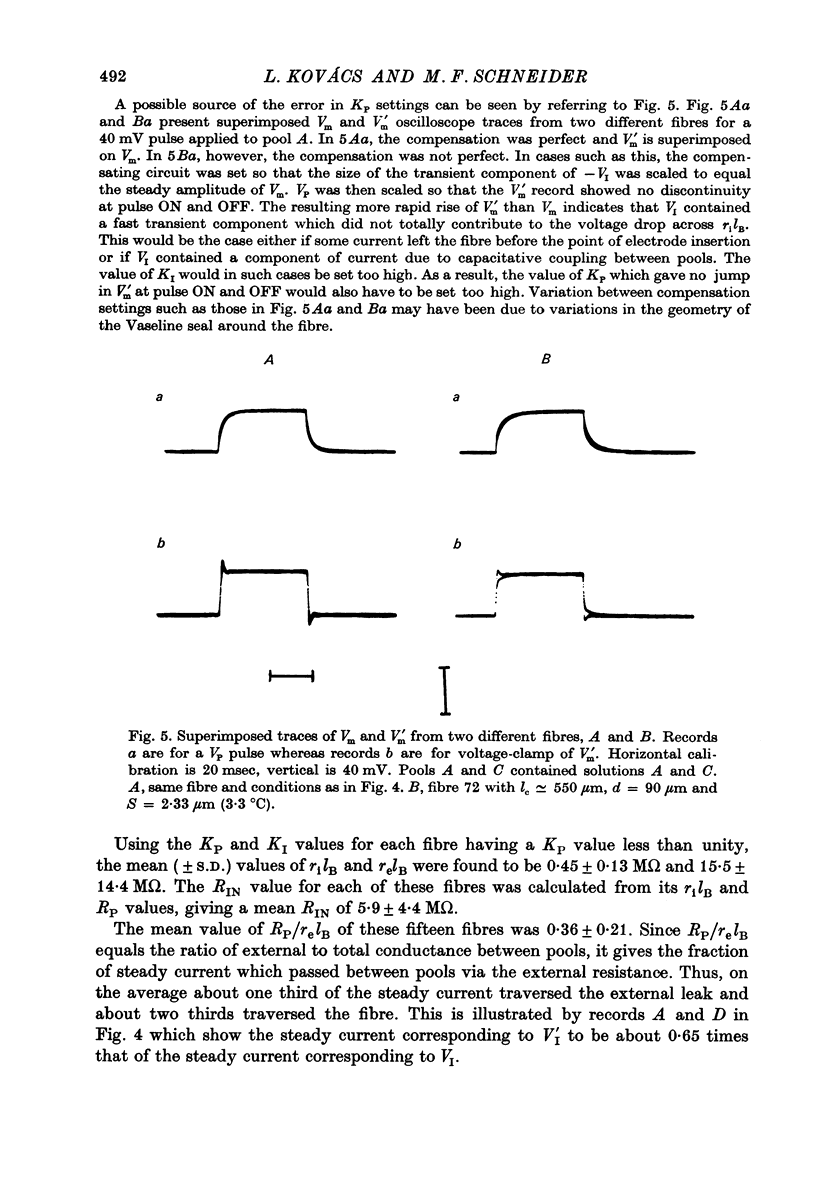
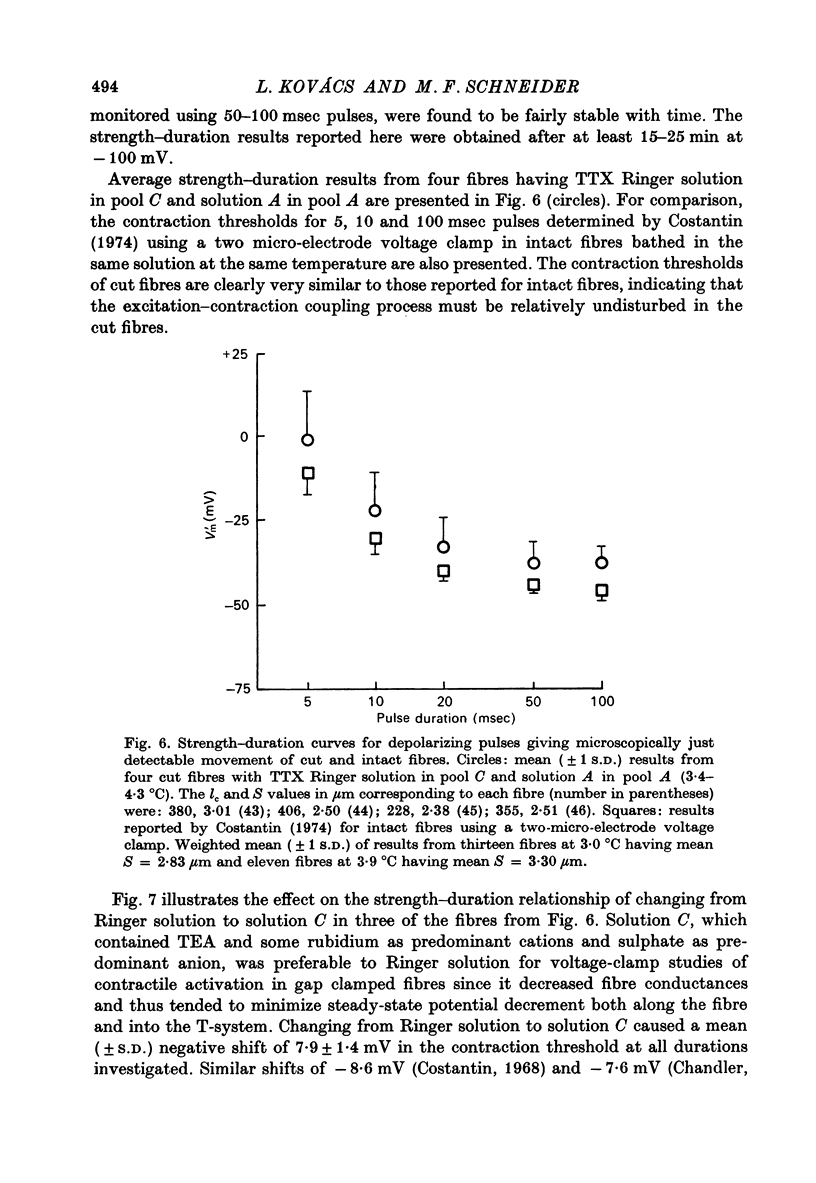
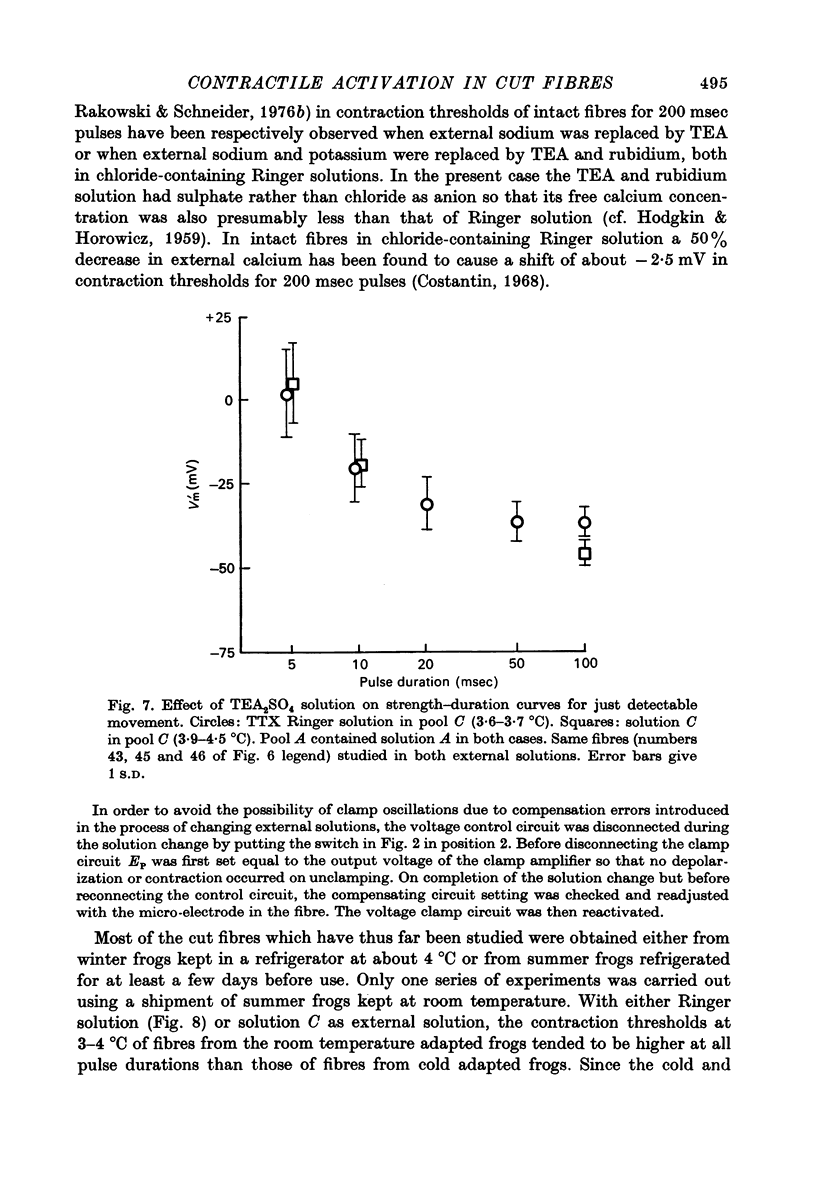
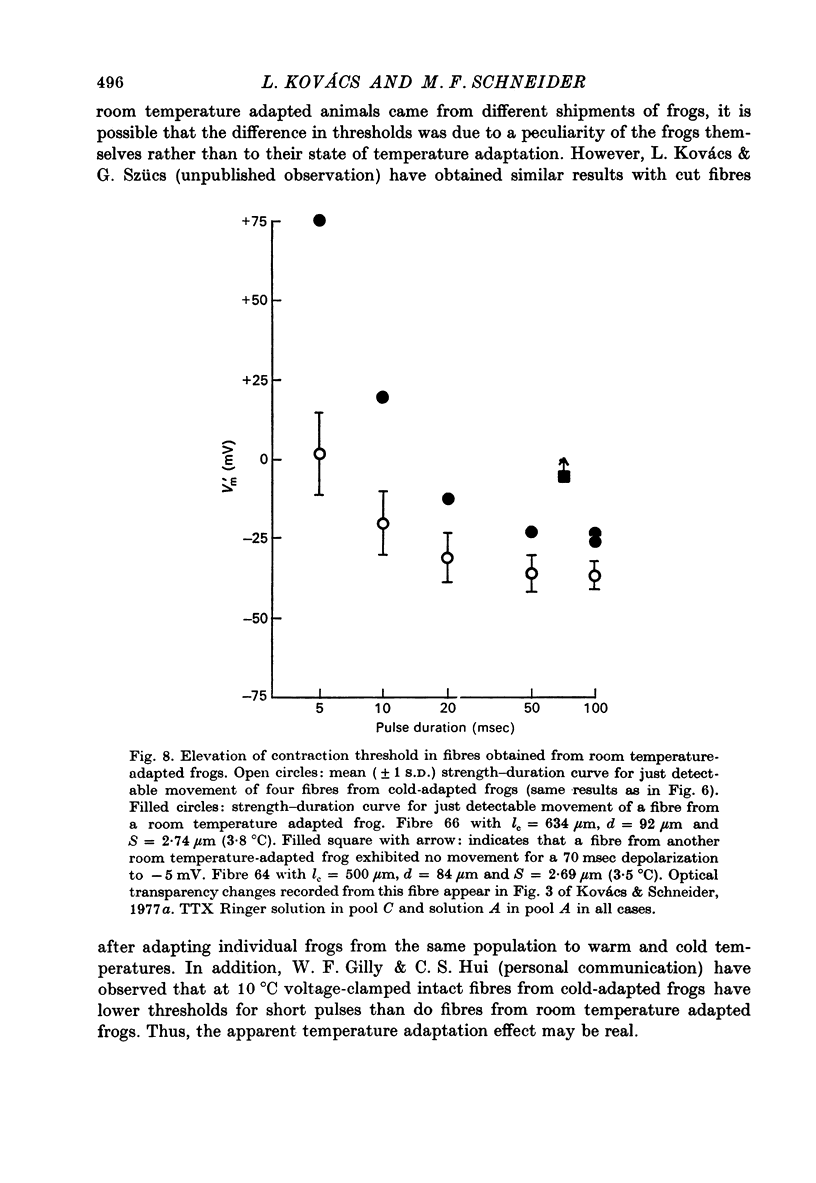
Abstract
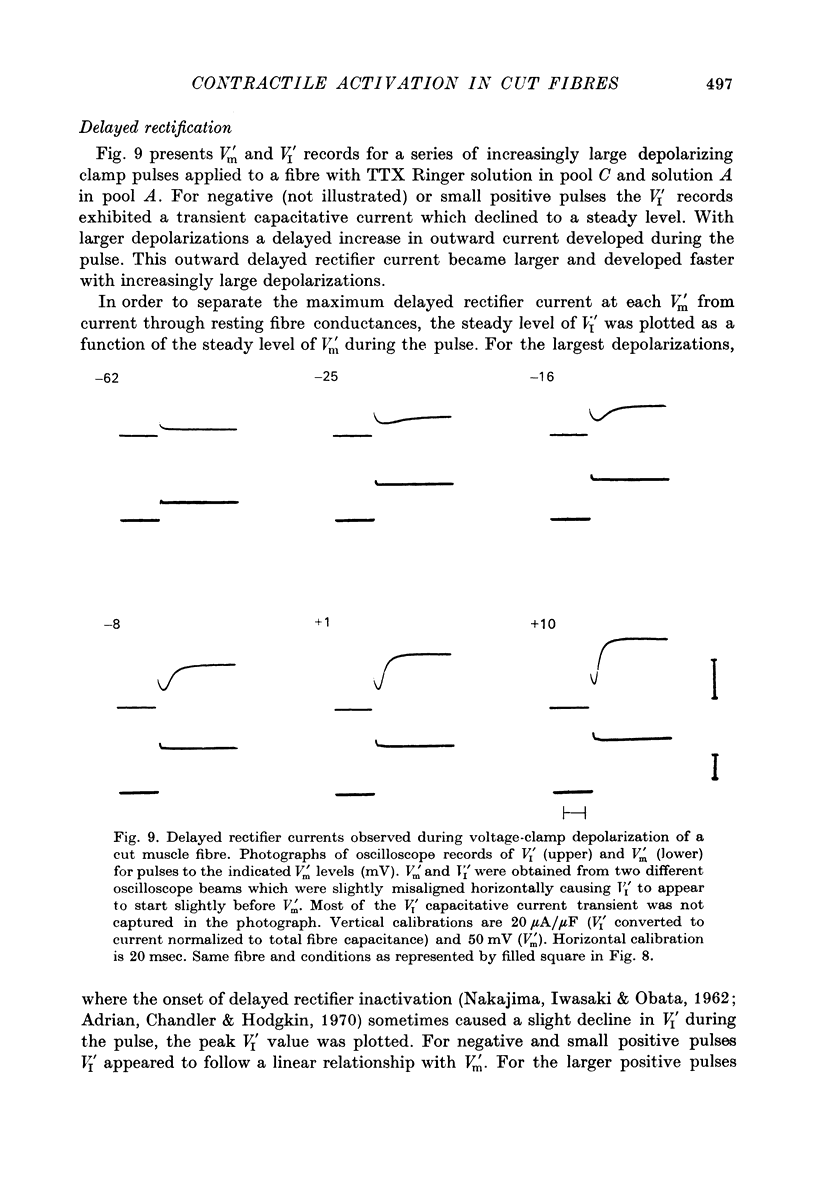
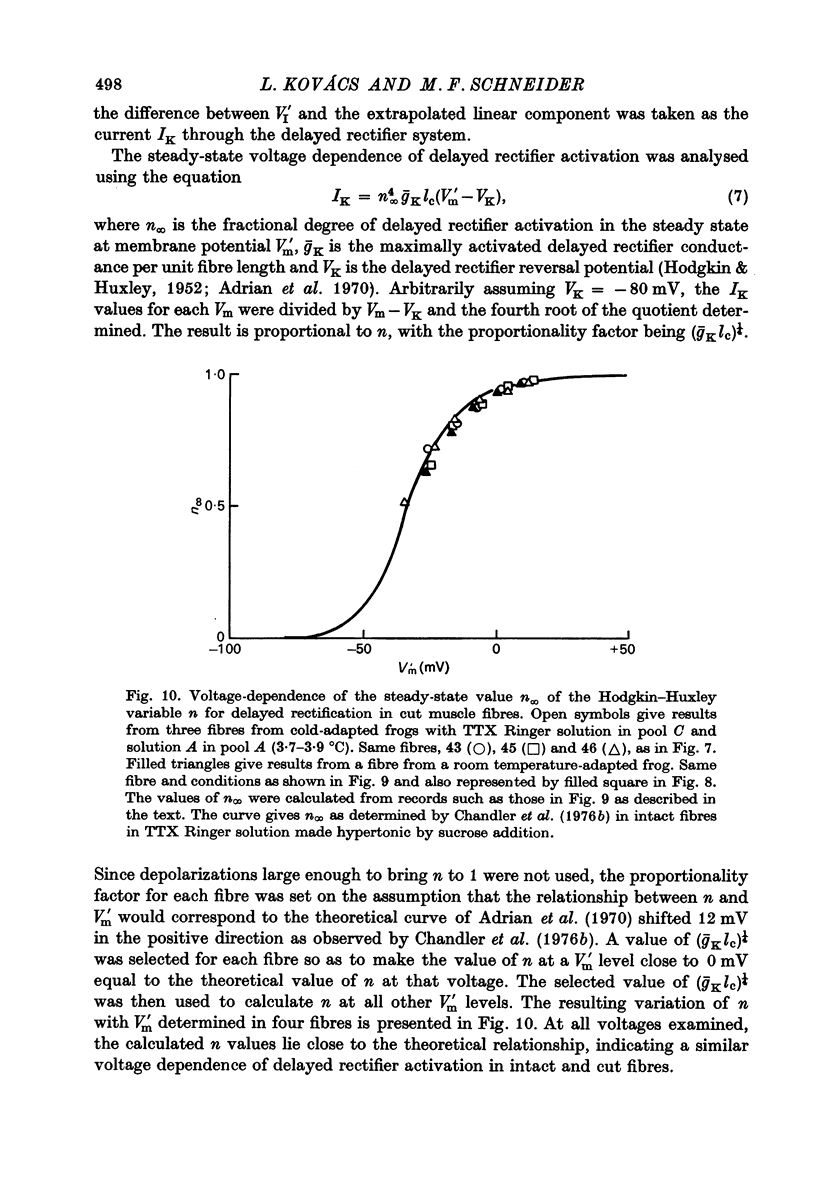
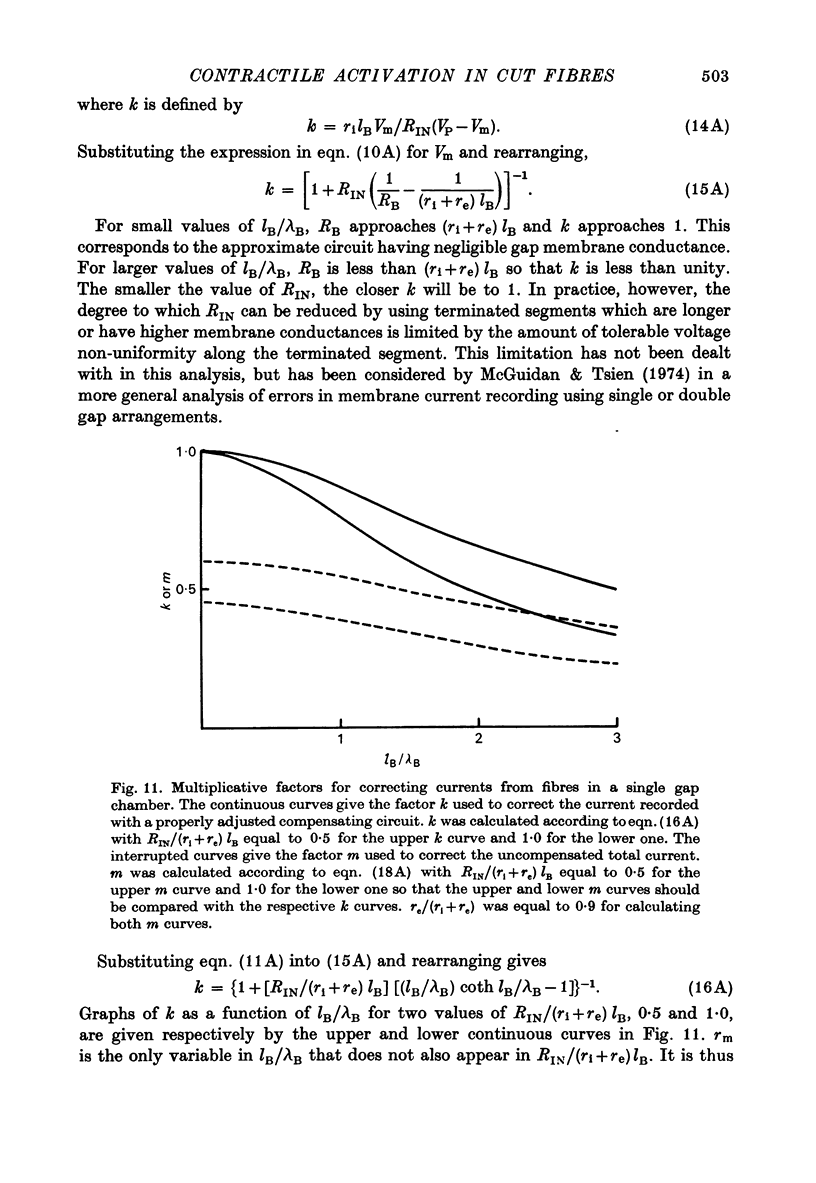
1. Single frog skeletal muscle fibres bathed in a relaxing solution were cut close to the tendon and mounted across a single Vaseline gap so that a short segment of intact terminated fibre extended beyond one side of the gap. 2. A compensating circuit, set with a micro-electrode in the terminated fibre segment, was used both to correct total current for external current crossing the gap and to correct pool voltage for the voltage drop across the fibre segment in the gap. 3. The micro-electrode was then removed and the fibre voltage-clamped using the compensating circuit. This allowed movement without damage under controlled voltage. 4. Strength-duration curves for contraction thresholds of cut fibres exposed externally to TTX Ringer solution and internally to a predominantly K glutamate solution were similar to strength-duration curves reported for intact fibres. 5. The change from TTX Ringer to a predominantly (TEA)2SO4 external solution shifted the strength-duration curve for cut fibre contraction thresholds in the negative direction as reported for intact fibres. 6. When studied at 3-4 degrees C, fibres from warm-adapted frogs appeared to have higher contraction thresholds than fibres from cold-adapted frogs. 7. Delayed rectifier currents recorded from cut fibres were similar to those reported for intact fibres.
Full text
PDF












Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adrian R. H., Chandler W. K., Hodgkin A. L. Voltage clamp experiments in striated muscle fibres. J Physiol. 1970 Jul;208(3):607–644. doi: 10.1113/jphysiol.1970.sp009139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adrian R. H., Freygang W. H. The potassium and chloride conductance of frog muscle membrane. J Physiol. 1962 Aug;163(1):61–103. doi: 10.1113/jphysiol.1962.sp006959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caputo C. The effect of caffeine and tetracaine on the time course of potassium contractures of single muscle fibres. J Physiol. 1976 Feb;255(1):191–207. doi: 10.1113/jphysiol.1976.sp011275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caputo C. The effect of low temperature on the excitation-contraction coupling phenomena of frog single muscle fibres. J Physiol. 1972 Jun;223(2):461–482. doi: 10.1113/jphysiol.1972.sp009858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler W. K., Rakowski R. F., Schneider M. F. A non-linear voltage dependent charge movement in frog skeletal muscle. J Physiol. 1976 Jan;254(2):245–283. doi: 10.1113/jphysiol.1976.sp011232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler W. K., Rakowski R. F., Schneider M. F. Effects of glycerol treatment and maintained depolarization on charge movement in skeletal muscle. J Physiol. 1976 Jan;254(2):285–316. doi: 10.1113/jphysiol.1976.sp011233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costantin L. L. Contractile activation in frog skeletal muscle. J Gen Physiol. 1974 Jun;63(6):657–674. doi: 10.1085/jgp.63.6.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costantin L. L., Podolsky R. J. Depolarization of the internal membrane system in the activation of frog skeletal muscle. J Gen Physiol. 1967 May;50(5):1101–1124. doi: 10.1085/jgp.50.5.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebashi S., Endo M. Calcium ion and muscle contraction. Prog Biophys Mol Biol. 1968;18:123–183. doi: 10.1016/0079-6107(68)90023-0. [DOI] [PubMed] [Google Scholar]
- Ebashi S., Endo M., Otsuki I. Control of muscle contraction. Q Rev Biophys. 1969 Nov;2(4):351–384. doi: 10.1017/s0033583500001190. [DOI] [PubMed] [Google Scholar]
- Endo M., Tanaka M., Ogawa Y. Calcium induced release of calcium from the sarcoplasmic reticulum of skinned skeletal muscle fibres. Nature. 1970 Oct 3;228(5266):34–36. doi: 10.1038/228034a0. [DOI] [PubMed] [Google Scholar]
- Ford L. E., Podolsky R. J. Calcium uptake and force development by skinned muscle fibres in EGTA buffered solutions. J Physiol. 1972 May;223(1):1–19. doi: 10.1113/jphysiol.1972.sp009830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAJDU S. Observations on the temperature dependence of the tension developed by the frog muscle. Arch Int Physiol. 1951 May;59(1):58–61. doi: 10.3109/13813455109146625. [DOI] [PubMed] [Google Scholar]
- HODGKIN A. L., HOROWICZ P. Potassium contractures in single muscle fibres. J Physiol. 1960 Sep;153:386–403. doi: 10.1113/jphysiol.1960.sp006541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., HOROWICZ P. The influence of potassium and chloride ions on the membrane potential of single muscle fibres. J Physiol. 1959 Oct;148:127–160. doi: 10.1113/jphysiol.1959.sp006278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F. A quantitative description of membrane current and its application to conduction and excitation in nerve. J Physiol. 1952 Aug;117(4):500–544. doi: 10.1113/jphysiol.1952.sp004764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F., KATZ B. Measurement of current-voltage relations in the membrane of the giant axon of Loligo. J Physiol. 1952 Apr;116(4):424–448. doi: 10.1113/jphysiol.1952.sp004716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUTTER O. F., NOBLE D. The chloride conductance of frog skeletal muscle. J Physiol. 1960 Apr;151:89–102. [PMC free article] [PubMed] [Google Scholar]
- HUXLEY A. F., TAYLOR R. E. Local activation of striated muscle fibres. J Physiol. 1958 Dec 30;144(3):426–441. doi: 10.1113/jphysiol.1958.sp006111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hille B., Campbell D. T. An improved vaseline gap voltage clamp for skeletal muscle fibers. J Gen Physiol. 1976 Mar;67(3):265–293. doi: 10.1085/jgp.67.3.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgkin A. L., Nakajima S. The effect of diameter on the electrical constants of frog skeletal muscle fibres. J Physiol. 1972 Feb;221(1):105–120. doi: 10.1113/jphysiol.1972.sp009742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jöbsis F. F., O'Connor M. J. Calcium release and reabsorption in the sartorius muscle of the toad. Biochem Biophys Res Commun. 1966 Oct 20;25(2):246–252. doi: 10.1016/0006-291x(66)90588-2. [DOI] [PubMed] [Google Scholar]
- Kovács L., Schneider M. F. Increased optical transparency associated with excitation--contraction coupling in voltage-clamped cut skeletal muscle fibres. Nature. 1977 Feb 10;265(5594):556–560. doi: 10.1038/265556a0. [DOI] [PubMed] [Google Scholar]
- McGuigan J. A. Some limitations of the double sucrose gap, and its use in a study of the slow outward current in mammalian ventricular muscle. J Physiol. 1974 Aug;240(3):775–806. doi: 10.1113/jphysiol.1974.sp010634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NAKAJIMA S., IWASAKI S., OBATA K. Delayed rectification and anomalous rectification in frog's skeletal muscle membrane. J Gen Physiol. 1962 Sep;46:97–115. doi: 10.1085/jgp.46.1.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima S., Bastian J. Double sucrose-gap method applied to single muscle fiber of Xenopus laevis. J Gen Physiol. 1974 Feb;63(2):235–256. doi: 10.1085/jgp.63.2.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima Y., Endo M. Release of calcium induced by 'depolarisation' of the sarcoplasmic reticulum membrane. Nat New Biol. 1973 Dec 19;246(155):216–218. doi: 10.1038/newbio246216a0. [DOI] [PubMed] [Google Scholar]
- New W., Trautwein W. Inward membrane currents in mammalian myocardium. Pflugers Arch. 1972;334(1):1–23. doi: 10.1007/BF00585997. [DOI] [PubMed] [Google Scholar]
- STEN-KNUDSEN O. Is muscle contraction initiated by internal current flow? J Physiol. 1960 May;151:363–384. doi: 10.1113/jphysiol.1960.sp006444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STEN-KNUDSEN O. The ineffectiveness of the `window field' in the initiation of muscle contraction. J Physiol. 1954 Aug 27;125(2):396–404. doi: 10.1113/jphysiol.1954.sp005167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider M. F., Chandler W. K. Effects of membrane potential on the capacitance of skeletal muscle fibers. J Gen Physiol. 1976 Feb;67(2):125–163. doi: 10.1085/jgp.67.2.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanfield P. R. The effect of the tetraethylammonium ion on the delayed currents of frog skeletal muscle. J Physiol. 1970 Jul;209(1):209–229. doi: 10.1113/jphysiol.1970.sp009163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor S. R., Rüdel R., Blinks J. R. Calcium transients in amphibian muscle. Fed Proc. 1975 Apr;34(5):1379–1381. [PubMed] [Google Scholar]


