: 10.1113/jphysiol.1978.sp012269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubel D. H., Wiesel T. N. Receptive fields and functional architecture of monkey striate cortex. J Physiol. 1968 Mar;195(1):215–243. doi: 10.1113/jphysiol.1968.sp008455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubel D. H., Wiesel T. N. Stereoscopic vision in macaque monkey. Cells sensitive to binocular depth in area 18 of the macaque monkey cortex. Nature. 1970 Jan 3;225(5227):41–42. doi: 10.1038/225041a0. [DOI] [PubMed] [Google Scholar]
- Zeki S. M. Colour coding in rhesus monkey prestriate cortex. Brain Res. 1973 Apr 27;53(2):422–427. doi: 10.1016/0006-8993(73)90227-8. [DOI] [PubMed] [Google Scholar]
- Zeki S. M. Colour coding in the superior temporal sulcus of rhesus monkey visual cortex. Proc R Soc Lond B Biol Sci. 1977 May 4;197(1127):195–223. doi: 10.1098/rspb.1977.0065. [DOI] [PubMed] [Google Scholar]
- Zeki S. M. Cortical projections from two prestriate areas in the monkey. Brain Res. 1971 Nov;34(1):19–35. doi: 10.1016/0006-8993(71)90348-9. [DOI] [PubMed] [Google Scholar]
- Zeki S. M. Functional organization of a visual area in the posterior bank of the superior temporal sulcus of the rhesus monkey. J Physiol. 1974 Feb;236(3):549–573. doi: 10.1113/jphysiol.1974.sp010452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeki S. M. Interhemispheric connections of prestriate cortex in monkey. Brain Res. 1970 Apr 1;19(1):63–75. doi: 10.1016/0006-8993(70)90237-4. [DOI] [PubMed] [Google Scholar]
- Zeki S. M. Representation of central visual fields in prestriate cortex of monkey. Brain Res. 1969 Jul;14(2):271–291. doi: 10.1016/0006-8993(69)90110-3. [DOI] [PubMed] [Google Scholar]
- Zeki S. M., Sandeman D. R. Combined anatomical and electrophysiological studies on the boundary between the second and third visual areas of rhesus monkey cortex. Proc R Soc Lond B Biol Sci. 1976 Nov 12;194(1117):555–562. doi: 10.1098/rspb.1976.0094. [DOI] [PubMed] [Google Scholar]
- Zeki S. M. Simultaneous anatomical demonstration of the representation of the vertical and horizontal meridians in areas V2 and V3 of rhesus monkey visual cortex. Proc R Soc Lond B Biol Sci. 1977 Feb 11;195(1121):517–523. doi: 10.1098/rspb.1977.0024. [DOI] [PubMed] [Google Scholar]
- Zeki S. M. The cortical projections of foveal striate cortex in the rhesus monkey. J Physiol. 1978 Apr;277:227–244. doi: 10.1113/jphysiol.1978.sp012270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeki S. M. The functional organization of projections from striate to prestriate visual cortex in the rhesus monkey. Cold Spring Harb Symp Quant Biol. 1976;40:591–600. doi: 10.1101/sqb.1976.040.01.055. [DOI] [PubMed] [Google Scholar]
- Zeki S. M. The secondary visual areas of the monkey. Brain Res. 1969 Apr;13(2):197–226. doi: 10.1016/0006-8993(69)90282-0. [DOI] [PubMed] [Google Scholar]
- Zeki S. M. The third visual complex of rhesus monkey prestriate cortex. J Physiol. 1978 Apr;277:245–272. doi: 10.1113/jphysiol.1978.sp012271. [DOI] [PMC free article] [PubMed] [Google Scholar]