Abstract
Host defense against Mycobacterium leprae infection is chiefly mediated by gamma interferon (IFN-γ)-secreting cytotoxic T cells. Since which antigen-presenting cell populations act to stimulate these T cells is not fully understood, we addressed the role of monocyte-derived dendritic cells (DCs). The DCs phagocytosed M. leprae and expressed bacterially derived antigens (Ags), such as phenolic glycolipid 1 (PGL-1), in the cytoplasm, as well as on the cell surface. The expression of HLA-ABC and -DR Ags on DCs was down-regulated by M. leprae infection, and that of CD86 was up-regulated, but not as fully as by Mycobacterium bovis BCG infection. Induction of CD83 expression required a large number of M. leprae cells. When a multiplicity of infection of >40 was used, the DCs induced a significant proliferative and IFN-γ-producing response in autologous T cells. However, these responses were significantly lower than those induced by BCG- or Mycobacterium avium-infected DCs. A CD40-mediated signaling in M. leprae-infected DCs up-regulated the expression of HLA Ags, CD86, and CD83 but did not enhance T-cell-stimulating ability. Therefore, M. leprae-infected DCs are less efficient at inducing T-cell responses. However, when the surface PGL-1 on M. leprae-infected DCs was masked by a monoclonal antibody, the DCs induced enhanced responses in both CD4+- and CD8+-T-cell subsets. M. leprae is a unique pathogen which remains resistant to DC-mediated T-cell immunity, at least in the early stages of infection.
Leprosy is a chronic infectious disease accompanied by irreversible peripheral-nerve damage and deformities (16, 17, 44). Since 1981, multidrug chemotherapy has been introduced by the World Health Organization for the elimination of leprosy in developing countries (51). However, at present, 2 to 3 million individuals are infected with Mycobacterium leprae, the causative agent of leprosy, and the detection of new cases continues to increase, reaching more than half a million cases each year (42, 52). Furthermore, no useful vaccines have been developed, and no successful immunotherapeutic tools against leprosy are yet available. Leprosy represents a type of disease in which clinical manifestations are associated with different levels of immune responses to M. leprae infection (36). One representative type is a tuberculoid leprosy, in which patients show cellular immunity against the bacteria and manifest a localized form of the disease with granulomatous pathological changes where a paucity of bacteria are observed. Another representative manifestation is lepromatous leprosy, in which patients show reduced levels or a complete lack of an effective cell-mediated immune response to M. leprae and suffer from more disseminated pathological changes in which an abundance of bacteria are usually involved.
Antigen (Ag)-specific gamma interferon (IFN-γ)-producing type 1 CD4+ T cells have been established as the host defense component most effective against infection by mycobacteria, such as Mycobacterium tuberculosis (1, 8, 32, 35). In addition, secreted IFN-γ plays an important role as an agent associated with activation of macrophages and intracellular bacterial killing (18, 28). However, quite recently, T-cell populations other than CD4+ T cells have been reevaluated with regard to protective antimycobacterial immunity (2, 20, 21, 41, 45). There is increasing evidence that mycobacterium-specific CD8+ T cells act not only as IFN-γ-secreting cells but also as a direct effector population (33, 43, 47). In the latter process, the activated CD8+ T cells kill mycobacteria through the actions of both perforin, a cytolytic molecule present in cytotoxic-T-lymphocyte granules, and granulysin, an antimicrobial peptide. Upon lysis of mycobacterium-infected cells, bacteria can be released, but those that escape from the actions of perforin and granulysin may be phagocytosed by macrophages, in which they are killed by IFN-γ-mediated mechanisms. However, it is still not fully determined which Ag-presenting cell (APC) populations work as stimulators of CD8+ T cells. Sieling et al. (39) reported recently that CD1+ CD83+ monocyte-derived dendritic cells (DCs) were observed in tuberculoid lesions of leprosy patients, and Yamauchi et al. (53) reported that T cells found in tuberculoid leprosy lesions expressed CD40 ligand, an important factor associated with the maturation and activation of DCs. These reports suggest that DCs are involved in protective immunity against M. leprae infection. Furthermore, among many well-known APCs, DCs are thought to be the most potent, since they can stimulate both naive and memory CD4+ and CD8+ T cells. The role of DCs in the development of various diseases and in the host defense against many pathological agents, including human T-lymphotropic virus type I, has been reported (24, 25).
In this study, we examined the sensitivity of monocyte-derived DCs from healthy individuals to M. leprae infection and also investigated the influence of mycobacterial infection on the APC function of DCs.
MATERIALS AND METHODS
Preparation of cells and bacteria.
Peripheral blood was provided under informed consent by >10 healthy but purified-protein-derivative-positive individuals. Peripheral blood mononuclear cells (PBMCs) were isolated using Ficoll-Paque Plus (Pharmacia, Uppsala, Sweden) and cryopreserved in liquid nitrogen until they were used, as previously described (23). Monocyte-derived DCs were differentiated from peripheral plastic-adherent cells as described previously (23, 24). Briefly, CD3+ T cells were removed in advance from either freshly isolated heparinized blood or cryopreserved PBMCs using immunomagnetic beads coated with an anti-CD3 monoclonal antibody (MAb) (Dynabeads 450; Dynal, Oslo, Norway). The CD3− fraction of the PBMCs was plated on collagen-coated plates and cultured for 60 min at 37°C. The non-plastic-adherent cells were then removed by extensive washing, and the remaining adherent cells were used as precursors of DCs. The plastic-adherent cells were cultured with 3 ml of RPMI 1640 medium containing 10% fetal calf serum and 1% penicillin G (Katayama Chemical, Osaka, Japan) for 5 days in the presence of 50 ng of recombinant granulocyte-macrophage colony-stimulating factor (Pepro Tech EC Ltd., London, England) and 10 ng of recombinant interleukin-4 (IL-4) (Pepro Tech) per ml. Recombinant granulocyte-macrophage colony-stimulating factor and recombinant IL-4 were supplied every 2 days, and 400 μl of medium was replaced as described previously (23, 24). In some cases, bacterium-infected or uninfected DCs were further treated with maturation and activation factors, including 10 μg of anti-CD40 MAb (PharMingen International, San Diego, Calif.) per ml, followed by overnight incubation in the presence of anti-mouse immunoglobulin G (IgG) polyclonal Ab (Jackson Immuno Research, West Grove, Pa.) or IL-12 (R&D Systems, Minneapolis, Minn.), IFN-γ (Genzyme/Techne, Cambridge, Mass.), lipopolysaccharide (LPS) (Escherichia coli 0111:B4; Difco Laboratories, Detroit, Mich.), tumor necrosis factor alpha (Boehringer Mannheim GmbH, Mannheim, Germany), and poly(I · C) (Amersham Pharmacia Biotech UK Ltd., Little Chalfont, Buckinghamshire, United Kingdom). Maturation of DCs was also conducted using a combination of anti-CD40 MAb and other reagents. Macrophages were also produced from plastic-adherent cells by culturing them in the presence of 20% fetal calf serum. CD4+ and CD8+ T cells, which were autologous to the DCs, were negatively purified from cryopreserved PBMCs by using immunomagnetic beads coated with MAbs to CD8 and CD4, respectively.
M. leprae cannot be cultivated or grown in vitro; therefore, M. leprae (Thai 53) was maintained and grown in BALB/c nu/nu mice. The bacteria isolated from the footpads of mice inoculated with M. leprae 1 year previously were counted by the method of Shepard and McRae (38) and were frozen at −80°C until they were used. The viability of M. leprae was 60% as assessed by a fluorescent diacetate-ethidium bromide test (18). Mycobacterium bovis BCG (Pasteur) and Mycobacterium avium (JATA 51) were used as control bacteria. They were cultured in vitro using Middlebrook 7H9 broth supplemented with 0.05% Tween 80 and albumin-dextrose-catalase. Both macrophages and DCs were counted and subsequently infected with bacteria by coculturing them at an appropriate multiplicity of infection (MOI). The MOI was determined based upon an assumption that all macrophages and DCs were susceptible to mycobacterial infection.
Analysis of cell surface and intracellular Ags.
Infection of DCs with M. leprae was assessed by staining M. leprae by the Ziehl-Neelsen method.
Evaluation of the phagocytosis of M. leprae by DCs was done using fluorescein isothiocyanate (FITC)-conjugated M. leprae. Bacteria (109/ml) were labeled by incubation with 0.5 mg of FITC per ml in 0.1 M carbonate buffer (pH 9.0) at 37°C for 2 h (13). The FITC-conjugated bacteria were washed three times and pulsed to immature DCs. Phagocytosis of M. leprae was determined using a fluorescence-quenching technique as reported previously (6, 11). In brief, quenching of nonphagocytosed membrane-bound FITC-conjugated M. leprae was done by treating the cells with 0.06% trypan blue for 5 min at 4°C.
The expression of cell surface Ags on DCs and macrophages was determined using a FACScalibur (Becton Dickinson Immunocytometry Systems, San Jose, Calif.). Dead cells were eliminated from the analysis by staining them with propidium iodide (Sigma Chemical Co., St. Louis, Mo.), and 104 live cells were analyzed. For analysis of cell surface Ags, the following MAbs were used: FITC-conjugated MAbs against HLA-ABC (G46-2.6; PharMingen), HLA-DR (L243; Becton Dickinson), CD14 (Leu-M3; Becton Dickinson), and CD40 (5C3; PharMingen); phycoerythrin-labeled MAbs against CD86 (IT2.2; PharMingen) and CD83 (HB15a; Immunotech, Marseille, France); and purified murine MAbs aainst CD1a (NS1/34; Serotec, Oxford, United Kingdom) and phenolic glycolipid-1 (PGL-1) (DZ2C11; a generous gift of H. Minagawa, Leprosy Research Center, Tokyo, Japan); they were followed by FITC-labeled goat F(ab′)2 anti-mouse IgG (Tago-immunologicals, Camarillo, Calif.). We also used sera from leprosy patients (generously provided by H. Minagawa); the sera (1 ml from each of 10 patients) were pooled and used to detect M. leprae-derived Ags, which were followed by FITC-conjugated murine anti-human immunoglobulins (Tago-immunologicals). In order to determine infection by M. leprae of DCs and macrophages, we performed intracellular staining of PGL-1 using FACScalibur. Briefly, DCs and macrophages pulsed with various doses of M. leprae were fixed in 2% formaldehyde and permeabilized using lysing solution (Becton Dickinson) and permeabilizing solution (Becton Dickinson). The fixed and pretreated cells were stained with anti-PGL-1 MAb, followed by FITC-labeled murine anti-human immunoglobulin Ab. The optimal concentrations of MAbs and patients' pooled sera were determined in advance.
Assessment of APC function of bacterium-infected DCs.
The ability of bacterium-infected DCs to stimulate autologous T cells was assessed using a mixed DC-autologous-T-cell reaction. DCs infected with bacteria for 48 h were treated with 50 μg of mitomycin C/ml and washed extensively to remove extracellular bacteria by centrifugation at 140 × g for 10 min and were used as stimulators. CD4+ and CD8+ T cells purified using immunomagnetic beads coated with MAbs were used as a responder population. Responder cells (105 per well) were plated in 96-well round-bottom tissue culture plates, and DCs were added to give a DC/responder CD4+-T-cell ratio of 1:20, 1:40, or 1:80 and a DC/responder CD8+-T-cell ratio of 1:10, 1:20, or 1:80. The T-cell proliferation during the last 10 h of a 4-day culture in the presence of 4% heat-inactivated human serum (a generous gift from Kagoshima Red Cross Blood Center) was quantified by incubating the cells with 1 μCi of [3H]thymidine/well. The results were expressed as the mean difference in counts per minute obtained from triplicate cultures.
Assessment of cytokine production.
The levels of the following cytokines were measured: IFN-γ and IL-10 produced by CD4+ and CD8+ T cells stimulated with M. leprae, M. avium, or M. bovis BCG-infected DCs, and IL-12 p70 and IL-10 produced by bacterium-infected DCs. Supernatant from DCs cocultured with T cells for 4 days and the 24-h culture supernatant of bacterium-infected DCs were collected, and the concentrations of cytokines were measured using an enzyme immunoassay. The quantification of IFN-γ was carried out using purified mouse anti-human IFN-γ MAb (NIB42; PharMingen International), biotinylated mouse anti-human IFN-γ MAb (4SB3; PharMingen International), and recombinant human IFN-γ protein (PharMingen International). The concentrations of IL-12 p70 and IL-10 were quantified using the enzyme assay kit Opt EIA Human IL-12 (p70) SET or Opt EIA Human IL-10 SET, respectively, available from PharMingen International.
Statistical analysis.
Student's t test was applied to demonstrate statistically significant differences.
RESULTS
Sensitivity of monocyte-derived DCs to infection with M. leprae in vitro.
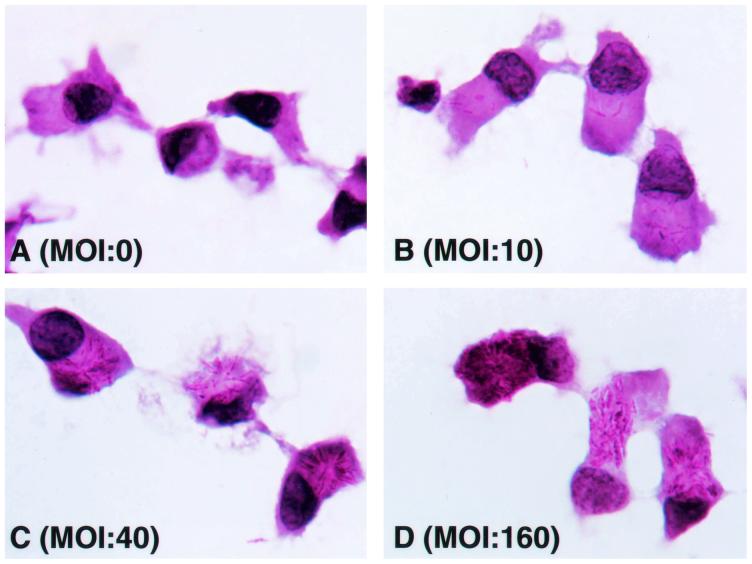
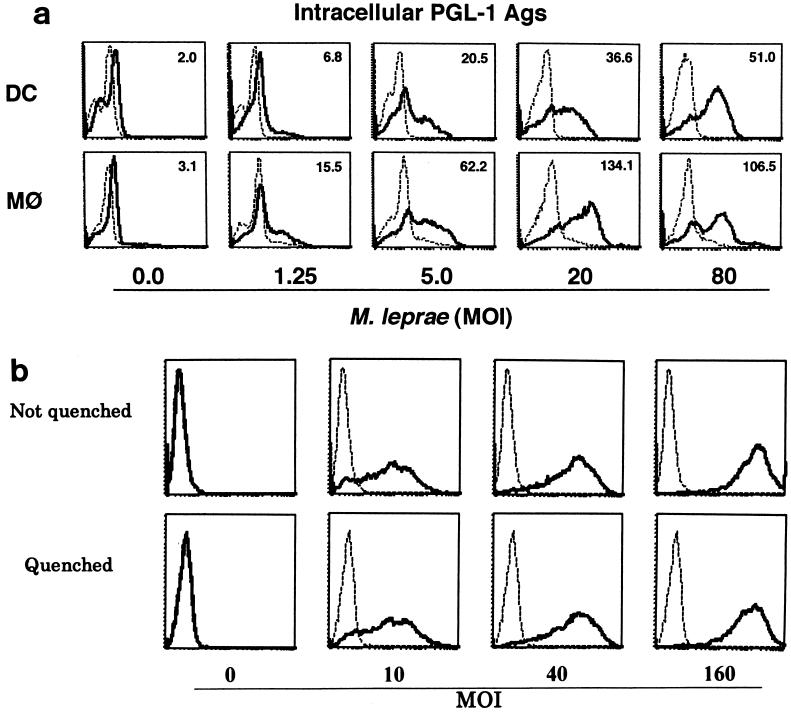
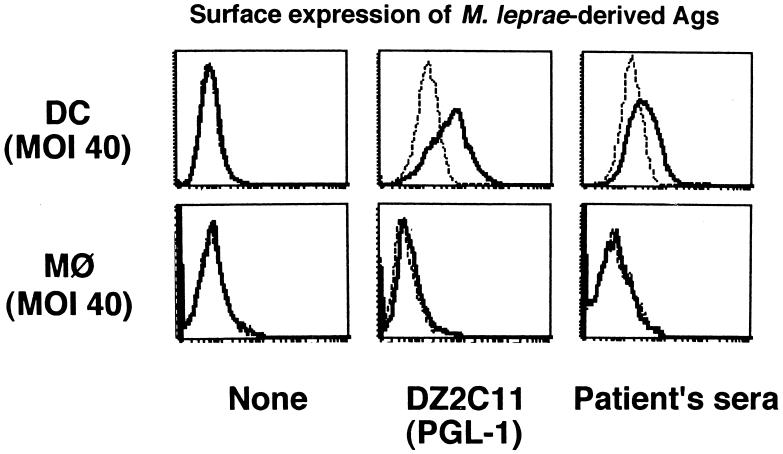
It is known that M. leprae infects monocytes/macrophages, but the sensitivity of the bacterium to DCs has not been extensively analyzed. Immature DCs were first examined morphologically. When M. leprae-pulsed immature DCs were stained with Ziehl-Neelsen stain, intracellular bacteria increased in a dose-dependent manner, and M. leprae was seen in most of the immature DCs (Fig. 1). Furthermore, electron microscopic observation proved that M. leprae was found inside DCs and not on the cell surface (not shown). To semiquantify the level of intracellular Ag of M. leprae, PGL-1, a specific marker of M. leprae, was stained (Fig. 2a). Macrophages were used as control cells. The Ag level of M. leprae in macrophages, represented by the mean fluorescence intensity, increased depending on the bacterial dose, and that in DCs showed a similar dose-dependent pattern, although the mean fluorescence intensity at each MOI in DCs seemed lower than that in macrophages. Similar results were obtained 24 and 48 h after infection with M. leprae (not shown). In order to reveal further evidence that M. leprae was internalized by DCs, surface FITC was quenched by exposure to trypan blue (Fig. 2b). Intracellular M. leprae, which was labeled with FITC, was detected when bacteria at an MOI of 10 were pulsed, and the fluorescence intensity increased in a manner dependent on the number of FITC-labeled M. leprae cells. Furthermore, there was no difference in fluorescence intensity between quenched and unquenched DCs. Next, the possibility of M. leprae-derived molecules being expressed on DC surfaces was examined by using a MAb against PGL-1 and pooled leprosy patient sera. On the surfaces of M. leprae-infected macrophages, neither Ag was detected, but Ags recognized by MAb to PGL-1 (DZ2C11) or pooled sera were detected on the surfaces of infected DCs (Fig. 3).
FIG. 1.
Visualization of phagocytosed M. leprae in DCs. Aliquots of DCs were spread on glass slides and subjected to staining. The DCs were air dried, fixed with 10% buffered formalin, and stained with carbol fuchsin, followed by hematoxylin counterstaining. Magnification, ×1,000.
FIG. 2.
(a) Intracellular expression of PGL-1 Ag. DCs and macrophages (MΦ) were differentiated in vitro from monocytes from healthy individuals and were infected with M. leprae at the indicated doses. Intracellular PGL-1 in DCs and macrophages 2 days postinfection was detected using FACScalibur. Dashed line, control MAb; solid line, DZ2C11 anti-PGL-1 MAb. A representative experiment of three independent experiments is shown. (b) Phagocytosis of FITC-conjugated M. leprae by DCs. Immature DCs differentiated from monocytes of healthy individuals were incubated with the indicated doses of FITC-conjugated M. leprae. The DCs were washed three times, and surface FITC was quenched by exposure to trypan blue. The cells phagocytosing the bacteria were determined by fluorescence-activated cell sorter analysis. Dashed lines, DCs unpulsed with FITC-conjugated M. leprae; solid lines, DCs pulsed with FITC-conjugated M. leprae.
FIG. 3.
Surface expression of M. leprae-derived Ags on DCs and macrophages. DCs and macrophages were differentiated from monocytes donated by healthy individuals and were infected at an M. leprae MOI of 40. Two days after infection, the cells were stained with DZ2C11 and pooled sera from 10 leprosy patients. Dashed lines, control MAb or sera from uninfected donors; solid lines, DZ2C11 or pooled sera from leprosy patients. A representative of three independent experiments is shown.
Ag-presenting function of DCs infected with M. leprae in vitro.
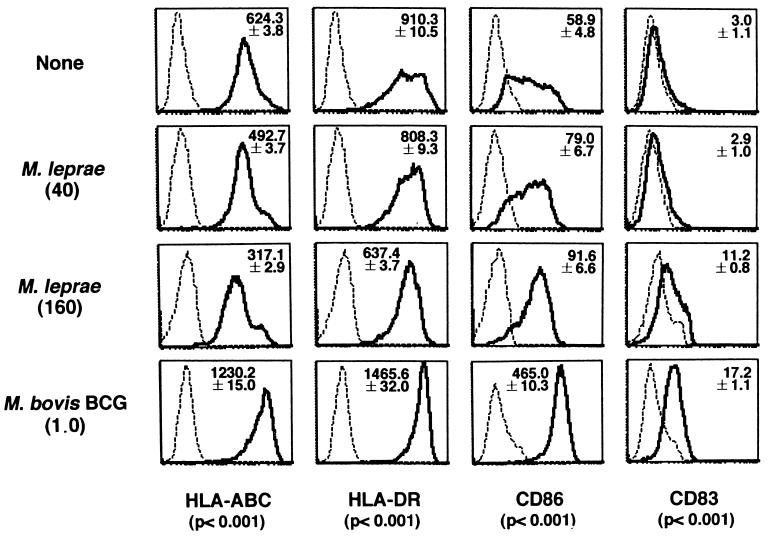
The surface expression of APC function-associated molecules on M. leprae-infected DCs was determined using BCG as a control (Fig. 4). All DCs infected and uninfected by mycobacteria expressed CD1a and completely lost CD14 expression (data not shown). However, DCs infected with BCG in the immature state expressed high levels of major histocompatibility complex (MHC) class I, class II, and CD86 Ags without any specific maturation factors and also expressed CD83. In contrast, the expression levels of MHC class I and class II Ags was down-regulated by M. leprae infection in a bacterial-dose-dependent manner. The expression of CD86 increased depending on the dose of M. leprae, and expression of CD83 was detected only when a high dose of M. leprae (MOI, 160) was pulsed. When M. leprae-infected DCs were compared with DCs infected with BCG (MOI, 1.0), all of the parameters examined were lower in the former than in the latter. DCs infected with a higher dose of M. leprae at MOIs of 40 and 160 showed no cytopathic effects or annexin-V-positive apoptotic cell death (data not shown). In order to further analyze the APC function of M. leprae-infected DCs, the ability of DCs to stimulate autologous CD4+ and CD8+ T cells was examined (Table 1). We used M. avium as a second bacterial control, since most healthy Japanese donors have been primed with BCG. The DCs infected with control bacteria strongly stimulated both autologous CD4+ and CD8+ T cells. However, the propensity of autologous T cells to respond to M. leprae-infected DCs was significantly lower than the response induced by DCs infected with BCG or M. avium. Similar results were obtained with freshly isolated M. leprae cells, which are 60 to 70% viable (not shown). M. leprae-infected macrophages did not stimulate either T-cell subset. The apparent T-cell responses to M. leprae-infected DCs were observed only when an MOI of 160 was used to infect DCs (the T-cell/DC ratios were 40 for CD4+ T cells and 20 for CD8+ T cells). All of the T-cell-proliferative responses to bacterium-infected DCs were at least partially blocked by MAbs to CD86 and MHC Ags. Furthermore, DCs pulsed with culture supernatant of cultured M. leprae did not induce high T-cell proliferation (not shown). We investigated the production of IFN-γ by T cells stimulated with infected DCs (Table 2). While DCs infected with control bacteria induced significant IFN-γ production, neither of the T-cell subsets produced significant amounts of IFN-γ upon stimulation with autologous DCs when an MOI of M. leprae of up to 40 was pulsed, and still less of the cytokine was produced, compared to production by control mycobacteria, when an M. leprae MOI of 160 was used. Finally, the production of immunomodulatory cytokines, the IL-12 p70 heterodimer and IL-10, by DCs was examined. DCs infected with M. leprae did not produce significant levels of these cytokines. Furthermore, T cells stimulated with the infected DCs did not produce significant levels of IL-10 (data not shown).
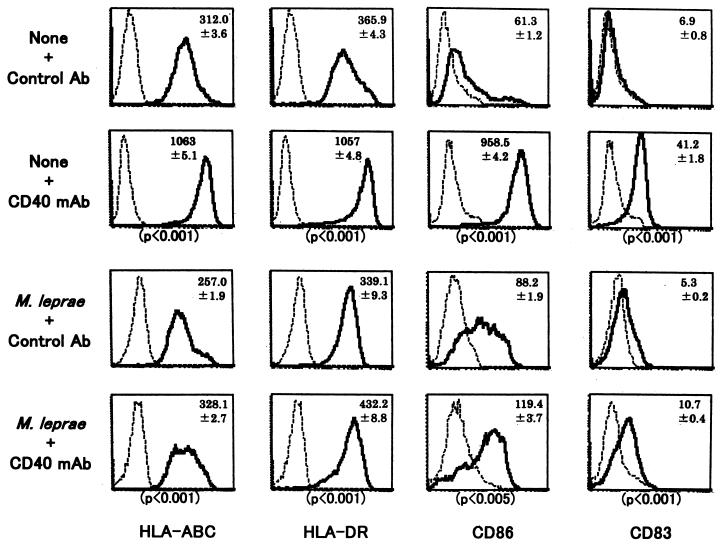
FIG. 4.
Surface expression of various molecules on uninfected DCs or DCs infected with M. leprae or M. bovis BCG. Immature DCs differentiated from healthy donors' monocytes were either left uninfected or infected with M. leprae (MOIs, 40 and 160) or BCG (MOI, 1.0) and were stained with the indicated MAbs. Dashed lines, control MAb; solid lines, indicated MAbs. The assays were done in triplicate, and the mean fluorescence intensity ± standard deviation of the mean is shown. The P values show the statistical difference between M. leprae (MOI, 160) and M. bovis BCG (MOI, 1.0). A representative of three independent experiments is shown.
TABLE 1.
APC function of M. leprae-infected DCsa
| Bacterium | Dose (MOI) | Responder [3H]thymidine uptake (103 cpm)
|
|||
|---|---|---|---|---|---|
| CD4
|
CD8
|
||||
| 20k | 40 | 10 | 20 | ||
| None | NAj | 7.9 ± 0.8 | 4.8 ± 0.6 | 3.0 ± 0.3 | 2.7 ± 0.6 |
| M. leprae | 10 | 8.8 ± 0.3 | 6.3 ± 0.5 | 3.3 ± 0.2 | 4.3 ± 0.4 |
| 40 | 25.2 ± 4.1 | 9.9 ± 1.1 | 17.2 ± 0.8 | 7.4 ± 0.9 | |
| 160 | 29.1 ± 6.7b,c | 16.8 ± 2.7d,e | 20.1 ± 1.2f,g | 13.5 ± 1.9h,i | |
| M. bovis BCG | 0.25 | 92.4 ± 8.8b | 65.8 ± 4.9d | 124.1 ± 7.9f | 119.6 ± 9.1h |
| 1.0 | 104.6 ± 9.3 | 66.7 ± 8.1 | 137.1 ± 10.1 | 108.2 ± 12.8 | |
| M. avium | 0.25 | 84.1 ± 6.2c | 51.1 ± 4.8e | 61.2 ± 4.3g | 33.6 ± 3.8i |
| 1.0 | 107.7 ± 7.8 | 70.2 ± 5.8 | 67.3 ± 5.2 | 51.1 ± 4.3 | |
Responder CD4+ and CD8+ T cells (105/well) were stimulated for 4 days with autologous DCs at the indicated T-cell/DC ratios. Monocyte-derived immature DCs were exposed to various doses of mycobacteria on day 3, and 5-day-cultured DCs were used as a stimulator. Representative results of three separate experiments are shown. The assays were done in triplicate, and the results were expressed as the mean ± standard deviation.
P < 0.001.
P < 0.001.
P < 0.001.
P < 0.005.
P < 0.005.
P < 0.005.
P < 0.005.
P < 0.005.
NA, not applicable.
T-cell/DC ratio.
TABLE 2.
IFN-γ production by T cells stimulated with autologous M. leprae-infected DCsa
| Bacterium | Dose (MOI) | Responder IFN-γ production (103 pg/ml)
|
|||
|---|---|---|---|---|---|
| CD4
|
CD8
|
||||
| 20j | 40 | 10 | 20 | ||
| M. leprae | 10 | 0.2 ± 0.0 | 0.2 ± 0.0 | 0.2 ± 0.0 | 0.2 ± 0.0 |
| 40 | 0.6 ± 0.0 | 0.3 ± 0.0 | 0.4 ± 0.0 | 0.2 ± 0.0 | |
| 160 | 4.0 ± 0.1b,c | 1.4 ± 0.2d,e | 3.7 ± 0.2f,g | 2.8 ± 0.3h,i | |
| M. bovis BCG | 0.25 | 22.4 ± 0.9b | 11.0 ± 0.7d | 35.6 ± 1.1f | 29.2 ± 1.8h |
| 1.0 | 22.9 ± 0.3 | 15.4 ± 0.8 | 78.6 ± 2.9 | 36.6 ± 1.7 | |
| M. avium | 0.63 | 57.0 ± 7.3c | 45.3 ± 3.8e | 102.9 ± 9.9g | 68.0 ± 6.8i |
| 2.5 | 104.3 ± 9.5 | 74.1 ± 6.3 | 138.2 ± 9.8 | 110.0 ± 9.7 | |
Responder CD4+ and CD8+ T cells (105/well) were stimulated for 4 days with autologous DCs at the indicated T-cell/DC ratios. Immature DCs were pulsed with various doses of mycobacteria on day 3, and 5-day-cultured DCs were used as a stimulator. Culture supernatants of DCs and T-cell cultures were collected on day 4, and the concentration of IFN-γ was determined by enzyme-linked immunosorbent assay. Representative results of three separate experiments are shown. The assays were done in triplicate, and the results were expressed as mean ± standard deviation.
P < 0.001.
P < 0.01.
P < 0.001.
P < 0.005.
P < 0.001.
P < 0.005.
P < 0.005.
P < 0.005.
T-cell/DC ratio.
Effects of maturation and activation factors on the APC function of M. leprae-infected DCs.
The possibility of DCs not being fully matured by M. leprae infection was examined using CD40 MAb, which leads DCs to terminal maturation states (Fig. 5 and Table 3). Uninfected DCs had increased expression of HLA-ABC and -DR, CD86, and CD83 Ags after treatment with CD40 MAb, and the M. leprae-infected DCs also had up-regulated expression after the MAb treatment. We then checked the effect of anti-CD40 MAb treatment on the ability of the infected DCs to stimulate T cells (Table 3). No apparent enhancement of the APC function of DCs pulsed with an M. leprae MOI of either 40 or 160 was observed through CD40 signaling, while the APC function of DCs pulsed with control Ag (ovalbumin) was up-regulated (not shown). In addition, neither LPS (Table 3), IL-12, IFN-γ, tumor necrosis factor alpha, nor poly(I · C) alone or in combination with anti-CD40 MAb up-regulated the T-cell-stimulating function of M. leprae-infected DCs (data not shown).
FIG. 5.
Influence of CD40 MAb treatment on the expression of various molecules on M. leprae-infected DCs. Immature DCs obtained from healthy donors were infected with M. leprae (MOI, 40) and were further treated with control Ab or MAb to CD40, followed by anti-mouse IgG Ab. The surface expression of various molecules on both DCs was determined. Dashed lines, control MAb; solid lines, indicated MAb. The assay was done in triplicate, and the mean fluorescence intensity ± standard deviation of the mean is shown. A representative of three independent experiments is shown.
TABLE 3.
Resistance of M. leprae-infected DCs to T-cell-mediated activation signala
| Bacterium | Dose (MOI) | Maturation factor | Responder [3H]thymidine uptake (103 cpm)
|
|||
|---|---|---|---|---|---|---|
| CD4
|
CD8
|
|||||
| 20b | 40 | 10 | 20 | |||
| M. leprae | 40 | None | 26.8 ± 0.3 | 10.5 ± 0.7 | 10.8 ± 0.4 | 3.1 ± 0.1 |
| 160 | 47.2 ± 0.8 | 26.4 ± 1.1 | 13.5 ± 0.6 | 12.2 ± 0.3 | ||
| 40 | CD40 MAb (10 μg/ml) | 28.3 ± 0.4 | 12.9 ± 0.6 | 5.6 ± 0.4 | 5.0 ± 0.4 | |
| 160 | 38.7 ± 0.9 | 18.5 ± 0.8 | 12.2 ± 0.9 | 10.1 ± 0.9 | ||
| 40 | LPS (10 ng/ml) | 23.9 ± 0.7 | 18.8 ± 0.5 | 12.1 ± 0.4 | 3.0 ± 0.3 | |
| 160 | 26.7 ± 0.6 | 21.3 ± 0.5 | 14.3 ± 0.2 | 9.9 ± 0.8 | ||
Responder CD4+ and CD8+ T cells (105/well) were stimulated for 4 days with autologous mature DCs at the indicated T-cell/DC ratios. Immature DCs were infected with M. leprae (MOI, 40 or 160) on day 3, matured using the reagents indicated on day 4, and used as a stimulator on day 5. Representative results of three separate experiments, using different donors, are shown. The assays were done in triplicate, and the results were expressed as mean ± standard deviation.
T-cell/DC ratio.
Masking effect of PGL-1 Ag expression on M. leprae-infected DCs.
Since it has been reported that purified PGL-1 suppresses the proliferative response of T cells to mitogens (29, 34), we examined whether PGL-1 on DCs was the factor involved in suppression of T-cell responses. To this end, we treated M. leprae-infected DCs with an MAb to PGL-1 immediately before coculture with T cells. While the PGL-1 MAb treatment did not affect the proliferative response of autologous T cells to DCs unexposed to any bacteria, or DCs pulsed with an unrelated Ag (ovalbumin), the treatment induced significantly higher CD4+- and CD8+-T-cell proliferative responses to M. leprae-infected DCs (Table 4). The treatment of infected DCs with control IgG did not enhance the T-cell responses.
TABLE 4.
Blocking effect of PGL-1 on T-cell-proliferative responses to M. leprae-infected DCsa
| Ag | Responder (T cell/DC) | Ab (μg/ml) [3H]thymidine uptake (103 cpm)
|
|||||
|---|---|---|---|---|---|---|---|
| None | Control IgG | DZ2C11 (1.0) | DZ2C11 (0.3) | DZ2C11 (1.0) | DZ2C11 (3.0) | ||
| None | CD4 (40) | 3.1 ± 0.1 | 4.3 ± 0.3 | 2.9 ± 0.8 | 3.3 ± 0.6 | 4.2 ± 0.4 | 5.2 ± 0.5 |
| CD8 (20) | 1.2 ± 0.1 | 0.9 ± 0.4 | 2.1 ± 0.5 | 2.7 ± 0.6 | 1.9 ± 0.5 | 2.4 ± 0.3 | |
| Ovalbumin (100 μg/ml) | CD4 (40) | 40.6 ± 3.7 | 34.9 ± 2.9 | 38.7 ± 5.2 | 39.9 ± 4.6 | 43.2 ± 3.8 | 45.3 ± 4.9 |
| CD8 (20) | 10.3 ± 1.2 | 13.5 ± 2.0 | 15.3 ± 2.2 | 12.6 ± 1.9 | 12.9 ± 2.0 | 16.3 ± 3.3 | |
| M. leprae (MOI, 40) | CD4 (40) | 10.9 ± 2.1 | 14.6 ± 1.9b,c | 23.3 ± 1.6b | 31.4 ± 3.1c | 42.8 ± 1.8 | 43.9 ± 3.8 |
| CD8 (20) | 8.2 ± 0.9 | 12.1 ± 2.0d,e | 16.9 ± 1.9d | 19.9 ± 1.3e | 22.8 ± 1.6 | 25.4 ± 2.3 | |
Responder T cells (105/well) were stimulated for 4 days with autologous DCs (5 × 103/well). Immature DCs were pulsed with M. leprae (MOI, 40) on day 3 and used as a stimulator on day 5 immediately after being treated with MAb to PGL-1 (DZ2C11) or control IgG. Representative results of three separate experiments are shown. The assays were done in triplicate, and the results are expressed as mean ± standard deviation.
P < 0.0005.
P < 0.005.
P < 0.0005.
P < 0.005.
DISCUSSION
Clarification of host defense mechanisms against M. leprae infection is required in order to develop immunotherapeutic tools. The cellular factor of the immunological response, in particular Ag-specific IFN-γ-producing CD4+ T cells and cytotoxic CD8+ T cells producing perforin and granulysin, has been established as an essential component of the protective immune response against M. leprae infection (1, 32, 33, 35, 43, 47). In this study, we focused on APCs capable of stimulating these T cells. In our hands, macrophages, when infected with M. leprae, expressed minimum mycobacterial Ags on the surface (Fig. 3) and did not stimulate autologous T cells (not shown). Therefore, it may be inferred that macrophages play a minor role. On the other hand, several reports indicate that monocyte-derived DCs infected with M. tuberculosis or BCG vigorously stimulate autologous CD4+ and CD8+ T cells and induce massive IFN-γ production (4, 5, 7, 12, 14, 19, 22, 27, 46, 48). Hence, DCs seem to be closely associated with host defense. In fact, DCs are capable of inducing protective immunity against M. tuberculosis infection in a rodent model (46). Similar results were observed in this study, as DCs were quite efficient in the induction of T-cell responses against M. avium and BCG. In leprosy, although CD1+ CD83+ monocyte-derived DCs have been found in a localized tuberculoid-form leprosy lesion, in which cellular immune reactions are observed (39, 50), the role of DCs against M. leprae infection has not been fully clarified.
The DCs infected with M. leprae in vitro are considered to be DCs because they express CD1a (data not shown) and CD83 (Fig. 3). When we examined the influence of mycobacterial infection on DCs, there were marked differences between M. leprae and BCG: while BCG up-regulated DCs to express enhanced levels of MHC class I, class II, and CD86 Ags upon infection and induced vigorous T-cell responses similar to those in previous reports (5, 7, 19), M. leprae down-regulated the expression of MHC class I and class II Ags and did not drastically up-regulate CD86 expression. These inefficient up-regulations may have resulted in less competent T-cell responses to M. leprae. Also, the production of IFN-γ by T cells required an unphysiologically high dose of M. leprae. These results led to the conclusion that M. leprae is a pathogen not capable of being efficiently utilized by DCs as a host defense APC component.
Several possibilities might be considered to explain the less efficient DC-mediated T-cell responses. (i) M. leprae was not phagocytosed by DCs and just adhered to the surfaces of DCs. This possibility is unlikely, because DCs are reported to be capable of phagocytosing mycobacteria, such as BCG (15), and Ziehl-Neelsen staining of DCs (Fig. 1) and electromicroscopic analysis (not shown) revealed that M. leprae was found inside DCs and not on the cell surface. Furthermore, we detected intracytoplasmic fluorescence, which was not reduced by trypan blue quenching, when DCs were pulsed with FITC-conjugated M. leprae (Fig. 2b). Therefore, we conclude that M. leprae is internalized by DCs. (ii) Although M. leprae-infected DCs expressed Ags that are recognized with pooled sera obtained from leprosy patients, the DCs might lack the expression of important T-cell epitopes. This also seems unlikely, since CD86 Ag-dependent responses of CD4+ and CD8+ T cells against DCs pulsed with M. leprae were observed (not shown). When we used DCs pulsed with heat-killed M. leprae as stimulators of autologous T cells, we observed no significant proliferation of the T cells. However, DCs pulsed with the cell membrane or cytosol fraction of M. leprae did competently stimulate T cells (not shown). Therefore, we may infer that some, but not all, T-cell epitopes are expressed on DCs. The difference in the extents of the T-cell responses induced by infection of DCs in M. avium and M. leprae could be due to the presence and/or absence of T cells primed with mycobacterium-derived Ags. This also seems unlikely, since most healthy Japanese individuals are certainly primed with BCG, which shares several proteins with both M. avium and M. leprae, although it has not yet been determined how many of the commonly expressed proteins contribute to T-cell priming. (iii) M. leprae might require more efficient maturation and activation factors for DCs besides the ones examined in this study. CD40 ligand is believed to play a central role in DC maturation (31, 37) and did enhance the APC function of DCs infected with M. tuberculosis (5). However, the cross-linking of CD40 molecules on M. leprae-infected DCs enhanced the expression of CD86 and the other Ags examined but did not reach the level required for up-regulation of the T-cell response. The combination of CD40 MAb with IFN-γ, IL-12, and LPS was also not effective (data not shown). While 19-kDa lipoprotein inhibited MHC class II expression (30) but CD40L enhanced the APC function of infected DCs in tuberculosis (5), both MHC class I and II molecule expressions were down-regulated by M. leprae infection of DCs, and CD40 MAb could not rescue their T-cell-stimulating abilities. Therefore, different mechanisms might be involved in the reduction of MHC molecule expression, and different factors might be required for an induction of vigorous T-cell proliferation in M. leprae. (iv) M. leprae might be a pathogen that takes an extremely long time to be processed in DCs. It is known that in macrophages, pathogens such as M. avium replicate in phagosomes, to some extent, that minimize contact with late endosomal-lysosomal compartments (49). We do not know if this is the case with M. leprae in DCs, and we have studies under way to examine this possibility using M. avium as a reference mycobacterium. (v) The last and, as far as this investigation is concerned, most likely explanation is that M. leprae cells contain components which prevent the tight interaction of DCs with T cells or which suppress T-cell responses. IL-10 and transforming growth factor β are known to be among these immunosuppressive mediators (3, 9, 10). However, the possibility of IL-10 being a direct immunosuppressing mediator is unlikely, because it was not produced by stimulated T cells or by DCs infected with mycobacteria, and anti-IL-10 MAb did not up-regulate T-cell responses (data not shown). We examined PGL-1 as a candidate factor which prevents T-cell responses, since it has been reported that purified PGL-1 suppresses the proliferative response of murine and human T cells to mitogens (29, 34) and PGL-1-mediated suppression seems to be a phenomenon specific to M. leprae. The T-cell suppression is reported to be induced by T-cell recognition of the terminal trisaccharide of PGL-1, which is a molecule providing specificity to PGL-1 (26). Furthermore, M. leprae-infected DCs expressed PGL-1 on their surfaces (Fig. 3), although the exact mechanism of the expression is not clear. The masking of PGL-1 on the DC surface by an MAb resulted in a convincing T-cell response to M. leprae-infected DCs, in terms of both proliferation (Table 4) and IFN-γ production (data not shown).
Taken together, these data indicate that M. leprae might be a unique pathogenic mycobacterium in that its individual components rather than the whole infectious bacterium were more efficient at stimulating T cells in combination with DCs. This is supported by the report that an M. leprae-derived lipid Ag could efficiently stimulate T cells through the CD1 pathway (39, 40).
Acknowledgments
We acknowledge the contribution of N. Makino to the preparation of the manuscript. We also thank H. Minagawa (Leprosy Research Center) for donating the MAb DZ2C11 and the Japanese Red Cross Society for kindly providing us PBMCs from healthy donors.
This work was supported in part by a Grant-in-Aid for Research on Emerging and Reemerging Infectious Diseases and by a Grant-in-Aid for Research on HIV/AIDS from the Ministry of Health, Labor, and Welfare of Japan.
Editor: S. H. E. Kaufmann
REFERENCES
- 1.Barnes, P. F., J. S. Abrams, S. Lu, P. A. Sieling, T. H. Rea, and R. L. Modlin. 1993. Patterns of cytokine production by Mycobacterium tuberculosis-reactive human T-cell clones. Infect. Immun. 61:197-203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Canaday, D. H., C. Ziebold, E. H. Noss, K. A. Chervenak, C. V. Harding, and W. H. Boom. 1999. Activation of human CD8+ αβ TCR+ cells by Mycobacterium tuberculosis via an alternate class I MHC antigen-processing pathway. J. Immunol. 162:372-379. [PubMed] [Google Scholar]
- 3.Caux, C., C. Massacrier, B. Vanbervliet, C. Barthelemy, Y. J. Kiu, and J. Banchereau. 1994. Interleukin 10 inhibits T cell alloreaction induced by human dendritic cells. Int. Immunol. 6:1177-1185. [DOI] [PubMed] [Google Scholar]
- 4.Demangel, C., A. G. Bean, E. Martin, C. G. Feng, A. T. Kamath, and W. J. Britton. 1999. Protection against aerosol Mycobacterium tuberculosis infection using Mycobacterium bovis Bacillus Calmette Guerin-infected dendritic cells. Eur. J. Immunol. 29:1972-1979. [DOI] [PubMed] [Google Scholar]
- 5.Demangel, C., U. Palendira, C. G. Feng, A. W. Heath, A. G. Bean, and W. J. Britton. 2001. Stimulation of dendritic cells via CD40 enhances immune responses to Mycobacterium tuberculosis infection. Infect. Immun. 69:2456-2460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fattorossi, A., R. Nisini, J. G. Pizzolo, and R. D'Amelio. 1989. New, simple flow cytometry technique to discriminate between internalized and membrane-bound particles in phagocytosis. Cytometry 10:320-325. [DOI] [PubMed] [Google Scholar]
- 7.Feng, C. G., C. Demangel, A. T. Kamath, M. Macdonald, and W. J. Britton. 2001. Dendritic cells infected with Mycobacterium bovis bacillus Calmette Guerin activate CD8(+) T cells with specificity for a novel mycobacterial epitope. Int. Immunol. 13:451-458. [DOI] [PubMed] [Google Scholar]
- 8.Flynn, J. L., J. Chan, K. J. Triebold, D. K. Dalton, T. A. Stewart, and B. R., Bloom. 1993. An essential role for interferon-γ in resistance to Mycobacterium tuberculosis infection. J. Exp. Med. 178:2249-2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Förtsch, D., M. Röllinghoff, and S. Stenger. 2000. IL-10 converts human dendritic cells into macrophage-like cells with increased antibacterial activity against virulent Mycobacterium tuberculosis. J. Immunol. 165:978-987. [DOI] [PubMed] [Google Scholar]
- 10.Goulart, I. M. B., J. R. Mineo, and N. T. Foss. 2000. Production of transforming growth factor-beta 1 (TGF-β1) by blood monocytes from patients with different clinical forms of leprosy. Clin. Exp. Immunol. 122:330-334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hed, J., G. Hallden, S. G. O. Johansson, and P. Larsson. 1987. The use of fluorescence quenching in flow cytofluorometry to measure the attachment and ingestion phases in phagocytosis in peripheral blood without prior cell separation. J. Immunol. Methods 101:119-125. [DOI] [PubMed] [Google Scholar]
- 12.Henderson, R. A., S. C. Watkins, and J. L. Flynn. 1997. Activation of human dendritic cells following infection with Mycobacterium tuberculosis. J. Immunol. 159:635-643. [PubMed] [Google Scholar]
- 13.Hmana, Z., R. Gabathuler, W. A. Jefferies, G. de Jong, and N. E. Reiner. 1998. Attenuation of HLA-DR expression by mononuclear phagocytes infected with Mycobacterium tuberculosis is related to intracellular sequestration of immature class II heterodimers. J. Immunol. 161:4882-4893. [PubMed] [Google Scholar]
- 14.Hope, J. C., L. S. Kwong, P. Sopp, R. A. Collins, and C. J. Howard. 2000. Dendritic cells induce CD4+ and CD8+ T-cell responses to Mycobacterium bovis and M. avium antigens in Bacille Calmette Guerin vaccinated and nonvaccinated cattle. Scand. J. Immunol. 52:285-291. [DOI] [PubMed] [Google Scholar]
- 15.Inaba, K., M. Inaba, M. Naito, and R. M. Steinman. 1993. Dendritic cell progenitors phagocytose particulates, including bacillus Calmette-Guerin organisms, and sensitize mice to mycobacterial antigens in vivo. J. Exp. Med. 178:479-488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Job, C. K. 1989. Nerve damage in leprosy. Int. J. Lepr. 57:532-539. [PubMed] [Google Scholar]
- 17.Johnson, P. C. 1997. Peripheral nerve pathology, p. 1233-1323. In R. L. Davis and D. M. Robertson (ed.), Neuropathology, 3rd ed. William and Wilkins, Baltimore Md.
- 18.Katoch, V. M., K. Katoch, U. Ramanathan, V. D. Sharma, C. T. Shivannavar, A. K. Datta, and V. P. Bharadwaj. 1989. Effect of chemotherapy on viability of Mycobacterium leprae as determined by ATP content, morphological index and FDA-EB fluorescent staining. Int. J. Lepr. 57:615-621. [PubMed] [Google Scholar]
- 19.Kim, K. D., H. G. Lee, J. K. Kim, S. N. Park, I. S. Choe, Y. K. Choe, S. J. Kim, E. Lee, and J. S. Lim. 1999. Enhanced antigen-presenting activity and tumour necrosis factor-alpha-independent activation of dendritic cells following treatment with Mycobacterium bovis bacillus Calmette-Guerin. Immunology 97:626-633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ladel, C. H., S. Daugelat, and S. H. Kaufmann. 1995. Immune response to Mycobacterium bovis bacilli Calmette-Guerin infection in major histocompatibility complex class I- and II-deficient knock-out mice: contribution of CD4 and CD8 T cells to acquired resistance. Eur. J. Immunol. 25:377-384. [DOI] [PubMed] [Google Scholar]
- 21.Lewinsohn, D. M., M. R. Alderson, A. L. Briden, S. R. Riddell, S. G. Reed, and K. H. Grabstein. 1998. Characterisation of human CD8+ T cells reactive with Mycobacterium tuberculosis-infected antigen presenting cells. J. Exp. Med. 187:1633-1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lewinsohn, D. M., A. L. Briden, S. G. Reed, K. H. Grabstein, and M. R. Alderson. 2000. Mycobacterium tuberculosis-reactive CD8+ T lymphocytes: the relative contribution of classical versus nonclassical HLA restriction. J. Immunol. 165:925-930. [DOI] [PubMed] [Google Scholar]
- 23.Makino, M., and M. Baba. 1997. Establishment of a cryopreservation method of human peripheral blood mononuclear cells for efficient production of dendritic cells. Scand. J. Immunol. 45:618-622. [DOI] [PubMed] [Google Scholar]
- 24.Makino, M., S. Shimokubo, S. Wakamatsu, S. Izumo, and M. Baba. 1999. The role of HTLV-I-infected dendritic cells in the development of HTLV-I-associated myelopathy/tropical spastic paraparesis. J. Virol. 73:4575-4581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Makino, M., S. Wakamatsu, S. Shimokubo, N. Arima, and M. Baba. 2000. Production of functionally deficient dendritic cells from HTLV-I-infected monocytes: implication for the dendritic cell defect in adult T cell leukemia. Virology 274:140-148. [DOI] [PubMed] [Google Scholar]
- 26.Mehra, V., P. J. Brennan, E. Rada, J. Convit, and B. R. Bloom. 1984. Lymphocyte suppression in leprosy induced by unique M. leprae glycolipid. Nature 308:194-196. [DOI] [PubMed] [Google Scholar]
- 27.Mittal, A., and I. Nath. 1987. Human T cell proliferative responses to particulate microbial antigens are supported by populations enriched in dendritic cells. Clin. Exp. Immunol. 69:611-617. [PMC free article] [PubMed] [Google Scholar]
- 28.Newport, M. J., C. M. Huxley, S. Huston, C. M. Hawrylowicz, B. A. Oostra, R. Williamson, and M. Levin. 1996. A mutation in the interferon-γ-receptor gene and susceptibility to mycobacterial infection. N. Engl. J. Med. 335:1941-1949. [DOI] [PubMed] [Google Scholar]
- 29.Nomaguchi, H., Y. Dohi, N. Ohno, T. Fujiwara, and T. Ito. 1989. Suppression of Con A response of mouse lymphocytes with unique M. leprae glycolipid. Jpn. J. Lepr. 58:191-196. [DOI] [PubMed] [Google Scholar]
- 30.Noss, E. H., R. K. Pai, T. J. Sellati, J. D. Radolf, J. Belisle, D. T. Golenbock, W. H. Boom, and C. V. Harding. 2001. Toll-like receptor 2-dependent inhibition of macrophage class II MHC expression and antigen processing by 19-kDa lipoprotein of Mycobacterium tuberculosis. J. Immunol. 167:910-918. [DOI] [PubMed] [Google Scholar]
- 31.O'Doherty, U., R. M. Steinman, M. Peng, P. U. Cameron, S. Gezelter, I. Kopeloff, W. J. Swiggard, M. Pope, and N. Bhardwaj. 1993. Dendritic cells freshly isolated from human blood express CD4 and mature into typical immunostimulatory dendritic cells after culture in monocyte-conditioned medium. J. Exp. Med. 178:1067-1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Orme, I. M., A. D. Roberts, J. P. Griffen, and J. S. Abrams. 1993. Cytokine secretion by CD4 T lymphocytes acquired in response to Mycobacterium tuberculosis infection. J. Immunol. 151:518-525. [PubMed] [Google Scholar]
- 33.Pena, S. V., D. A. Hanson, B. A. Carr, T. J. Goralski, and A. M. Krensky. 1997. Processing, subcellular localization, and function of 519 (granulysin), a human late T cell activation molecule with homology to small, lytic, granule proteins. J. Immunol. 158:2680-2688. [PubMed] [Google Scholar]
- 34.Prasad, H. K., R. S. Mishra, and I. Math. 1987. Phenolic glycolopid-I of Mycobacterium leprae induces general suppression of in vitro concanavalin a responses unrelated to leprosy type. J. Exp. Med. 165:239-244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ravn, P., H. Boesen, B. K. Pedersen, and P. Andersen. 1997. Human T cell responses induced by vaccination with Mycobacterium bovis BCG. J. Immunol. 158:1949-1955. [PubMed] [Google Scholar]
- 36.Ridley, D. S., and W. H. Jopling. 1966. Classification of leprosy according to immunity: a five-group system. Int. J. Lepr. Other Mycobact. Dis. 34:255-273. [PubMed] [Google Scholar]
- 37.Roake, J. A., A. S. Rao, P. J. Morris, C. P. Larsen, D. F. Hankins, and J. M. Austyn. 1995. Dendritic cell loss from non-lymphoid tissues after systemic administration of lipopolysaccharide, tumor necrosis factor, and interleukin 1. J. Exp. Med. 181:2237-2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shepard, C. C., and D. H. McRae. 1971. Hereditary characteristic that varies among isolates of Mycobacterium leprae. Infect. Immun. 3:121-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sieling, P. A., D. Jullien, M. Dahlem, T. F. Tedder, T. H. Rea, R. L. Modlin, and S. A. Porcelli. 1999. CD1 expression by dendritic cells in human leprosy lesions: correlation with effective host immunity. J. Immunol. 162:1851-1858. [PubMed] [Google Scholar]
- 40.Sieling, P. A., M.-T. Ochoa, D. Jullien, D. S. Leslie, S. Sabet, J.-P. Rosat, A. E. Burdick, T. H. Rea, M. B. Brenner, S. A. Porcelli, and R. L. Modlin. 2000. Evidence for human CD4+ T cells in the CD1-restricted repertoire: derivation of mycobacteria-reactive T cells from leprosy lesions. J. Immunol. 164:4790-4796. [DOI] [PubMed] [Google Scholar]
- 41.Smith, S. M., R. Brookes, M. R. Klein, A. S. Malin, P. T. Lukey, A. S. King, G. S. Ogg, A. V. S. Hill, and H. M. Dockrell. 2000. Human CD8+ CTL specific for the mycobacterial major secreted antigen 85A. J. Immunol. 165:7088-7095. [DOI] [PubMed] [Google Scholar]
- 42.Smith, W. C. 1997. We need to know what is happening to the incidence of leprosy. Lepr. Rev. 68:195-200. [DOI] [PubMed] [Google Scholar]
- 43.Stenger, S., D. A. Hanson, R. Teitelbaum, P. Dewan, K. R. Niazi, C. J. Froelich, T. Ganz, S. Thoma-Uszynski, A. Melián, C. Bogdan, S. A. Porcelli, B. R. Bloom, A. M. Krensky, and R. L. Modlin. 1998. An antimicrobial activity of cytolytic T cells mediated by granulysin. Science 282:121-125. [DOI] [PubMed] [Google Scholar]
- 44.Stoner, G. L. 1979. Importance of the neural predilection of Mycobacterium leprae in leprosy. Lancet 10:994-996. [DOI] [PubMed] [Google Scholar]
- 45.Tan, J. S., D. H. Canaday, W. H. Boom, K. N. Balaji, S. K. Schwander, and E. A. Rich. 1997. Human alveolar T lymphocyte responses to Mycobacterium tuberculosis antigens: role for CD4+ and CD8+ cytotoxic T cells and relative resistance of alveolar macrophages to lysis. J. Immunol. 159:290-297. [PubMed] [Google Scholar]
- 46.Tascon, R. E., C. S. Soares, S. Ragno, E. Stavropoulos, E. M. Hirst, and M. J. Colston. 2000. Mycobacterium tuberculosis-activated dendritic cells induce protective immunity in mice. Immunology 99:473-480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Thoma-Uszynski, S., S. Stenger, and R. L. Modlin. 2000. CTL-mediated killing of intracellular Mycobacterium tuberculosis is independent of target cell nuclear apoptosis. J. Immunol. 165:5773-5779. [DOI] [PubMed] [Google Scholar]
- 48.Tsuji, S., M. Matsumoto, O. Takeuchi, S. Akira, I. Azuma, A. Hayashi, K. Toyoshima, and T. Seya. 2000. Maturation of human dendritic cells by cell wall skeleton of Mycobacterium bovis bacillus Calmette-Guerin: involvement of toll-like receptors. Infect. Immun. 68:6883-6890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ullrich, H.-J., W. L. Beatty, and D. G. Russell. 2000. Interaction of Mycobacterium avium-containing phagosomes with the antigen presentation pathway. J. Immunol. 165:6073-6080. [DOI] [PubMed] [Google Scholar]
- 50.Verghese, S., D. G. Healey, J. Curtis, and J. L. Turk. 1988. Accessory cell function of dendritic cells from lymph nodes containing Mycobacterium leprae induced granulomas. Int. Arch. Allergy Appl. Immunol. 87:392-399. [DOI] [PubMed] [Google Scholar]
- 51.World Health Organization. 1982. Chemotherapy of leprosy for control programmes. W. H. O. technical report series no. 675. World Health Organization, Geneva, Switzerland. [PubMed]
- 52.World Health Organization. 2000. Leprosy—global situation. Wkly. Epidemiol. Rec. 75:225-232. [Google Scholar]
- 53.Yamauchi, P. S., J. R. Bleharski, K. Uyemura, J. Kim, P. A. Seiling, A. Miller, H. Brightbill, K. Schlienger, T. H. Rea, and R. L. Modlin. 2000. A role for CD40-CD40 ligand interactions in the generation of type 1 cytokine responses in human leprosy. J. Immunol. 165:1506-1512. [DOI] [PubMed] [Google Scholar]