Abstract
The YadA protein is a major adhesin of Yersinia pseudotuberculosis that promotes tight adhesion to mammalian cells by binding to extracellular matrix proteins. In this study, we first addressed the possibility of competitive interference of YadA and the major invasive factor invasin and found that expression of YadA in the presence of invasin affected neither the export nor the function of invasin in the outer membrane. Furthermore, expression of YadA promoted both bacterial adhesion and high-efficiency invasion entirely independently of invasin. Antibodies against fibronectin and β1 integrins blocked invasion, indicating that invasion occurs via extracellular-matrix-dependent bridging between YadA and the host cell β1 integrin receptors. Inhibitor studies also demonstrated that tyrosine and Ser/Thr kinases, as well as phosphatidylinositol 3-kinase, are involved in the uptake process. Further expression studies revealed that yadA is regulated in response to several environmental parameters, including temperature, ion and nutrient concentrations, and the bacterial growth phase. In complex medium, YadA production was generally repressed but could be induced by addition of Mg2+. Maximal expression of yadA was obtained in exponential-phase cells grown in minimal medium at 37°C, conditions under which the invasin gene is repressed. These results suggest that YadA of Y. pseudotuberculosis constitutes another independent high-level uptake pathway that might complement other cell entry mechanisms (e.g., invasin) at certain sites or stages during the infection process.
Many pathogenic bacteria possess multifunctional non-pilus adhesins on the cell surface that mediate tight adhesion of the microbes to eukaryotic cells. These proteinaceous structures often also promote internalization of the bacteria into the eukaryotic cells following the adhesion process (41). Invasion may protect the bacteria against host clearance mechanisms and enable them to penetrate epithelial cell layers for subsequent dissemination. The enteropathogenic Yersinia spp. Yersinia enterocolitica and Y. pseudotuberculosis cause a number of enteric diseases ranging from enteritis, diarrhea, and lymphadenitis to autoimmune disorders (8). They possess two different non-pilus-associated adhesins, invasin and YadA, anchored to the outer membrane. In both organisms, they mediate initial adhesion, uptake, and transfer of the bacteria through M cells intercalating the epithelial layer of the intestine and establish the extracellular colonization of underlying lymphatic tissues and organs, such as the liver and spleen.
The surface protein invasin of Y. pseudotuberculosis was shown to be the most efficient factor that promotes binding and internalization of yersiniae into mammalian cells (28). It is particularly important for the initial step of invasion by its interaction with M cells (36, 43). Functional and structural analyses of Y. pseudotuberculosis invasin have shown that the N-terminal region of the protein is anchored in the outer membrane, whereas the C-terminal portion is surface exposed and forms five globular, predominantly β-stranded, subdomains that project from the outer membrane (20). The two most extreme C-terminal domains are absolutely required for cell penetration and promote cell attachment and entry by binding to at least five different members of the β1 integrin receptor family (27, 33). Furthermore, the second domain, extruding from the outer membrane, has the capacity to mediate invasin oligomerization and significantly enhances the invasin-mediated uptake process (17).
After invasion of the intestinal epithelial layer, the YadA protein seems to predominate as adhesin in infected tissue. YadA mediates adherence to epithelial cells, professional phagocytes, and extracellular matrix (ECM) proteins such as fibronectin and collagen. YadA also promotes autoagglutination and mediates serum resistance (18). It has been shown that YadA of Y. enterocolitica densely covers the bacterial surface by forming a capsule-like structure of lollipop-shaped surface projections, which might mask the lipopolysaccharides and protect the bacteria from the complement system and defensin lysis (23). The YadA polypeptides are thought to form oligomers with apparent molecular masses of 160 to 250 kDa, depending on the Yersinia species and the serotype (53). The lollipop structure of YadA of Y. enterocolitica consists of an outer membrane anchor domain at the C terminus, an intermediate segment forming a pillar-like stalk, and a bulky N-terminal head structure that promotes tight adherence to host cells (e.g., neutrophils) and ECM proteins. Although YadA promotes significant attachment to eukaryotic cells, its contribution to bacterial entry seemed to be relatively small and was only apparent in the absence of invasin (5, 57).
The yadA gene and a complex set of operons involved in the expression of virulence proteins known as Yersinia outer membrane proteins (Yops) are encoded on virulence plasmid pIB1. Two of the Yop proteins, YopE and YopH, directly antagonize the uptake process (46). YopE is a cytotoxin that disrupts the eukaryotic cytoskeleton by blocking actin polymerization and thereby prevents the host cell phagocytic mechanisms. YopH encodes a tyrosine phosphatase that interferes with cell signal transduction molecules, such as pFAK125 and pCas130, involved in the invasin-mediated uptake process (3, 4). The yopE, yopH, and yadA genes are coordinately controlled by VirF, a positive transcriptional activator protein, and are simultaneously induced by growth at 37°C (31). Because of its cell binding characteristics and its coexpression with the Yop proteins at 37°C, YadA was considered to be the dominant adhesin that mediates intimate attachment and colonization of host tissue after the transfer of yersiniae through M cells. Thereby, it would promote binding to neutrophils and macrophages underlying the M cells and could support the delivery of the anti-invasive YopH and YopE proteins and prevent uptake and killing by professional phagocytes.
For efficient invasion by yersiniae, a significant number of invasin molecules have to be accessible for the interaction with β1 receptors (17, 55). Maximal expression of invasin at moderate temperatures, conditions predominantly found outside the host, might guarantee rapid invasion and transcytosis through M cells after oral infection (40). However, induction of the major adhesin YadA at 37°C in the host will lead to the formation of surface projections (20 to 23 nm), slightly longer than invasin (18 nm), that will cover the outer membrane of the organism in a capsule-like fashion (20, 23). It has been shown that O-antigen production can interfere with the function of Y. pseudotuberculosis invasin (56); however, nothing is known about the interplay of YadA and invasin in the bacterial outer membrane. In this study, we addressed the possibility of competitive interference of YadA and invasin of Y. pseudotuberculosis and found that, even upon strong overexpression, invasin-mediated uptake is not negatively affected by the YadA protein. In contrast, our data demonstrated that YadA of Y. pseudotuberculosis constitutes a second highly efficient uptake pathway that is able to complement invasin-mediated cell entry, e.g., under conditions in which invasin synthesis is repressed.
MATERIALS AND METHODS
Bacterial strains, cell culture, media, and growth conditions.
The strains used in this study are listed in Table 1. Overnight cultures of Escherichia coli were routinely grown at 37°C, Yersinia strains were grown at 25°C in LB (Luria-Bertani) broth and minimal medium A (MMA) (38, 48) supplemented with 0.2% glucose and 0.2% Casamino Acids. The antibiotics used for bacterial selection were as follows: ampicillin, 100 μg/ml; chloramphenicol, 30 μg/ml; kanamycin, 50 μg/ml; gentamicin, 50 μg/ml.
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Description | Source or reference |
|---|---|---|
| Bacterial strains | ||
| E. coli K-12 CC118 | F− Δ(ara-leu)7697 Δ(lacZ)74 Δ(phoA)20 araD139 galE galK thi rpsE rpoB arfE(Am) recA1 | 34 |
| Y. pseudotuberculosis | ||
| YPIII | pIB1; wild type | 7 |
| YP1 | pIB1 inv::phoA(60)a Kanr | 40 |
| YP28 | pIB1 yadA::TnphoA(27)b Kanr | This study |
| YP29 | pIB1 yadA::Cm Cmlr | Dorothy Fallows |
| YP30 | pIB1 inv::Kan Kanr | Dorothy Fallows |
| YP31 | pIB1−inv::Tn10 Tetr | Dorothy Fallows |
| YP32 | pIB1 yadA::Cml inv::Kan Kanr Cmlr | Dorothy Fallows |
| Plasmids | ||
| pACYC184 | cloning vector; p15a Cmr Tetr | 12 |
| pAM226 | pACYC184 inv+ Cmr | 36 |
| pAY04 | pACYC184 yadA+ Cmr | 57 |
| pBAD18 | Cloning vector; pBAD Ampr | 19 |
| pGP704 | R6K-based suicide vector; Ampr | 39 |
| pPD259 | pAY04 yadA::TnphoA(27)a Cmr Kanr | This study |
| pPD260 | pGP704 yadA::TnphoA(27)a Ampr Kanr | This study |
| pPD284 | pBAD18 yadA+ Apr | This study |
| pRI203 | pBR325 inv+ Apr | 28 |
| pYV260 | pIB1 yadA::TnphoA(27)a Kanr | This study |
inv-phoA fusion expressed from the inv promoter; the number in parentheses indicates the codon of inv fused to phoA.
yadA-phoA fusion expressed from the yadA promoter; the number in parentheses indicates the codon of yadApst6 fused to TnphoA.
Human HEp-2 cells were cultured in RPMI 1640 medium (Invitrogen) supplemented with 5% newborn calf serum (Invitrogen) and 2 mM l-glutamine at 37°C in the presence of 5% CO2.
DNA manipulations and sequence analysis.
All DNA manipulations, restriction digestions, ligations, and transformations were performed by standard techniques as described previously (38, 48). Plasmid DNA was purified with QIAprep plasmid spin columns as described by the manufacturer. Restriction and DNA-modifying enzymes were purchased from Boehringer Mannheim or New England Biolabs and used in accordance with the manufacturer's instructions. The oligonucleotides used for amplification by PCR and sequencing were purchased from Metabion (Martinsried, Germany). PCRs were routinely performed with a 100-μl mixture volume for 25 cycles with AmpliTaq polymerase (Perkin-Elmer) or ProofStart polymerase (Qiagen) in accordance with the manufacturer's instructions in a Primus DNA thermal cycler (MWG-Biotech). PCR products were purified with the QIAquick kit (Qiagen) before and after restriction digestion of the amplification product. Sequencing reactions were performed by GATC (Constance, Germany).
The plasmids used in this study are listed in Table 1. Plasmids pPD259 and pPD260 are derivatives of pAY04 and pGP704 carrying a TnphoA insertion in codon 27 of the yadA gene, generating an in-frame yadA-phoA fusion (for construction details, see below). pPD284 was constructed by inserting an NruI-XbaI fragment of pAY04 containing the yadA gene of Y. pseudotuberculosis into pBAD18 cut with SmaI and XbaI.
Construction of the virulence plasmid-encoded yadA-phoA translational fusion.
To isolate yadA fusions to the phoA indicator gene, a Tn5 IS50L::phoA (TnphoA) derivative was inserted into yadA+ plasmid pAK04 by transposition as previously described (34). Active phoA+ Kanr colonies were selected, and insertions in the yadA gene were sequenced. One TnphoA insertion, yadA::TnphoA (27) in plasmid pPD259, generated an in-frame yadA-phoA fusion after codon 27 of yadA. This translational fusion, carried on a 5.0-kb NruI fragment of pPD259, was subcloned into the EcoRV site of suicide vector pGP704 (39). The resulting plasmid, pPD260, was transferred into Y. pseudotuberculosis YPIII, and transconjugants were selected on Yersinia selective agar (Oxoid) as described previously (39). The transconjugants (YP28) yielded a merodiploid virulence plasmid, pYP260 (yadA+ yadA-phoA), that harbored a wild-type copy of yadA and the yadA-phoA fusion. The appropriate genetic structure of the recombinant virulence plasmid was proven by Southern blot hybridization.
Alkaline phosphatase assays.
Alkaline phosphatase activity was measured in permeabilized cells as described previously (40). The activities were calculated as follows: optical density at 420 nm (OD420), × 6.46 × OD578 × Δt (minutes) × volume (milliliters). Assays of triplicate cultures grown under the conditions indicated were performed. Y. pseudotuberculosis YPIII assayed under the identical conditions had a low detectable background level of alkaline phosphatase activity that was subtracted from the values presented.
Isolation of outer membrane fractions.
The preparation of the outer membranes was performed with some modifications as previously described (7). Cultures (100 ml) were grown to stationary phase in LB medium and centrifuged at 4°C. The bacterial pellet was resuspended in 10 ml of TEM buffer (10 mM Tris-HCl, 5 mM EDTA [pH 7.8], 1 mM β-mercaptoethanol). Subsequently, the bacteria were lysed by being passed three times through a French press at 103,500 kPa. Cell debris were separated by low-speed centrifugation, and membranes were pelleted from the soluble fraction in a Sorvall OTD65B ultracentrifuge at 100,000 × g for 1 h. The membrane fraction was resuspended in 10 ml of SM buffer (0.5% Sarkosyl, 1 mM β-mercaptoethanol). Total membranes were incubated overnight at 4°C, and the solution was subsequently centrifuged at 100,000 × g for 1 h. The final outer membrane pellet was resuspended in 200 μl of sample buffer (100 mM Tris-HCl [pH 6.8], 2% sodium dodecyl sulfate [SDS], 10% glycerol, 3% dithiothreitol, 0.001% bromophenol blue) (48) and analyzed by gel electrophoresis.
Gel electrophoresis, preparation of cell extracts, and Western blotting.
Bacteria were grown under various environmental conditions as described above. The OD of the cultures was adjusted, and a 1-ml aliquot was withdrawn from each culture. The cells were collected by centrifugation, resuspended in 100 μl of sample buffer (48), and lysed at 95°C for 5 min. To reduce the viscosity of the cell extracts, 3 μl of Benzonase (Merck) was added to the samples, which were incubated at 37°C for 15 min. To visualize the YadA protein, the cell extracts were loaded onto SDS-10% polyacrylamide gels. The proteins were separated by electrophoresis and stained with Coomassie blue. The YadA protein was identified as 180- to 200-kDa protein complexes in the gel as described previously (7, 29), and the identities of the complexes were confirmed by Western blotting with a polyclonal antibody raised against the C-terminal fragment of YadA (see below). For immunological detection of invasin protein, the outer membrane preparation was electrophoretically separated on SDS-10% polyacrylamide gels and the proteins were transferred onto an Immobilon membrane (Millipore). The bound proteins were then probed with a monoclonal antibody (MAb), 3A2, directed against invasin (17). The antigen-antibody complexes were visualized with a secondary goat alkaline phosphatase antibody (Sigma) with 5-bromo-4-chloro-3-indolylphosphate and nitroblue tetrazolium (Boehringer Mannheim) as substrates.
Antibodies against YadA of Y. pseudotuberculosis.
Polyclonal antibodies to YadA were generated in a New Zealand White rabbit (Trenzyme, Constance, Germany). A 200-μg sample of a purified 332-amino-acid C-terminal fragment of the YadA protein in 1× sample buffer was injected subcutaneously into the rabbit. The rabbit was given boosters of 50 μg after 2 and 4 weeks, and at 5 weeks, the rabbit was sacrificed and the antiserum was collected. For further purification, the serum was incubated with precipitated cell lysates of a pIB1-cured Y. pseudotuberculosis strain. As a negative control, serum was taken from preimmune blood of the rabbit.
Cell adhesion and invasion assay.
In preparation of the cell adhesion and uptake assay, 5 × 10 4 HEp-2 cells were seeded and grown overnight in individual wells of 24-well cell culture plates (Nunc). Cell monolayers were washed three times with phosphate-buffered saline and incubated in binding buffer (RPMI 1640 medium supplemented with 20 mM HEPES [pH 7.0] and 0.4% bovine serum albumin) before the addition of bacteria. For the inhibitor studies, increasing concentrations (0 to 100 μM) of tyrphostin, genistein, herbimycin A (BRL Gibco), staurosporine, wortmannin, and cytochalasin D (Sigma) or different antibody dilutions (P4C10 against β1 integrins, 3E1 against human fibronectin) with or without human cellular fibronectin (Sigma) at 10 μg/ml were added to the binding buffer, which was incubated for 1 h at 37°C before the addition of bacteria. Approximately 5 × 106 bacteria were added to the monolayer, which was incubated at 20°C to test for cell binding or at 37°C to test for invasion (40). At 1 h postinfection, the cells were washed extensively with phosphate-buffered saline. The total number of adherent bacteria was determined by cell lysis with 0.1% Trizon X-100 and plating on bacterial medium. Bacterial uptake was assessed 60 min after infection as the percentage of bacteria that survived killing by the addition of the antibiotic gentamicin to the external medium, as described previously (17). For each strain, the relative level of bacterial adhesion and uptake was determined by calculating the number of CFU relative to the total number of bacteria introduced onto monolayers. The experiments were routinely performed in triplicate.
RESULTS
YadA expression does not interfere with invasin function.
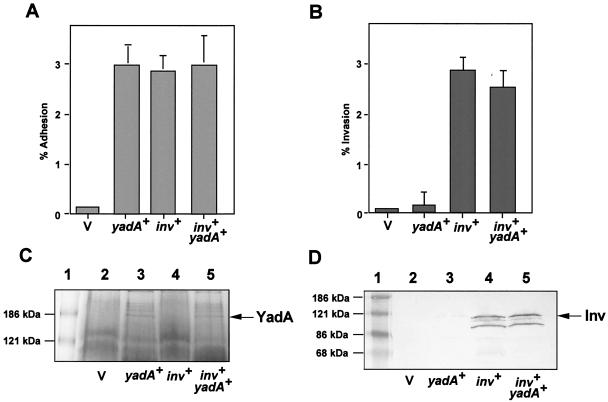
YadA and invasin are both anchored in the outer membrane of yersiniae. In order to test whether the synthesis of YadA negatively interferes with invasin function, we expressed both yadA and invasin simultaneously in E. coli K-12. This allowed the analysis of YadA- and invasin-mediated adhesion and uptake without other interfering components of the Yersinia virulence system (i.e., Yop proteins) known to affect the internalization process. To do this, we transformed medium-copy plasmids pAY04 (yadA+) and pRI203 (inv+) into E. coli strain CC118 and analyzed the adhesion and invasion of the resulting E. coli strains and compared them with those of E. coli expressing only YadA or invasin. As shown in Fig. 1A, the YadA- and invasin-mediated adhesion properties of the E. coli strains were almost identical (2.8 to 3%) and did not increase when both molecules were expressed simultaneously. Presumably, all of the bacteria that contacted the cells remained bound. Invasin-promoted uptake was about 3%, indicating that all of the bacteria that bound to the cells by invasin were also internalized (Fig. 1B). In contrast, YadA-mediated cell entry of E. coli was hardly detectable (<0.1%), which is in full agreement with previous reports on YadA-mediated cell entry (5, 22, 57). When both adhesive factors were expressed simultaneously, neither the adhesion nor the invasion efficiency of the invasin-mediated uptake process was significantly reduced (Fig. 1A and B). To ensure that the amounts of both invasin and YadA were unchanged in the different strains, outer membrane fractions of the bacterial cultures used for the assays were prepared and analyzed by SDS-polyacrylamide gel electrophoresis and Western blotting in parallel (Fig. 1C and D). As described previously (29, 30), the YadA molecules oligomerized into higher-molecular-weight complexes that could not be resolved in sample buffer and were easily detectable as 180- to 200-kDa protein complexes. Figure 1C and D further illustrate that the amounts of invasin and YadA in the outer membrane were identical, independent of whether the adhesins were expressed individually or together. These data demonstrate that, under these conditions, YadA expression affects neither the synthesis nor the export of the invasin protein into the outer membrane. Our results also indicate that there is no steric hindrance of the large complexes in the outer membrane, since YadA does not interfere with invasin-mediated cell entry.
FIG. 1.
YadA production and outer membrane incorporation do not interfere with invasin function. The cell adhesion (A) and invasion efficiency (B) of E. coli strains CC118/pACYC184, CC118/pRI203 (inv+), CC118/pAY04 (yadA+), and CC118/pAY04/pRI203 (inv+ yadA+) are documented. About 5 × 106 bacteria of an overnight culture grown at 37°C were used to challenge 5 × 104 HEp-2 cells, which were incubated for 1 h at 20°C to determine cell attachment or at 37°C to monitor invasion efficiency. The total number of surface-attached bacteria was determined by plating the bacteria after separation from cells, and the number of intracellular bacteria was determined by the gentamicin protection assay as previously described (17). Each value is the mean result of three independent assays done in triplicate. Outer membrane fractions of E. coli strains CC118/pACYC184 (lane 2), CC118/pAY04 (yadA+) (lane 3), CC118/pRI203 (inv+) (lane 4), and CC118/pAY04/pRI203 (inv+ yadA+) (lane 5) were tested. Outer membranes of the strains were prepared in parallel, and equal amounts were separated on a SDS-10% polyacrylamide gel and stained with Coomassie blue to visualize YadA (C) or transferred onto an Immobilon membrane to detect invasin with MAb 3A2 (D) (17). A prestained molecular weight marker was loaded in lane 1, and the positions of the marker proteins are indicated on the left. The positions of the invasin and YadA proteins are indicated on the right. V, vector.
Overexpression of the yadA gene dramatically enhances YadA-mediated cell entry.
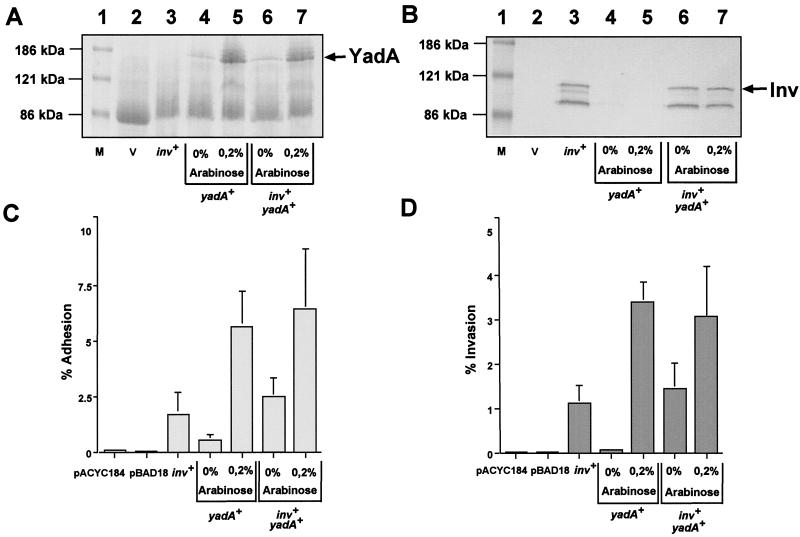
The previous experiment was performed with an E. coli strain expressing yadA from a low-copy plasmid. As efficient yadA transcription in yersiniae requires the virulence plasmid-encoded VirF activator protein, yadA expression from this plasmid might not be sufficient to mimic yadA induction in E. coli. To overcome this problem, the yadA gene was cloned under the control of the arabinose-inducible pBAD promoter (pPD284) (19). Similarly to the experiments described above, plasmids pAM226 (inv+) and/or pPD284 (pBAD::yadA) were transformed into E. coli K-12. To induce yadA expression, 0.2% arabinose was added to the bacterial growth medium. First, outer membrane fractions of the individual cultures were prepared and analyzed by SDS- polyacrylamide gel electrophoresis and Western blotting to compare the amounts of invasin and YadA in the bacterial cell envelope. Identical amounts of YadA protein were identified in the outer membranes of the yadA+ strain (CC118/pPD284) and the yadA+ inv+ strain (CC118/pPD284/pAM226) (Fig. 2A). Furthermore, the amount of YadA was significantly increased upon addition of arabinose and no significant difference in the amount of invasin was detected between the yadA mutant and yadA+ strains, even after induction of yadA expression (Fig. 2B). As the amount of neither invasin nor YadA was affected by the synthesis of the other adhesin, this system was suitable for analysis of whether an increase in YadA, mimicking capsule formation in vivo, reduces invasin-mediated uptake into epithelial cells. The adhesion and invasion of E. coli expressing YadA or/and invasin in the presence or absence of arabinose were tested. As expected, E. coli K-12 harboring the empty vectors pACYC184 and pBAD18 did not bind and enter HEp-2 cells. In contrast, E. coli expressing invasin (CC118/pAM226) showed significant adherence to HEp-2 cells (>2.0%) and 70% of the bound bacteria were internalized after 1 h (Fig. 2C). As expected, the adhesion rate of E. coli expressing small amounts of YadA (CC118/pPD284, without arabinose) was very low (0.5%) but the cell binding of this culture was increased 10-fold by the addition of arabinose. When both inv and yadA were expressed simultaneously, without or with arabinose, cell adhesion was about 2.5 and 7%, respectively. This is equivalent to the sum of the individual YadA- and invasin-mediated adhesion rates, indicating an additive effect. As shown in Fig. 2D, the invasion efficiency of the yadA+ strains was about 0.07% but increased dramatically to 3 to 3.5% (50-fold) upon the addition of arabinose, independently of whether invasin was present or not. Thus, induction of YadA expression seems to result in a dramatic increase in invasion efficiency, indicating that the adhesive molecule YadA represents a separate highly efficient invasive factor itself. In fact, by addition of increasing concentrations of arabinose (0 to 1.0%), augmenting the number of E. coli K-12 cells expressing YadA, invasion of HEp-2 cells could be continuously raised from 0.05 to 4% (data not shown).
FIG. 2.
Overexpression of the yadA gene dramatically enhances YadA-mediated cell entry. E. coli strains CC118/pACYC184 (lane 2), CC118/pAY04 (yadA+) (lane 3 and 4), CC118/pRI203 (inv+) (lane 3), and CC118/pAY04/pRI203 (inv+ yadA+) (lane 5 and 6) were grown in LB medium with or without 0.2% arabinose as indicated. Outer membranes of the strains were prepared and separated on an SDS-10% polyacrylamide gel, and the YadA (A) and invasin (B) proteins were detected as described in the legend to Fig. 1. A prestained molecular weight marker (M) was loaded in lane 1, and the positions of the standard proteins are indicated on the left. The positions of the invasin and YadA proteins are indicated on the right. The cell adhesion (C) and invasion efficiency (D) of the various E. coli strains were determined in parallel as described in the legend to Fig. 1. Each value represents the mean of three independent assays done in triplicate. V, vector.
Analysis of environmental control of yadA expression of Y. pseudotuberculosis.
Previous analysis showed that YadA and invasin constitute independent, complementary high-level uptake pathways that are likely to operate at different stages during infection. To analyze whether YadA might be active in different host environments than invasin, we wanted to characterize the environmental control of yadA expression in vitro and compare the regulation pattern with that of invasin analyzed in a previous study (40). To do this, we constructed a virulence plasmid-encoded yadA-phoA translational fusion and transferred it onto virulence plasmid pIB1. A single recombination event yielded merodiploid Y. pseudotuberculosis strain YP28 harboring the yadA-phoA fusion and the yadA wild-type copy on pIB1 (pYV260). The localization of the yadA-phoA fusion in YP28 was verified by Southern blot analysis (data not shown).
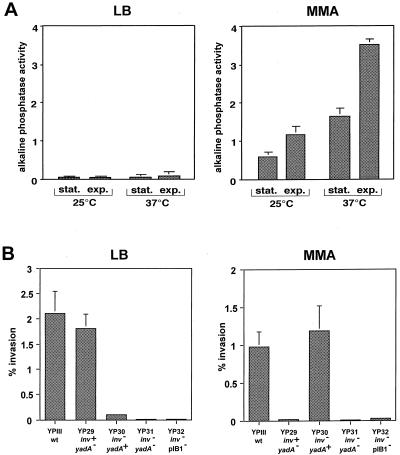
Several environmental factors, including temperature, growth phase, and growth media, have been described previously to affect invasin expression (40). To test their effect on yadA expression, we grew YP28 in complex medium (LB) or in bacterial MMA and determined the alkaline phosphatase activity of the cultures and compared them with regard to temperature and growth phase control. Under all of the conditions tested, yadA expression was strongly repressed in bacteria grown in LB medium whereas YadA synthesis was significantly induced in bacteria grown in MMA (Fig. 3A). A similarly induced yadA expression pattern was also found in other bacterial minimal media (M9, M63, and morpholinepropanesulfonic acid [MOPS]) and different cell culture media, such as RPMI 1640 medium and Dulbecco modified Eagle medium (data not shown). Furthermore, YadA synthesis in MMA was dependent on the temperature and growth phase of the culture. As shown in Fig. 3A, the highest level of yadA expression was found in exponential-phase cells grown at 37°C. This is in contrast to the invasin gene of Y. pseudotuberculosis, which was strongly repressed under these conditions and whose maximal expression has been demonstrated in stationary-phase cells grown in LB medium at 25°C (40).
FIG. 3.
Effect of growth medium on yadA expression and entry of Y. pseudotuberculosis into mammalian cells. (A) Strain YP28 (yadA-phoA) was grown in LB broth and MMA supplemented with 0.2% glucose and 0.2% Casamino Acids under the growth conditions indicated. Stationary-phase (stat.) bacteria had an OD600 of 4.0 to 4.5 and exponential-phase (exp.) cultures were harvested at an OD600 of 0.6 when alkaline phosphatase activity was determined. Each value represents the average ± the standard deviation of three different experiments performed in triplicate. (B) Strains YPIII (inv+ yadA+), YP29 (inv+ yadA::Cmr), YP30 (inv::Kanr yadA+), YP31 (inv::Kanr yadA::Cmr), and YP32 (inv::Kanr pIB1−) were grown in LB broth at 25°C or in minimal medium at 37°C overnight. About 5 × 106 bacteria were used to challenge 5 × 104 HEp-2 cells, which were incubated for 1 h at 20°C to determine cell attachment or at 37°C to monitor invasion efficiency. The total numbers of adherent and internalized bacteria were determined as described in the legend to Fig. 1. Each value represents the mean of three independent assays done in triplicate. wt, wild type.
Effects of Mg2+ and other ions on yadA expression.
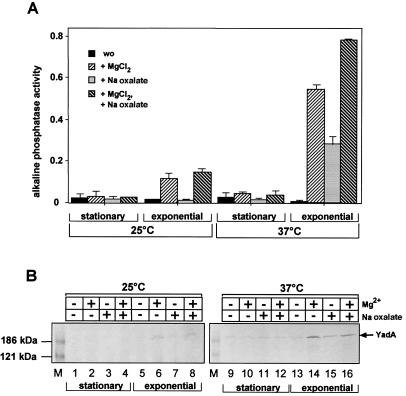
Previous work demonstrated that the production of the coordinately controlled Yop effector proteins only occurs in the absence of Ca2+ (31, 32, 52). Further regulation studies investigating the influence of ions on yadA expression revealed that YadA production is strongly Mg2+ dependent. This is especially apparent during growth in rich media, where expression of yadA can be induced more than 25-fold at certain ion concentrations. As shown in Fig. 4A, maximal yadA expression of YP28 in LB medium was detected in the presence of 20 mM MgCl2 and 20 mM Na oxalate, which reduce the amount of free Ca2+ ions in the growth medium. The expression of yadA under these conditions was induced primarily by the addition of Mg2+ ions but could be further increased by the reduction of free Ca2+ ions; the effect of both ions was additive. However, induction only occurred at 37°C when the cultures were in the exponential growth phase and the overall level of yadA expression was still significantly lower than during growth in minimal medium (Fig. 4A). The expression pattern correlated with the level of YadA in Y. pseudotuberculosis wild-type strain YPIII seen on Western blots (Fig. 4B). Large amounts of the YadA protein complexes could only be visualized in exponential-phase cells grown at 37°C in the presence of Mg2+, whereas very small amounts of YadA were detected when one condition (stationary phase or 25°C) was not inducing. These results were consistent with previous reports on temperature effects on the amount of YadA in the Y. pseudotuberculosis outer membrane (7, 29) and demonstrated that yadA expression is also strongly ion dependent and under growth phase control.
FIG. 4.
Effects of temperature, growth phase, and ion concentration on yadA expression. (A) Overnight and exponential-phase cultures of YP28 (yadA-phoA) grown at 25 or 37°C in LB medium were incubated without (wo) or with 20 mM MgCl2 or 20 mM Na oxalate or with both 20 mM MgCl2 and 20 mM Na oxalate for 3 h. Subsequently, the alkaline phosphatase activity of the cultures was determined. (B) Cell extracts from equal amounts of Y. pseudotuberculosis YPIII bacteria grown in LB medium under the conditions indicated were prepared, and the OD of the cultures was adjusted. Equal amounts of the cell extracts were separated on an SDS-10% polyacrylamide gel and transferred onto an Immobilon membrane. The YadA protein was detected by a polyclonal antibody directed against a C-terminal fragment of the YadA protein. Lanes: 1 to 8, YPIII grown at 25°C; 9 to 16, YPIII grown at 37°C. A minus sign indicates without, and a plus sign indicates with, the addition of 20 mM MgCl2 or 20 mM Na oxalate to the growth medium. A prestained protein molecular size marker was loaded on the left (M). The position of the YadA protein is indicated on the right.
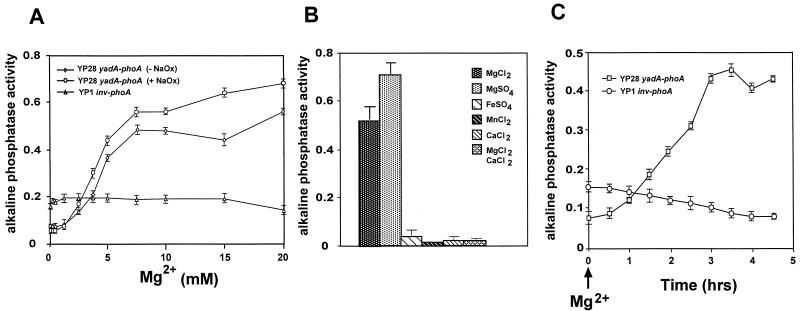
To further analyze the influence of Mg2+ on yadA expression, we gradually increased the amount of magnesium ions in the LB growth medium in the presence or absence of the Ca2+ chelator Na oxalate. As shown in Fig. 5A, yadA expression increased proportionally to the Mg2+ concentration of the growth medium. Maximal activation of yadA was achieved with an [Mg2+] of >7 mM, whereas growth with an [Mg2+] of <1 mM led to repression of yadA expression. Somewhat higher yadA expression levels occurred upon addition of Na oxalate. In contrast, the expression of a phoA control fusion to the inv gene (YP1) was not changed by the presence of Mg2+ and/or Na oxalate. Furthermore, yadA induction was independent of the anion and was induced by MgSO4 and MgCl2 to a similar level. Moreover, yadA activation at 37°C seems to be Mg2+ specific since the addition of other divalent cations, such as Mn2+, Ca2+, and Fe2+, had no inducing effect (Fig. 5B). However, Mg2+-mediated yadA induction could be abolished by the addition of high concentrations of CaCl2. We further investigated the kinetics of yadA induction by Mg2+ in vitro. Mg2+ was added to YP28 (yadA-phoA) 1 h after dilution in fresh LB medium incubated at 37°C, and the alkaline phosphatase activity of the fusion strain was determined at various times thereafter (Fig. 5C). The activity of the yadA-phoA fusion increased about 30 min after the addition of Mg2+. It rose constantly and reached a maximum after 3 h. In contrast, the inv-phoA control fusion of YP1 decreased slightly, which is consistent with the repression of inv expression in exponentially grown yersiniae described earlier (40).
FIG. 5.
Effects of Mg2+ and other ions on yadA expression. (A) The alkaline phosphatase activity of strain YP28 (yadA-phoA) was determined in exponential phase at 37°C at increasing concentrations of Mg2+ (0 to 20 mM) with or without 20 mM Na oxalate (NaOx). Strain YP1 (inv-phoA) was used as a control grown in increasing concentrations of Mg2+ (0 to 20 mM) with 20 mM Na oxalate. (B) The alkaline phosphatase activity of YP28 (yadA-phoA) was determined in exponential-phase cultures grown at 37°C in the presence of the divalent cations indicated. Each value represents the average ± the standard deviation of three different experiments performed in triplicate. (C) The alkaline phosphatase activity of YP28 (yadA-phoA) was determined in exponential-phase cells at 37°C after the addition of 20 mM MgCl2. Strain YP1 (inv-phoA) was used as a control.
Induction of yadA expression is sufficient to mediate high-level uptake of Y. pseudotuberculosis into mammalian cells.
Previous experiments demonstrated that yadA is significantly induced under growth conditions in which the invasin gene is fully repressed (40). This provided conditions for analysis of whether YadA of Y. pseudotuberculosis can compensate for invasin in an environment in which invasin is not active. To do this, we determined the capacity of various Y. pseudotuberculosis strains defective in the yadA or/and inv gene to adhere to and enter epithelial cells after their cultivation under conditions favoring either invasin or YadA expression. To induce invasin synthesis, the Yersinia strains were grown in LB medium at 25°C; to activate yadA expression, cultures were grown in minimal medium at 37°C. As shown in Fig. 3B, significant invasion efficiencies (>1.8%) of the cultures grown in LB medium were only detected with Y. pseudotuberculosis strains harboring the inv gene (YPIII [inv+ yadA+] and YP29 [inv+ yadA]). inv mutant strain YP30, however, showed significantly less uptake (0.1%), whereas inv yadA double-mutant strains (YP32 [inv yadA] and YP31 [inv pIB1−]) did not promote detectable entry of attached bacteria (<0.003%). In contrast, about 1% of the yadA+ Yersinia strains (YPIII [inv+ yadA+] and YP30 [inv yadA+]) grown in minimal medium were internalized into HEp-2 cells independent of the presence of the inv gene, whereas no invasion was observed with yadA mutant strains, even when the inv gene was present (YP29 [inv+ yadA], YP31 [inv pIB1−], and YP32 [inv yadA]; Fig. 3B). Our results indicate that the YadA protein can promote high-efficiency uptake of yersiniae independently of invasin after yadA expression is induced and a certain amount of the protein is exposed on the bacterial cell surface. A temperature of 37°C and nutrients/ions in the close vicinity of host tissues could trigger YadA synthesis in vivo.
YadA-mediated invasion of epithelial cells via fibronectin bound to β1 integrins.
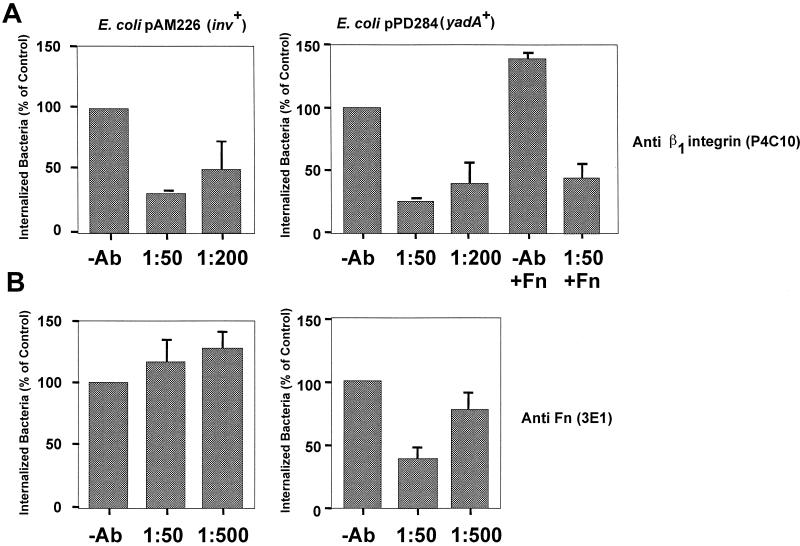
To compare YadA-mediated uptake and invasin-mediated uptake, we investigated the YadA entry mechanism in further detail. To determine whether high-level cell entry mediated by YadA occurs mainly via β1 integrin receptors like invasin (27), HEp-2 monolayers were preincubated with MAbs specific for β1 integrins and then challenged with E. coli K-12/pPD284 (yadA+) grown in the presence of arabinose. The ability of the antibodies to inhibit invasion was tested in a gentamicin protection assay. E. coli K-12/pAM226 expressing the invasin protein was used as a positive control. Figure 6A illustrates that increasing concentrations of MAb P4C10, which neutralized the activity of β1 integrins expressed by HEp-2 cells, significantly reduced both high-level YadA- and invasin-promoted cell entry. In contrast, a MAb against β3 or β4 integrins had no significant effect on bacterial uptake (data not shown). These results suggest that YadA mediated its biological activity mainly through the interaction with β1 integrin receptors.
FIG. 6.
YadA-mediated invasion of epithelial cells via fibronectin (Fn) molecules. About 5 × 104 HEp-2 cells were preincubated for 30 min at 37°C in RPMI 1640 medium supplemented with 0.4% bovine serum albumin and 20 mM HEPES (pH 7.0) without or with human cellular fibronectin (Fn) and for 30 min with certain dilutions of MAb (Ab) P4C10 directed against β1 integrins (A) or 3E1 directed against human fibronectin (B). Subsequently, about 5 × 106 bacteria of strains CC118/pAM226 (inv+), grown in LB medium, and CC118/pPD284 (yadA+), grown in LB medium supplemented with 0.2% arabinose, were added to the cell monolayer, which was incubated for 1 h at 20°C to determine cell attachment or at 37°C to monitor invasion efficiency. The total number of adherent bacteria was determined by plating the bacteria after separation from cells, and the number of intracellular bacteria was determined by the gentamicin protection assay as described in reference 17. Each value represents the mean of three independent assays done in triplicate.
As β1 integrins are transmembrane receptors for a variety of ECM proteins, such as collagen and fibronectin, that are efficiently bound by YadA (18), it seemed possible that interaction of YadA with integrins is indirect and occurs via an ECM bridging mechanism. To test this, we assayed uptake into HEp-2 cells with E. coli expressing YadA in the presence of MAb 3E1 directed against human fibronectin (Fig. 6B). Gentamicin protection assays revealed that addition of the antibody significantly reduced YadA-mediated uptake into HEp-2 cells in a concentration-dependent manner. In contrast, it did not reduce invasin-mediated bacterial uptake, which is known to be induced by direct interaction of the invasin protein with β1 integrin receptors. On the contrary, the antibody stimulated invasin-promoted cell entry, presumably by sequestering fibronectin competing with invasin for β1 integrin receptors. To further test the role of fibronectin, HEp-2 cells were preincubated with human cellular fibronectin in the presence and absence of function-blocking anti-β1 integrin MAb P4C10. Addition of fibronectin resulted in a slight increase in YadA-mediated invasion, which was significantly blocked when MAb P4C10 was added prior to fibronectin (Fig. 6A). In contrast, the anti β1 integrin antibody seemed to have no effect on YadA-mediated uptake when added after incubation with fibronectin (data not shown). According to these results, a sandwich model can be proposed in which YadA of yersiniae first interacts with the matrix molecule fibronectin, which subsequently associates with the cell adhesion receptors.
Efficient YadA-mediated invasion is impaired by inhibitors of tyrosine kinase, Ser/Thr kinase, and phosphatidylinositol 3-kinase (PI3K) activities.
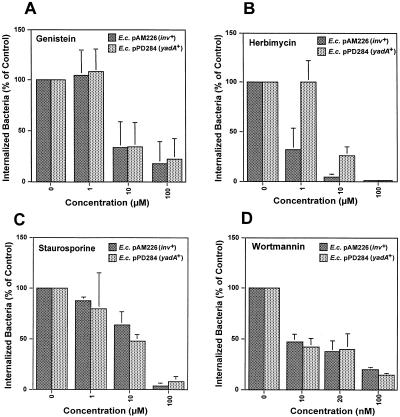
Further studies were done to identify host signaling events of human epithelial cells induced by the YadA-mediated invasion process of Y. pseudotuberculosis. Protein phosphorylation seems to be a key event in many receptor or bacterial internalization processes, including that of yersiniae (6, 45, 54). To investigate whether YadA-mediated entry of Y. pseudotuberculosis requires phosphorylation of cellular proteins, we preincubated HEp-2 cells with different amounts of the tyrosine kinase inhibitors herbimycin A (1 to 100 μM), tyrphostin (1 to 100 μM), and genistein (1 to 20 μM) and the Ser/Thr kinase inhibitor staurosporine (1 to 100 μM) prior to infection. As shown in Fig. 7 (data not shown for tyrphostin), all of the phosphokinase inhibitors tested significantly impaired invasion mediated by YadA or invasin, as judged by gentamicin survival assays 1 h after initiation of infection (Fig. 7). In contrast, the number of cell-associated bacteria and the viability of the cells were not reduced in the presence of the toxins (data not shown). Treatment of the HEp-2 cells with genistein, tyrphostin, and staurosporine caused inhibition of invasion by YadA or invasin in a similar manner (Fig. 7), whereas herbimycin, a toxin that significantly inhibits Src-like tyrosine kinases, was more effective on invasin-mediated uptake; 1 μM herbimycin A reduced cell entry of invasin-expressing bacteria to 40% but had no detectable effect on YadA-promoted bacterial uptake. This indicated that the signal transduction pathways induced during the YadA- and invasin-mediated entry processes are similar but may not involve entirely identical signaling molecules.
FIG. 7.
YadA-mediated invasion requires tyrosine kinases, Ser/Thr kinases, and the PI3K of the host cells. About 5 × 104 HEp-2 cells were preincubated for 30 min at 37°C in RPMI 1640 medium supplemented with 0.4% bovine serum albumin and 20 mM HEPES (pH 7.0) with increasing concentrations of the tyrosine kinase inhibitors genistein (A) and herbimycin (B), the Ser/Thr kinase inhibitor staurosporine (C), and the PI3K inhibitor wortmannin (D). Subsequently, about 5 × 106 bacteria of strains CC118/pAM226 (inv+), grown in LB medium, and CC118/pPD284 (yadA+), grown in LB medium supplemented with 0.2% arabinose, were added to the cell monolayer, which was incubated for 1 h at 37°C to monitor invasion efficiency. The number of intracellular bacteria was determined by the gentamicin protection assay as described in reference 17. Each value represents the mean of three independent assays done in triplicate. E.c., E. coli.
A signaling protein that couples tyrosine phosphorylation events to actin rearrangements during bacterial entry is PI3K (10). PI3K has been shown to be involved in the internalin B- and invasin-mediated uptake processes of Listeria monocytogenes and Y. enterocolitica, respectively (9, 49). To investigate whether activation of this PI3K activity is also required for YadA-mediated uptake, we tested the effect of the PI3K inhibitor wortmannin on entry via YadA. A 10 to 100 nM wortmannin concentration greatly reduced entry of the bacteria into HEp-2 cells (90% inhibition) (Fig. 7D). These results were similar to those obtained with the invasin-expressing E. coli strain and are in full agreement with previous studies on invasin-mediated uptake of yersiniae (37, 49). We conclude that YadA-promoted cell entry, like invasin-promoted cell entry, occurs through a PI3K-dependent signaling pathway and requires phosphokinases and actin polymerization.
DISCUSSION
Colonization of the interior surface of the intestine by enteropathogenic Yersinia spp. is an essential step in the process of infection. A variety of virulence factors, PsaA, Ail, YadA, and invasin, have been identified, all of which promote attachment of the bacteria to different cell types in vitro. Of these factors, invasin also allowed efficient uptake of yersiniae into mammalian cells (26, 43). In contrast, the YadA protein promoted significant attachment to eukaryotic cells but its contribution to bacterial entry, relative to that of invasin, seemed small (5, 22, 57). Experiments in this study demonstrated that YadA of Y. pseudotuberculosis is an adhesin that has the capacity to mediate very efficient high-level uptake into epithelial cells. Expression of yadA, driven by an inducible promoter, conferred high cell entry proficiency on E. coli K-12. Furthermore, a Yersinia mutant strain lacking the inv gene was still internalized efficiently into human cells; the prerequisite is that yadA expression is induced under the prevailing environmental conditions. Thus, it is possible that the function of YadA as a highly efficient invasive factor has been overlooked and/or underestimated when YadA was not fully expressed or active.
In this study, we analyzed the environmental signals that affect the synthesis of YadA and found that, in Y. pseudotuberculosis, yadA expression was maximally induced in bacterial minimal or cell culture medium. This is in full agreement with studies on Y. enterocolitica showing that the strongest synthesis of surface fibrillae could be seen with bacteria grown in minimal medium (29, 52). In contrast, in complex medium, yadA was generally repressed and could only be induced in exponential-phase cells at 37°C by the addition of Mg2+. Thus, yadA expression seems to follow an induction pattern that is the opposite of that of invasin of Y. pseudotuberculosis, which is predominantly expressed in stationary-phase bacteria grown in complex medium at 25°C (40). Previous analysis demonstrated that YadA synthesis correlates with the production of the virulence plasmid-encoded Yop effector proteins. It was shown that the transcriptional activator protein VirF activates and the histone-like protein YmoA negatively modulates yadA and yop transcription in response to temperature (31). The mechanism of VirF- and YmoA-mediated thermoregulation of yadA transcription seems to involve temperature-induced changes in the DNA topology of the yadA promoter, making it more accessible to VirF (13, 14). Whether this regulatory process is affected by the ion and nutrient contents of the medium and the bacterial growth phase is unknown. YadA production in Y. enterocolitica is also temperature regulated, but unlike the yop genes, it is independent of Ca2+ ions (29, 52). We found that in Y. pseudotuberculosis, the presence of high concentrations of Ca2+ seems to negatively affect yadA expression in LB medium. However, in contrast to the yop regulon, yadA expression in Y. pseudotuberculosis does not seem to be strictly dependent on the absence of Ca2+, since yadA is induced in defined media, such as M9, RPMI 1640, and Dulbecco modified Eagle medium, even in the presence of Ca2+ (P. Dersch, unpublished results). This indicates that temperature control and ion control constitute independent regulatory mechanisms that may allow YadA production independently of antiphagocytic Yop synthesis. The Mg2+ concentration is usually higher in the mammalian extracellular compartments (1 mM) than in the intracellular compartments (<100 μM) (44), and this could stimulate YadA synthesis outside cells. Since yadA induction by Mg2+ is relatively slow and the Mg2+ concentrations that affect yadA expression in vitro are rather high, it is unclear if the same signals modulate expression of yadA during the course of infection. It is likely that they mimic or support other environmental changes upon contact with host tissues.
In agreement with previous studies (50), our data indicate that YadA and invasin of Y. pseudotuberculosis establish two independent alternative pathways for cellular penetration, any of which could be used for translocation from the lumen of the intestine into the Peyer's patches before dissemination into the liver and spleen. Another major conclusion of this study is that incorporation of YadA into the bacterial outer membrane can replace invasin-mediated cell adhesion and entry. Presumably, if one pathway is repressed, the other could compensate for its absence. Invasin is maximally expressed under conditions found outside the host and may be advantageous for rapid and efficient transcytosis through the epithelial layer early in the infectious process (35, 43). Conversely, YadA synthesis is induced at 37°C and may function at a later time or at other sites during infection. The expression of independent uptake pathways may also explain why the absence of invasin in Y. pseudotuberculosis inv strains only delays but does not prevent the onset of disease after oral infection without overtly affecting the 50% lethal dose of the organism (36, 47). Y. pseudotuberculosis inv strains are extremely inefficient at translocation from the lumen of the intestine into the Peyer's patches and mesenteric lymph nodes upon oral inoculation, but the infection process may only be retarded until sufficient YadA and/or other uptake factors are produced to allow higher numbers of bacteria to translocate M cells and overwhelm host defenses. It is very likely that YadA is not the only invasive factor that can compensate for the loss of invasin function, since an inv yadA double mutant of Y. pseudotuberculosis is not avirulent and maintains almost the same virulence as the wild-type strain (21).
In contrast to YadA of Y. pseudotuberculosis, expression of yadA of Y. enterocolitica did not induce detectable uptake of adherent bacteria into HEp-2 cells; it reduced bacterial entry, most likely mediated by invasin (22). The reason for this difference is not clear, but it is possible that the YadA protein of Y. enterocolitica lacks determinants that are important for the ability of YadA of Y. pseudotuberculosis to promote cellular entry. Furthermore, different properties of the invasin molecule of enteropathogenic Yersinia species may contribute to the different phenotypes observed (16).
In analogy to invasin, YadA mediates bacterial cell entry via β1 integrin receptors, which link ECM components to the cytoskeleton (24). This is in agreement with a previous study by Bliska et al. demonstrating that YadA interaction with cells involves β1 integrins (5). Our antibody neutralization experiments gave a first indication that, in contrast to invasin, the YadA-β1 integrin interaction may not be direct but occurs via a trimolecular ECM bridging mechanism. First, YadA seems to interact with ECM molecules (e.g., fibronectin) synthesized and localized on the surface of the epithelial cells and presumably this interaction then triggers transmembrane and intracellular signaling cascades via the β1 integrin receptors bound to the ECM molecules. A number of other microbial pathogens are known to produce adhesive factors that bind ECM proteins and promote cell uptake by bridging ECM molecules. For instance, attachment and cell entry of Neisseria gonorrhoeae, Streptococcus pyogenes, and Staphylococcus aureus occur by interaction of their adhesins, e.g., Opa, FnBPs/Sfb1, and F1 protein, with vitronectin, fibronectin, or collagen (15, 42, 51).
Another important point concerns the intracellular signal transduction pathways induced by YadA binding to ECM-β1 integrin complexes. E. coli and yersiniae expressing small amounts of YadA promoted tight adhesion to mammalian cells but showed very low levels of invasiveness, whereas higher concentrations of YadA conferred high levels of invasiveness. Results obtained in recent years provide evidence that efficient bacterial internalization by invasin is determined by its high affinity for integrins. It also involves invasin-induced β1 integrin clustering, as a domain of invasin that promotes homotypic interaction significantly enhances bacterial uptake (17, 55). Similarly, YadA oligomers with multiple ECM-binding domains might interact with several ECM-β1 integrin complexes, inducing receptor multimerization. This would explain the capacity of YadA to mediate efficient cell entry only when larger amounts of the adhesin are exposed on the bacterial cell surface. Several tyrosine kinases have been shown to be activated by ligation of β1 integrin receptors during cell spreading on ECM, cell migration, and cell differentiation (24). We have shown that bacterial uptake promoted by YadA can be inhibited by the addition of protein tyrosine kinase inhibitors to epithelial cells, suggesting that interaction of YadA with β1 integrins also activates tyrosine kinases, which may then stimulate other signaling pathways downstream. Our inhibitor experiments with wortmannin further demonstrated that one of the signaling pathways seems to involve PI3K. This signaling protein is implicated in actin polymerization and endocytic events and is activated upon receptor stimulation and tyrosine phosphorylation. In fact, PI3K can interact with focal adhesion kinase pFAK125, which associates with β1 integrins (11). Its participation in induced bacterial uptake into mammalian cells has also been demonstrated for other pathogens; e.g., PI3K activation is among the early signal transduction events that take place during internalin B-promoted uptake of L. monocytogenes (25). It is striking that the effects of the inhibitors on cell entry via YadA are very similar to those of invasin (Fig. 7). Thus, it is likely that similar signal transduction pathways of the mammalian cells are induced during both the YadA and invasin internalization processes. The invasin-mediated uptake pathway seems to involve pFAK125 and c-Src, two signaling molecules regulating the association of integrins with the actin cytoskeleton, PI3K, the small GTPase Rac-1, and the Arp2/3 complex (1, 2). Whether these signaling molecules also participate in the YadA-mediated uptake pathway is unknown and will be the subject of further studies.
Acknowledgments
We thank Dorothy Fallows for strains. We also thank Birgitta Beatrix, Martin Fenner, and Eckhard Strauch for helpful discussions and critical reading of the manuscript. We thank Regine Hengge-Aronis for her generous support and interest.
This work was supported by grant DE 616/2-1 from the Deutsche Forschungsgemeinschaft and the Gottfried-Wilhelm Leibniz award given to Regine Hengge-Aronis.
Editor: J. D. Clements
REFERENCES
- 1.Alrutz, M. A., and R. R. Isberg. 1998. Involvement of focal adhesion kinase in invasin-mediated uptake. Proc. Natl. Acad. Sci. USA 95:13658-13663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alrutz, M. A., A. Srivastava, K. W. Wong, C. D'Souza-Schorey, M. Tang, L. E. Ch'ng, S. B. Snapper, and R. R. Isberg. 2001. Efficient uptake of Yersinia pseudotuberculosis via integrin receptors involves a Rac1-Arp 2/3 pathway that bypasses N-WASP function. Mol. Microbiol. 42:689-703. [DOI] [PubMed] [Google Scholar]
- 3.Andor, A., K. Trulzsch, M. Essler, A. Roggenkamp, A. Wiedemann, J. Heesemann, and M. Aepfelbacher. 2001. YopE of Yersinia, a GAP for Rho GTPases, selectively modulates Rac-dependent actin structures in endothelial cells. Cell Microbiol. 3:301-310. [DOI] [PubMed] [Google Scholar]
- 4.Black, D. S., and J. B. Bliska. 1997. Identification of p130Cas as a substrate of Yersinia YopH (Yop51), a bacterial protein tyrosine phosphatase that translocates into mammalian cells and targets focal adhesions. EMBO J. 16:2730-2744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bliska, J. B., M. C. Copass, and S. Falkow. 1993. The Yersinia pseudotuberculosis adhesin YadA mediates intimate bacterial attachment to and entry into HEp-2 cells. Infect. Immun. 61:3914-3921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bliska, J. B., J. E. Galan, and S. Falkow. 1993. Signal transduction in the mammalian cell during bacterial attachment and entry. Cell 73:903-920. [DOI] [PubMed] [Google Scholar]
- 7.Bolin, I., I. Norlander, and H. Wolf-Watz. 1982. Temperature-inducible outer membrane protein of Yersinia pseudotuberculosis and Yersinia enterocolitica is associated with the virulence plasmid. Infect. Immun. 37:506-512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bottone, E. J. 1997. Yersinia enterocolitica: the charisma continues. Clin. Microbiol. Rev. 10:257-276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Braun, L., H. Ohayon, and P. Cossart. 1998. The InIB protein of Listeria monocytogenes is sufficient to promote entry into mammalian cells. Mol. Microbiol. 27:1077-1087. [DOI] [PubMed] [Google Scholar]
- 10.Carpenter, C. L., and L. C. Cantley. 1996. Phosphoinositide 3-kinase and the regulation of cell growth. Biochim. Biophys. Acta 1288:11-16. [DOI] [PubMed] [Google Scholar]
- 11.Carpenter, C. L., and L. C. Cantley. 1996. Phosphoinositide kinases. Curr. Opin. Cell Biol. 8:153-158. [DOI] [PubMed] [Google Scholar]
- 12.Cohen, S. N., and A. C. Y. Chang. 1977. Revised interpretation of the origin of the pSC101 plasmid. J. Bacteriol. 132:734-737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cornelis, G. R. 1993. Role of the transcription activator VirF and the histone-like protein YmoA in the thermoregulation of virulence functions in yersiniae. Zentbl. Bakteriol. 278:149-164. [DOI] [PubMed] [Google Scholar]
- 14.Cornelis, G. R., C. Sluiters, I. Delor, D. Geib, K. Kaniga, C. Lambert de Rouvroit, M. P. Sory, J. C. Vanooteghem, and T. Michiels. 1991. ymoA, a Yersinia enterocolitica chromosomal gene modulating the expression of virulence functions. Mol. Microbiol. 5:1023-1034. [DOI] [PubMed] [Google Scholar]
- 15.Dehio, M., O. G. Gomez-Duarte, C. Dehio, and T. F. Meyer. 1998. Vitronectin-dependent invasion of epithelial cells by Neisseria gonorrhoeae involves alpha(v) integrin receptors. FEBS Lett. 424:84-88. [DOI] [PubMed] [Google Scholar]
- 16.Dersch, P., and R. Isberg. 2000. An immunoglobin superfamily-like domain unique to the Yersinia pseudotuberculosis invasin protein is required for stimulation of bacterial uptake via integrin receptors. Infect. Immun. 68:2930-2938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dersch, P., and R. R. Isberg. 1999. A region of the Yersinia pseudotuberculosis invasin protein enhances integrin-mediated uptake into mammalian cells and promotes self-association. EMBO J. 18:1199-1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.El Tahir, Y., and M. Skurnik. 2001. YadA, the multifaceted Yersinia adhesin. Int. J. Med. Microbiol. 291:209-218. [DOI] [PubMed] [Google Scholar]
- 19.Guzman, L. M., D. Belin, M. J. Carson, and J. Beckwith. 1995. Tight regulation, modulation, and high-level expression by vectors containing the arabinose PBAD promoter. J. Bacteriol. 177:4121-4130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hamburger, Z. A., M. S. Brown, R. R. Isberg, and P. J. Bjorkman. 1999. Crystal structure of invasin: a bacterial integrin-binding protein. Science 286:291-295. [DOI] [PubMed] [Google Scholar]
- 21.Han, Y. W., and V. L. Miller. 1997. Reevaluation of the virulence phenotype of the inv yadA double mutants of Yersinia pseudotuberculosis. Infect. Immun. 65:327-330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Heesemann, J., and L. Gruter. 1987. Genetic evidence that the outer membrane protein YOP1 of Yersinia enterocolitica mediates adherence and phagocytosis resistance to human epithelial cells. FEBS Microbiol. Lett. 40:37-41. [Google Scholar]
- 23.Hoiczyk, E., A. Roggenkamp, M. Reichenbecher, A. Lupas, and J. Heesemann. 2000. Structure and sequence analysis of Yersinia YadA and Moraxella UspAs reveal a novel class of adhesins. EMBO J. 19:5989-5999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hynes, R. O. 1999. Cell adhesion: old and new questions. Trends Cell Biol. 9:33-37. [PubMed] [Google Scholar]
- 25.Ireton, K., B. Payrastre, H. Chap, W. Ogawa, H. Sakaue, M. Kasuga, and P. Cossart. 1996. A role for phosphoinositide 3-kinase in bacterial invasion. Science 274:780-782. [DOI] [PubMed] [Google Scholar]
- 26.Isberg, R. R. 1989. Determinants for thermoinducible cell binding and plasmid-encoded cellular penetration detected in the absence of the Yersinia pseudotuberculosis invasin protein. Infect. Immun. 57:1998-2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Isberg, R. R., and J. M. Leong. 1990. Multiple beta 1 chain integrins are receptors for invasin, a protein that promotes bacterial penetration into mammalian cells. Cell 60:861-871. [DOI] [PubMed] [Google Scholar]
- 28.Isberg, R. R., D. L. Voorhis, and S. Falkow. 1987. Identification of invasin: a protein that allows enteric bacteria to penetrate cultured mammalian cells. Cell 50:769-778. [DOI] [PubMed] [Google Scholar]
- 29.Kapperud, G., E. Namork, and H.-J. Skarpeid. 1985. Temperature-inducible surface fibrillae associated with the virulence plasmid of Yersinia enterocolitica and Yersinia pseudotuberculosis. Infect. Immun. 47:561-566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kapperud, G., T. Vardund, E. Skjerve, E. Hornes, and T. E. Michaelsen. 1993. Detection of pathogenic Yersinia enterocolitica in foods and water by immunomagnetic separation, nested polymerase chain reactions, and colorimetric detection of amplified DNA. Appl. Environ. Microbiol. 59:2938-2944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lambert de Rouvroit, C., C. Sluiters, and G. R. Cornelis. 1992. Role of the transcriptional activator, VirF, and temperature in the expression of the pYV plasmid genes of Yersinia enterocolitica. Mol. Microbiol. 6:395-409. [PubMed] [Google Scholar]
- 32.Lee, V. T., S. K. Mazmanian, and O. Schneewind. 2001. A program of Yersinia enterocolitica type III secretion reactions is activated by specific signals. J. Bacteriol. 183:4970-4978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Leong, J. M., R. S. Fournier, and R. R. Isberg. 1990. Identification of the integrin binding domain of the Yersinia pseudotuberculosis invasin protein. EMBO J. 9:1979-1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Manoil, C., and J. Beckwith. 1986. A genetic approach to analyzing membrane protein topology. Science 233:1403-1408. [DOI] [PubMed] [Google Scholar]
- 35.Marra, A., and R. R. Isberg. 1996. Analysis of the role of invasin during Yersinia pseudotuberculosis infection of mice. Ann. N. Y. Acad. Sci. 797:290-292. [DOI] [PubMed] [Google Scholar]
- 36.Marra, A., and R. R. Isberg. 1997. Invasin-dependent and invasin-independent pathways for translocation of Yersinia pseudotuberculosis across the Peyer's patch intestinal epithelium. Infect. Immun. 65:3412-3421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mecsas, J., B. Raupach, and S. Falkow. 1998. The Yersinia Yops inhibit invasion of Listeria, Shigella and Edwardsiella but not Salmonella into epithelial cells. Mol. Microbiol. 28:1269-1281. [DOI] [PubMed] [Google Scholar]
- 38.Miller, J. H. 1992. A short course in bacterial genetic: a laboratory manual and handbook for Escherichia coli and related bacteria. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 39.Miller, V. L., and J. J. Mekalanos. 1988. A novel suicide vector and its use in construction of insertion mutations: osmoregulation of outer membrane proteins and virulence determinants in Vibrio cholerae requires toxR. J. Bacteriol. 170:2575-2583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nagel, G., A. Lahrz, and P. Dersch. 2001. Environmental control of invasin expression in Yersinia pseudotuberculosis is mediated by regulation of RovA, a transcriptional activator of the SlyA/Hor family. Mol. Microbiol. 41:1249-1269. [DOI] [PubMed] [Google Scholar]
- 41.Oelschlaeger, T. A. 2001. Adhesins as invasins. Int. J. Med. Microbiol. 291:7-14. [DOI] [PubMed] [Google Scholar]
- 42.Ozeri, V., I. Rosenshine, D. F. Mosher, R. Fassler, and E. Hanski. 1998. Roles of integrins and fibronectin in the entry of Streptococcus pyogenes into cells via protein F1. Mol. Microbiol. 30:625-637. [DOI] [PubMed] [Google Scholar]
- 43.Pepe, J. C., and V. L. Miller. 1993. The biological role of invasin during a Yersinia enterocolitica infection. Infect. Agents Dis. 2:236-241. [PubMed] [Google Scholar]
- 44.Reinhart, R. A. 1988. Magnesium metabolism. A review with special reference to the relationship between intracellular content and serum levels. Arch. Intern. Med. 148:2415-2420. [DOI] [PubMed] [Google Scholar]
- 45.Rosenshine, I., V. Duronio, and B. B. Finlay. 1992. Tyrosine protein kinase inhibitors block invasin-promoted bacterial uptake by epithelial cells. Infect. Immun. 60:2211-2217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rosqvist, R., C. Persson, S. Hakansson, R. Nordfeldt, and H. Wolf-Watz. 1995. Translocation of the Yersinia YopE and YopH virulence proteins into target cells is mediated by YopB and YopD. Contrib. Microbiol. Immunol. 13:230-234. [PubMed] [Google Scholar]
- 47.Rosqvist, R., and H. Wolf-Watz. 1986. Cytotoxic effects of virulent Yersinia pseudotuberculosis on HeLa cells, p. 229-240. In D. L. Lark (ed.), Protein-carbohydrate interactions in biological systems. Academic Press Inc., London, United Kingdom.
- 48.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 49.Schulte, R., R. Zumbihl, D. Kampik, A. Fauconnier, and I. B. Autenrieth. 1998. Wortmannin blocks Yersinia invasin-triggered internalization, but not interleukin-8 production by epithelial cells. Med. Microbiol. Immunol. 187:53-60. [DOI] [PubMed] [Google Scholar]
- 50.Simonet, M., D. Mazigh, and P. Berche. 1984. Growth of Yersinia pseudotuberculosis in mouse spleen despite loss of a virulence plasmid of mol. wt. 47 × 106. J. Med Microbiol. 18:371-375. [DOI] [PubMed] [Google Scholar]
- 51.Sinha, B., P. P. Francois, O. Nusse, M. Foti, O. M. Hartford, P. Vaudaux, T. J. Foster, D. P. Lew, M. Herrmann, and K. H. Krause. 1999. Fibronectin-binding protein acts as Staphylococcus aureus invasin via fibronectin bridging to integrin α5β1. Cell Microbiol. 1:101-117. [DOI] [PubMed] [Google Scholar]
- 52.Skurnik, M. 1985. Expression of antigens encoded by the virulence plasmid of Yersinia enterocolitica under different growth conditions. Infect. Immun. 47:183-190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Skurnik, M., I. Bolin, H. Heikkinen, S. Piha, and H. Wolf-Watz. 1984. Virulence plasmid-associated autoagglutination in Yersinia spp. J. Bacteriol. 158:1033-1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tang, P., I. Rosenshine, and B. B. Finlay. 1994. Listeria monocytogenes, an invasive bacterium, stimulates MAP kinase upon attachment to epithelial cells. Mol. Biol. Cell 5:455-464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tran Van Nhieu, G., and R. R. Isberg. 1993. Bacterial internalization mediated by β1 chain integrins is determined by ligand affinity and receptor density. EMBO J. 12:1887-1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Voorhis, D. L. Dillon, S. Formal, S. B., and R. R. Isberg. 1991. An O antigen can interfere with the function of the Yersinia pseudotuberculosis invasin protein. Mol. Microbiol. 5:317-325. [DOI] [PubMed] [Google Scholar]
- 57.Yang, Y., and R. R. Isberg. 1993. Cellular internalization in the absence of invasin expression is promoted by the Yersinia pseudotuberculosis yadA product. Infect. Immun. 61:3907-3913. [DOI] [PMC free article] [PubMed] [Google Scholar]