Abstract
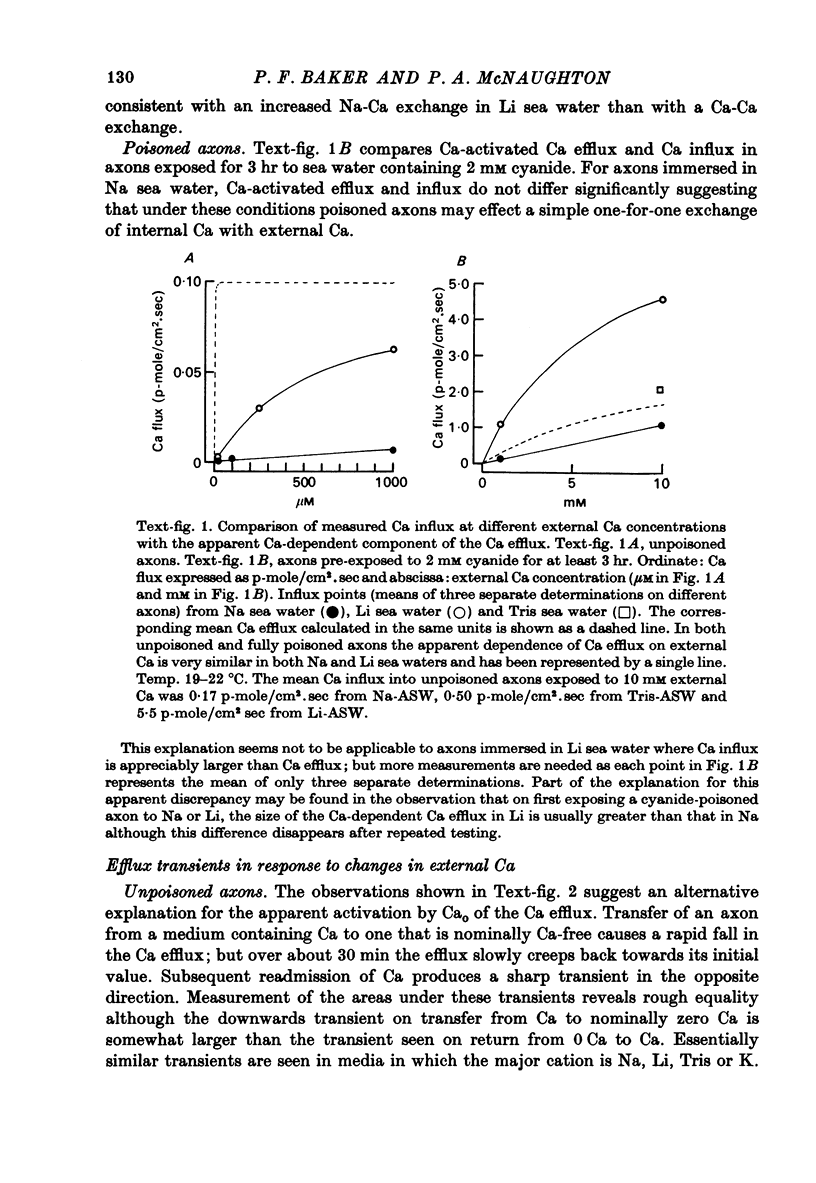
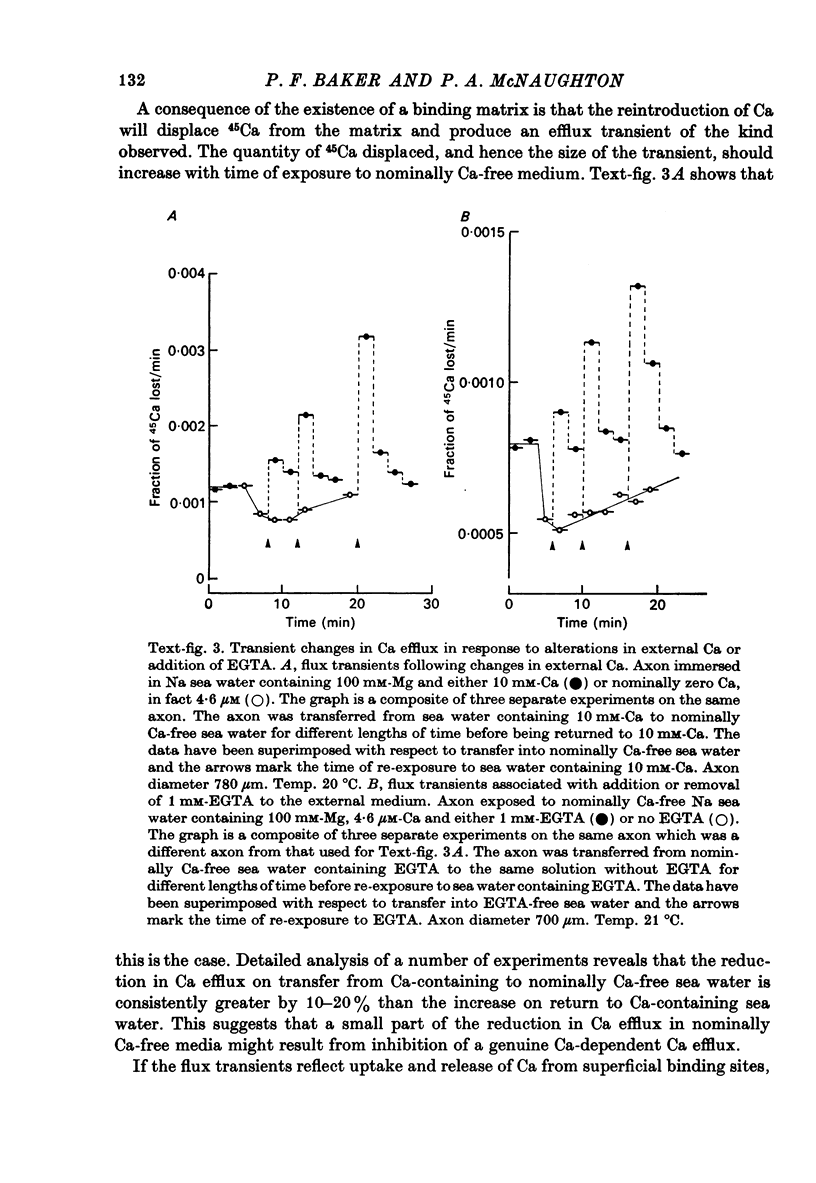
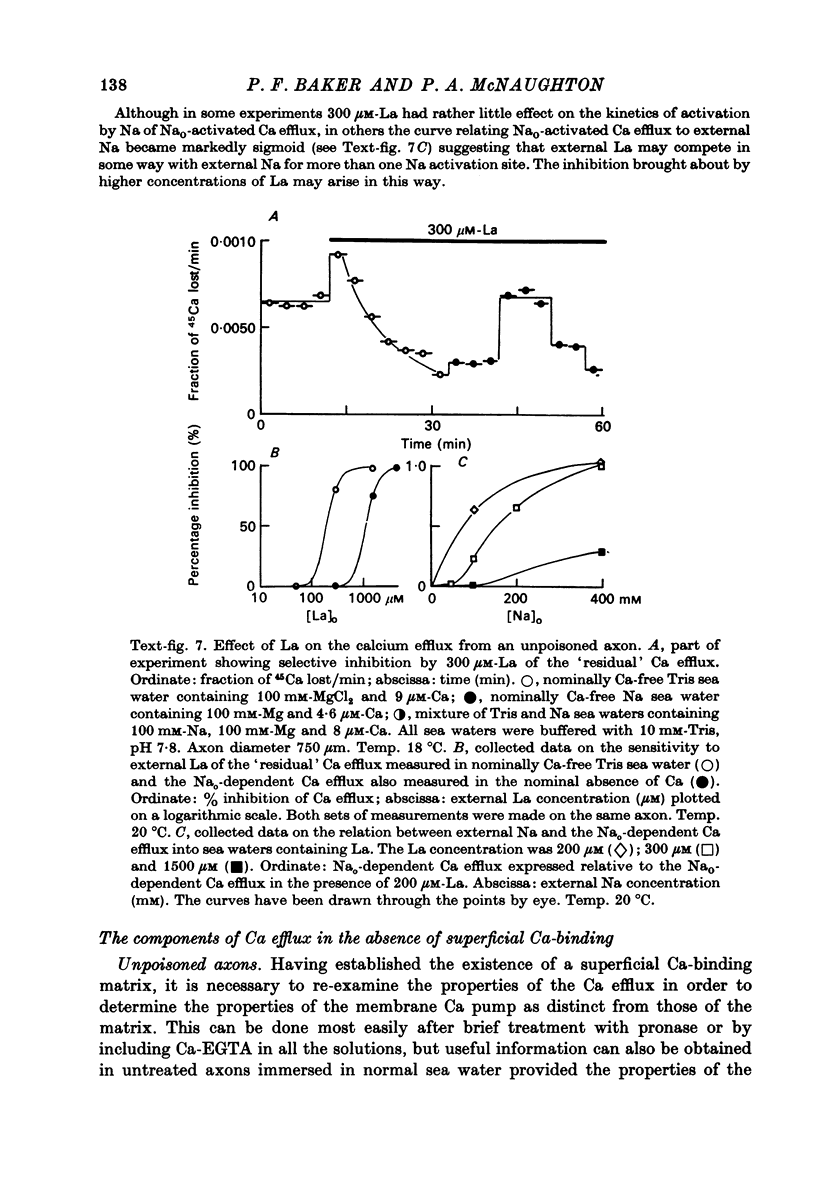
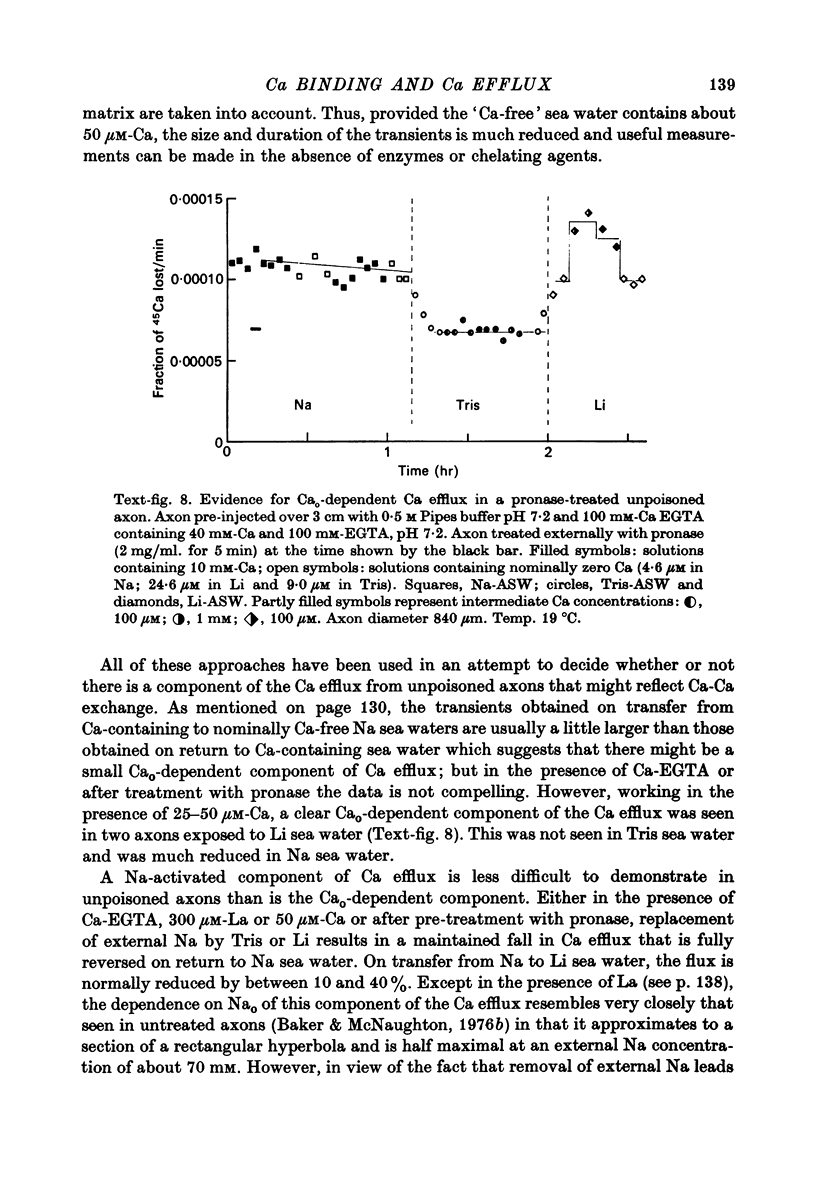
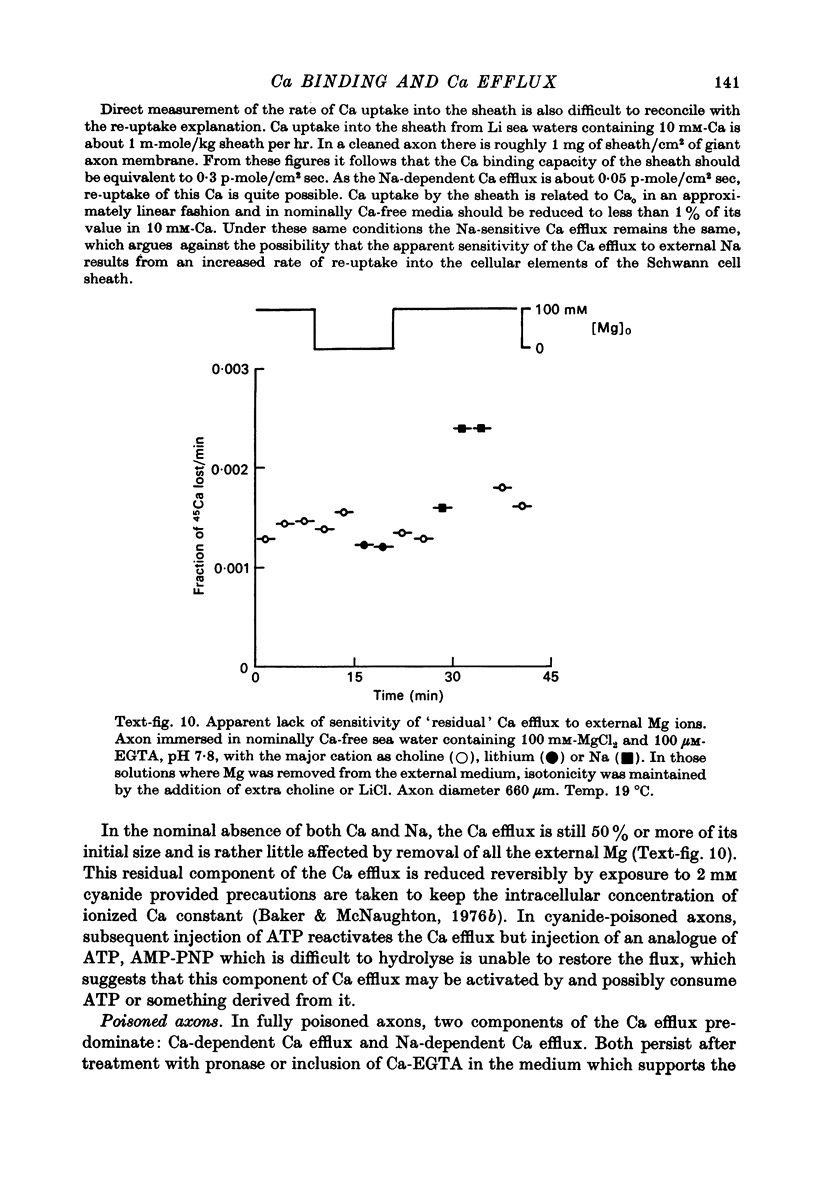
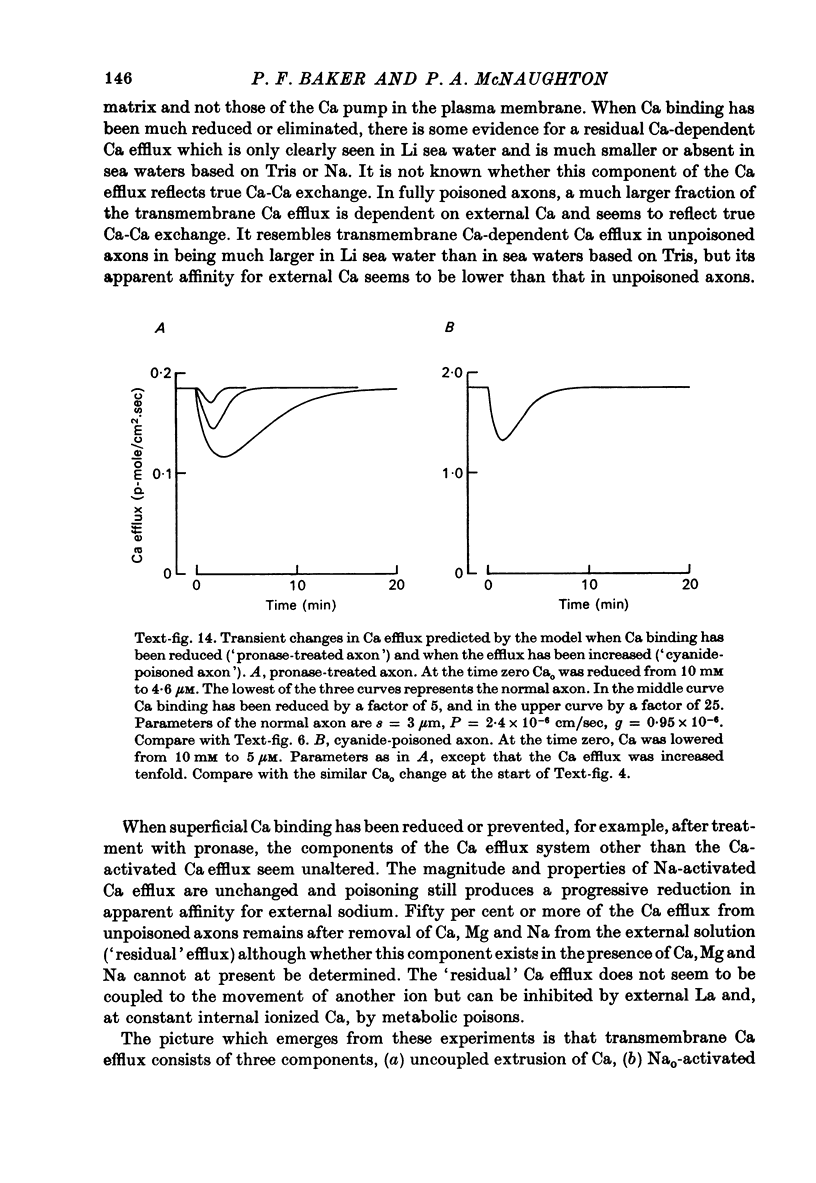
1. The Ca efflux from unpoisoned squid axons seems to consist of three components: Cao-dependent Ca efflux and a 'residual' flux that persists in the nominal absence of external Ca and Na and is little affected by the further removal of external Mg. 2. Evidence is presented to show that much of the apparent dependence on Cao of the Ca efflux from unpoisoned axons probably does not reflect a membrane process but results instead from the existence of a Ca-binding matrix external to the plasma membrane. Removal of external Ca produces a fall in efflux which is not maintained but returns to its original level over 20-60 min. Readmission of Ca produces only a transient increase in Ca efflux. Addition of EGTA to nominally Ca-free media also produces only a transient rise in Ca efflux. 3. Direct measurement of Ca binding to the surface of highly cleaned axons reveals appreciable binding over a wide range of Ca concentrations. A high affinity component of superficial binding can be recognized which has a capacity of about 60 p-mole/cm2 axon membrane and is half-maximal about 0.3 micrometer-Ca in Na-ASW. This component of binding is unaltered in cyanide-poisoned axons and in media in which Na is replaced isosmotically by Tris; but is reduced in the presence of 1 mM-La or after brief exposure of the axon to pronase. There is also a component of large capacity and lower affinity which was not saturated by 100 mM-Cao. 4. After brief pronase treatment the sensitivity of the Ca efflux to external Ca is markedly reduced although the Na-dependent Ca efflux persists apparently unaltered. 5. Addition of La produces a transient increase in Ca efflux followed by a maintained fall. 300 micrometer-La largely inhibits the component of the Ca efflux that persists in the nominal absence of external Na and Ca. Higher concentrations of La also inhibit Nao-dependent Ca efflux. 6. The Cao-dependent Ca efflux and Nao-dependent Ca efflux seen in poisoned axons persist in the presence of EGTA or after pronase treatment, suggesting that both are genuine membrane processes. 7. We conclude that in unpoisoned axons 50-90% of the Ca efflux can continue in the absence of external Ca, Na and Mg and may reflect an uncoupled extrusion of Ca. Most of the remaining efflux from these axons is Nao-dependent although a small Cao-dependent component can be distinguished in the absence of Na. Poisoning results in the loss of uncoupled Ca efflux, the appearance of appreciable Cao-dependent Ca efflux and alteration in the kinetics of Nao-dependent Ca efflux.
Full text
PDF


















Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker P. F., Blaustein M. P., Hodgkin A. L., Steinhardt R. A. The influence of calcium on sodium efflux in squid axons. J Physiol. 1969 Feb;200(2):431–458. doi: 10.1113/jphysiol.1969.sp008702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker P. F., McNaughton P. A. Kinetics and energetics of calcium efflux from intact squid giant axons. J Physiol. 1976 Jul;259(1):103–144. doi: 10.1113/jphysiol.1976.sp011457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker P. F., McNaughton P. A. Proceedings: Calcium-dependent calcium efflux from intact squid axons: Ca-Ca exchange or net extrusion? J Physiol. 1976 Jun;258(2):97P–98P. [PubMed] [Google Scholar]
- Baker P. F., Rubinson K. A. TTX resistant action potentials in crab nerve after treatment with Meerwein's reagent (proceedings). J Physiol. 1977 Mar;266(1):3P–4P. [PubMed] [Google Scholar]
- Blaustein M. P., Hodgkin A. L. The effect of cyanide on the efflux of calcium from squid axons. J Physiol. 1969 Feb;200(2):497–527. doi: 10.1113/jphysiol.1969.sp008704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinley F. J., Jr, Mullins L. J. Sodium extrusion by internally dialyzed squid axons. J Gen Physiol. 1967 Nov;50(10):2303–2331. doi: 10.1085/jgp.50.10.2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRANKENHAEUSER B., HODGKIN A. L. The after-effects of impulses in the giant nerve fibres of Loligo. J Physiol. 1956 Feb 28;131(2):341–376. doi: 10.1113/jphysiol.1956.sp005467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glynn I. M., Karlish S. J. The sodium pump. Annu Rev Physiol. 1975;37:13–55. doi: 10.1146/annurev.ph.37.030175.000305. [DOI] [PubMed] [Google Scholar]
- Levinson S. R., Meves H. The binding of tritiated tetrodotoxin to squid giant axons. Philos Trans R Soc Lond B Biol Sci. 1975 Jun 10;270(908):349–352. doi: 10.1098/rstb.1975.0014. [DOI] [PubMed] [Google Scholar]
- van Breemen C., Casteels R. The use of Ca-EGTA in measurements of 45Ca efflux from smooth muscle. Pflugers Arch. 1974 Apr 22;348(3):239–245. doi: 10.1007/BF00587414. [DOI] [PubMed] [Google Scholar]
- van Breemen C., De Weer P. Lanthanum inhibition of 45Ca efflux from the squid giant axon. Nature. 1970 May 23;226(5247):760–761. doi: 10.1038/226760a0. [DOI] [PubMed] [Google Scholar]