Abstract
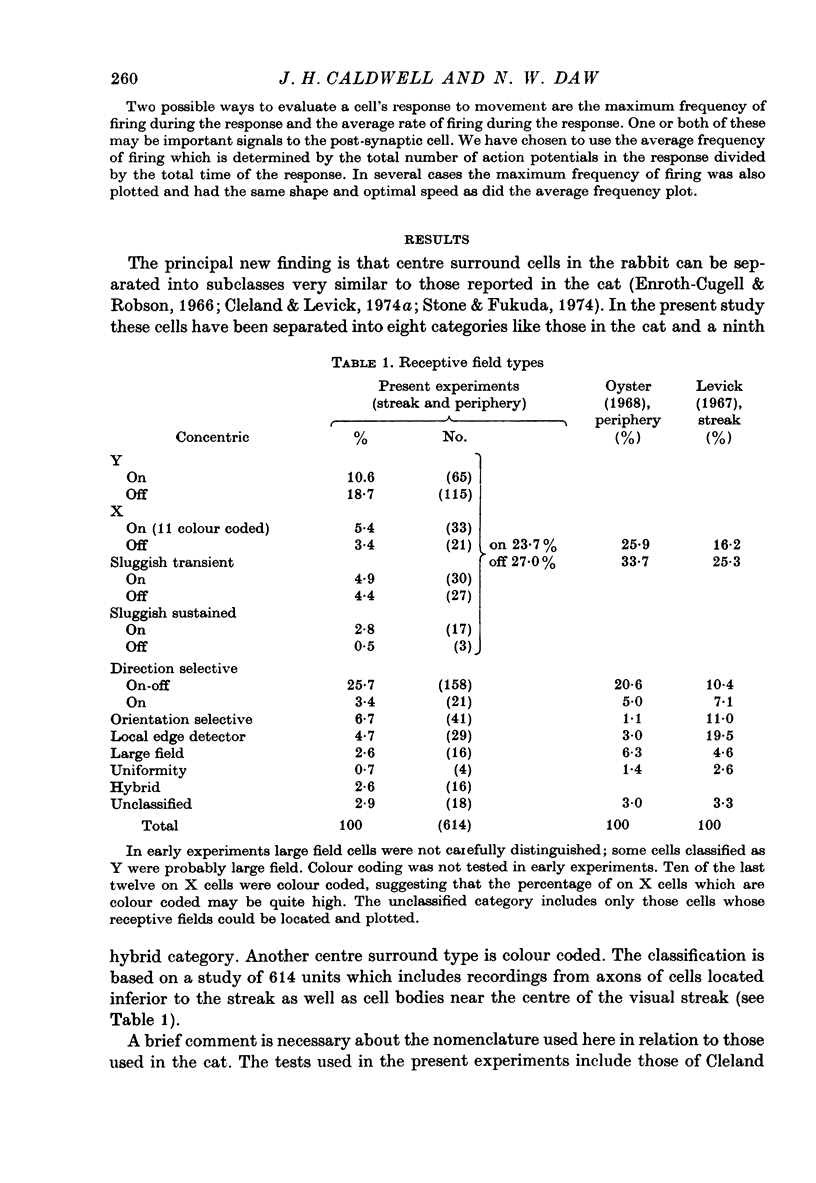
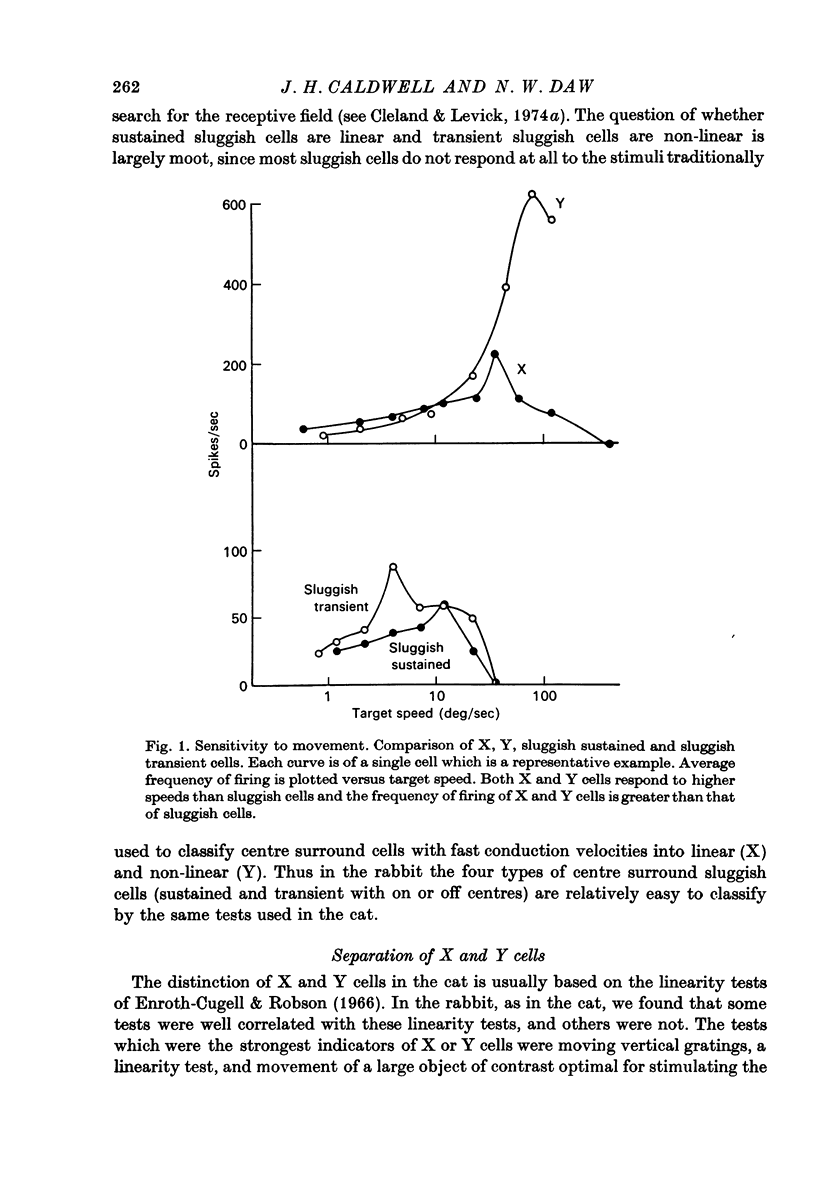
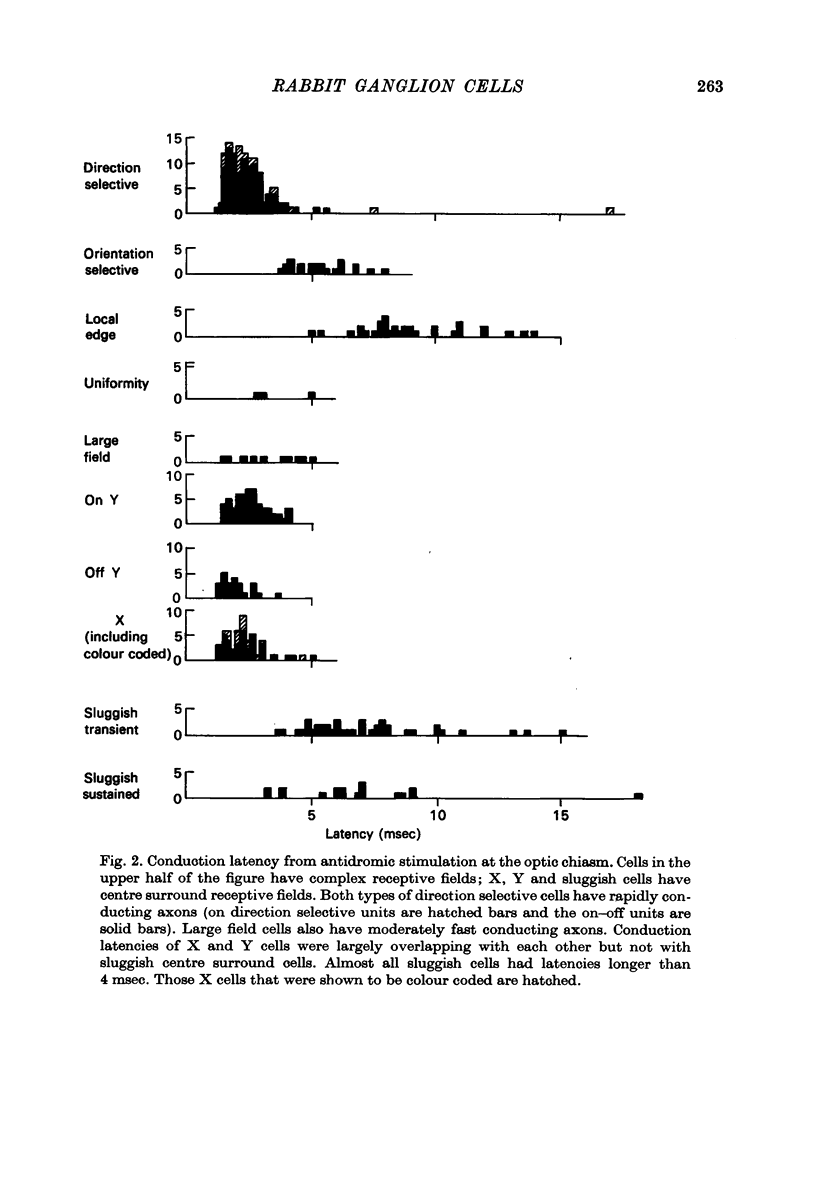
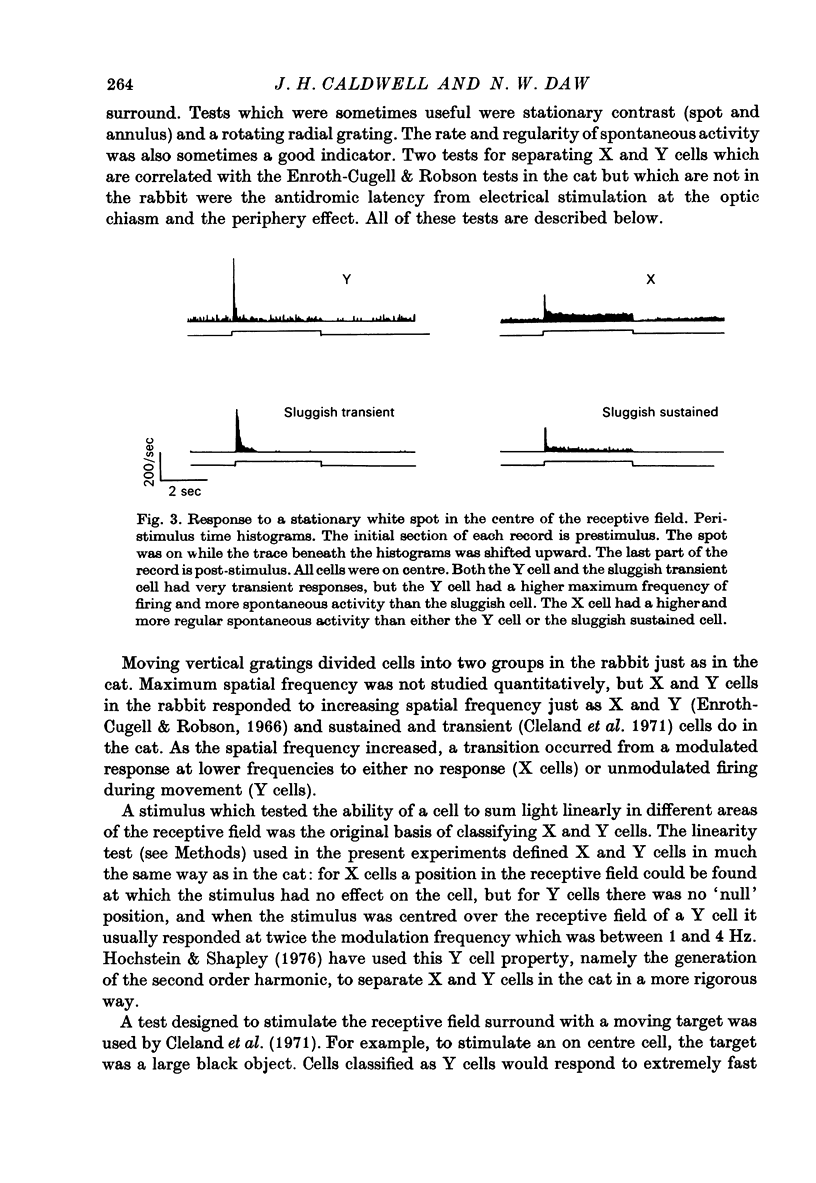
1. Receptive fields of centre surround cells in the rabbit retina were investigated. There is a clear distinction between cells with sluggish responses, low spontaneous activity and slow conduction velocity (centre surround sluggish cells) and cells with brisk responses, higher spontaneous activity and faster conduction velocity (X and Y cells). The sluggish cells can be divided into sustained and transient types. X and Y cells can be distinguished from each other by their responses to a moving linear grating, a large rapidly moving object and whether or not there is a response to the alternation of certain stimuli. Some times the response to a rotating radial grating, the rate of spontaneous activity, and whether or not the response to spots and annuli was sustained or transient could also be used to distinguish these two types. The antidromic latency from electrical stimulation of the optic chiasm and the periphery effect did not distinguish X from Y. 2. Eleven colour coded units were investigated. They all gave on responses to blue light in the centre of their receptive field and off responses to green light in the periphery of their receptive field. The blue pigment had a spectral sensitivity peaking at about 465 nm. The other pigment peaked near 500 nm, like the rods but gave a response at high mesopic and probably photopic levels. In some cases there was evidence for excitatory input from the green receptors to the centre of the receptive field. All the colour coded cells had rapidly conducting axons and were on centre X cells by all criteria. 3. Eighty-five cells various types other than colour coded were tested for their thresholds at 420 nm and 590 nm. In all cases the results were explained by a pigment peaking close to 500 nm, even at high mesopic and low photopic levels, which suggests the existence of cones with a cyan pigment in them. 4. Conduction latency from stimulation at the optic chiasm was measured for cells with centre surround receptive fields and cells with more complex receptive fields. Both 'on-off' and 'on' directionally sensitive cells have short conduction latencies, overlapping X and Y cells. Orientation selective cells and local edge detectors have long conduction latencies, overlapping centre surround sluggish cells. The sample of uniformity detectors was too small to characterize...
Full text
PDF












Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARLOW H. B., HILL R. M., LEVICK W. R. RETINAL GANGLION CELLS RESPONDING SELECTIVELY TO DIRECTION AND SPEED OF IMAGE MOTION IN THE RABBIT. J Physiol. 1964 Oct;173:377–407. doi: 10.1113/jphysiol.1964.sp007463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlow H. B., Levick W. R. The mechanism of directionally selective units in rabbit's retina. J Physiol. 1965 Jun;178(3):477–504. doi: 10.1113/jphysiol.1965.sp007638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop G. H., Clare M. H., Landau W. M. Further analysis of fiber groups in the optic tract of the cat. Exp Neurol. 1969 Jul;24(3):386–399. doi: 10.1016/0014-4886(69)90144-7. [DOI] [PubMed] [Google Scholar]
- Boycott B. B., Wässle H. The morphological types of ganglion cells of the domestic cat's retina. J Physiol. 1974 Jul;240(2):397–419. doi: 10.1113/jphysiol.1974.sp010616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell J. H., Daw N. W. Effects of picrotoxin and strychnine on rabbit retinal ganglion cells: changes in centre surround receptive fields. J Physiol. 1978 Mar;276:299–310. doi: 10.1113/jphysiol.1978.sp012234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell J. H., Daw N. W., Wyatt H. J. Effects of picrotoxin and strychnine on rabbit retinal ganglion cells: lateral interactions for cells with more complex receptive fields. J Physiol. 1978 Mar;276:277–298. doi: 10.1113/jphysiol.1978.sp012233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleland B. G., Dubin M. W., Levick W. R. Sustained and transient neurones in the cat's retina and lateral geniculate nucleus. J Physiol. 1971 Sep;217(2):473–496. doi: 10.1113/jphysiol.1971.sp009581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleland B. G., Levick W. R. Brisk and sluggish concentrically organized ganglion cells in the cat's retina. J Physiol. 1974 Jul;240(2):421–456. doi: 10.1113/jphysiol.1974.sp010617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleland B. G., Levick W. R. Properties of rarely encountered types of ganglion cells in the cat's retina and an overall classification. J Physiol. 1974 Jul;240(2):457–492. doi: 10.1113/jphysiol.1974.sp010618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleland B. G., Levick W. R., Sanderson K. J. Properties of sustained and transient ganglion cells in the cat retina. J Physiol. 1973 Feb;228(3):649–680. doi: 10.1113/jphysiol.1973.sp010105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conner J. D., MacLeod D. I. Rod photoreceptors detect rapid flicker. Science. 1977 Feb 18;195(4279):698–699. doi: 10.1126/science.841308. [DOI] [PubMed] [Google Scholar]
- DODT E., ELENIUS V. Change of threshold during dark adaptation measured with orange and blue light in cats and rabbits. Experientia. 1960 Jul 15;16:313–314. doi: 10.1007/BF02157769. [DOI] [PubMed] [Google Scholar]
- DODT E., WALTHER J. B. Photopic sensitivity mediated by visual purple. Experientia. 1958 Apr 15;14(4):142–143. doi: 10.1007/BF02157125. [DOI] [PubMed] [Google Scholar]
- Daw N. W., Pearlman A. L. Cat colour vision: one cone process or several? J Physiol. 1969 May;201(3):745–764. doi: 10.1113/jphysiol.1969.sp008785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daw N. W., Wyatt H. J. Raising rabbits in a moving visual environment: an attempt to modify directional sensitivity in the retina. J Physiol. 1974 Jul;240(2):309–330. doi: 10.1113/jphysiol.1974.sp010612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Monasterio F. M., Gouras P. Functional properties of ganglion cells of the rhesus monkey retina. J Physiol. 1975 Sep;251(1):167–195. doi: 10.1113/jphysiol.1975.sp011086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enroth-Cugell C., Robson J. G. The contrast sensitivity of retinal ganglion cells of the cat. J Physiol. 1966 Dec;187(3):517–552. doi: 10.1113/jphysiol.1966.sp008107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Famiglietti E. V., Jr, Kolb H. Structural basis for ON-and OFF-center responses in retinal ganglion cells. Science. 1976 Oct 8;194(4261):193–195. doi: 10.1126/science.959847. [DOI] [PubMed] [Google Scholar]
- Fukada Y. Receptive field organization of cat optic nerve fibers with special reference to conduction velocity. Vision Res. 1971 Mar;11(3):209–226. doi: 10.1016/0042-6989(71)90186-6. [DOI] [PubMed] [Google Scholar]
- Fukada Y., Saito H. Phasic and tonic cells in the cat's lateral geniculate nucleus. Tohoku J Exp Med. 1972 Feb;106(2):209–210. doi: 10.1620/tjem.106.209. [DOI] [PubMed] [Google Scholar]
- Fukuda Y. A three-group classification of rat retinal ganglion cells: histological and physiological studies. Brain Res. 1977 Jan 7;119(2):327–334. doi: 10.1016/0006-8993(77)90314-6. [DOI] [PubMed] [Google Scholar]
- Fukuda Y., Stone J. Retinal distribution and central projections of Y-, X-, and W-cells of the cat's retina. J Neurophysiol. 1974 Jul;37(4):749–772. doi: 10.1152/jn.1974.37.4.749. [DOI] [PubMed] [Google Scholar]
- GRANIT R., MARG E. Conduction velocities in rabbit's optic nerve and some observations on antidromic retinal spikes. Am J Ophthalmol. 1958 Nov;46(5 Pt 2):223–231. [PubMed] [Google Scholar]
- HILL R. M., MARG E. Single-cell responses of the nucleus of the transpeduncular tract in rabbit to monochromatic light on the retina. J Neurophysiol. 1963 Mar;26:249–257. doi: 10.1152/jn.1963.26.2.249. [DOI] [PubMed] [Google Scholar]
- HILL R. M. Unit responses of the rabbit lateral geniculate nucleus to monochromatic light on the retina. Science. 1962 Jan 12;135(3498):98–99. doi: 10.1126/science.135.3498.98. [DOI] [PubMed] [Google Scholar]
- Hochstein S., Shapley R. M. Quantitative analysis of retinal ganglion cell classifications. J Physiol. 1976 Nov;262(2):237–264. doi: 10.1113/jphysiol.1976.sp011594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman K. P., Stone J. Conduction velocity of afferents to cat visual cortex: a correlation with cortical receptive field properties. Brain Res. 1971 Sep 24;32(2):460–466. doi: 10.1016/0006-8993(71)90340-4. [DOI] [PubMed] [Google Scholar]
- Hoffmann K. P. Conduction velocity in pathways from retina to superior colliculus in the cat: a correlation with receptive-field properties. J Neurophysiol. 1973 May;36(3):409–424. doi: 10.1152/jn.1973.36.3.409. [DOI] [PubMed] [Google Scholar]
- Hoffmann K. P., Stone J., Sherman S. M. Relay of receptive-field properties in dorsal lateral geniculate nucleus of the cat. J Neurophysiol. 1972 Jul;35(4):518–531. doi: 10.1152/jn.1972.35.4.518. [DOI] [PubMed] [Google Scholar]
- Hughes A. Topographical relationships between the anatomy and physiology of the rabbit visual system. Doc Ophthalmol. 1971 Sep 12;30:33–159. doi: 10.1007/BF00142518. [DOI] [PubMed] [Google Scholar]
- Hughes A., Wassle H. The cat optic nerve: fibre total count and diameter spectrum. J Comp Neurol. 1976 Sep 15;169(2):171–184. doi: 10.1002/cne.901690204. [DOI] [PubMed] [Google Scholar]
- Ikeda H., Wright M. J. Receptive field organization of 'sustained' and 'transient' retinal ganglion cells which subserve different function roles. J Physiol. 1972 Dec;227(3):769–800. doi: 10.1113/jphysiol.1972.sp010058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakiela H. G., Enroth-Cugell C. Adaptation and dynamics in X-cells and Y-cells of the cat retina. Exp Brain Res. 1976 Feb 26;24(4):335–342. doi: 10.1007/BF00235001. [DOI] [PubMed] [Google Scholar]
- Levick W. R. Another tungsten microelectrode. Med Biol Eng. 1972 Jul;10(4):510–515. doi: 10.1007/BF02474199. [DOI] [PubMed] [Google Scholar]
- Levick W. R., Cleland B. G., Dubin M. W. Lateral geniculate neurons of cat: retinal inputs and physiology. Invest Ophthalmol. 1972 May;11(5):302–311. [PubMed] [Google Scholar]
- Levick W. R. Receptive fields and trigger features of ganglion cells in the visual streak of the rabbits retina. J Physiol. 1967 Feb;188(3):285–307. doi: 10.1113/jphysiol.1967.sp008140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MCILWAIN J. T. RECEPTIVE FIELDS OF OPTIC TRACT AXONS AND LATERAL GENICULATE CELLS: PERIPHERAL EXTENT AND BARBITURATE SENSITIVITY. J Neurophysiol. 1964 Nov;27:1154–1173. doi: 10.1152/jn.1964.27.6.1154. [DOI] [PubMed] [Google Scholar]
- Nuboer J. F. Spectral discrimination in a rabbit. Doc Ophthalmol. 1971 Sep 12;30:279–298. doi: 10.1007/BF00142525. [DOI] [PubMed] [Google Scholar]
- Oyster C. W., Takahashi E., Collewijn H. Direction-selective retinal ganglion cells and control of optokinetic nystagmus in the rabbit. Vision Res. 1972 Feb;12(2):183–193. doi: 10.1016/0042-6989(72)90110-1. [DOI] [PubMed] [Google Scholar]
- Oyster C. W., Takahashi E., Levick W. R. Information processing in the rabbit visual system. Doc Ophthalmol. 1971 Sep 12;30:161–204. doi: 10.1007/BF00142519. [DOI] [PubMed] [Google Scholar]
- Oyster C. W. The analysis of image motion by the rabbit retina. J Physiol. 1968 Dec;199(3):613–635. doi: 10.1113/jphysiol.1968.sp008671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe M. H., Stone J. Properties of ganglion cells in the visual streak of the cat's retina. J Comp Neurol. 1976 Sep 1;169(1):99–125. doi: 10.1002/cne.901690106. [DOI] [PubMed] [Google Scholar]
- Schiller P. H., Malpeli J. G. Properties and tectal projections of monkey retinal ganglion cells. J Neurophysiol. 1977 Mar;40(2):428–445. doi: 10.1152/jn.1977.40.2.428. [DOI] [PubMed] [Google Scholar]
- Sherman S. M., Norton T. T., Casagrande V. A. X- and Y-cells in the dorsal lateral geniculate nucleus of the tree shrew (Tupaia glis). Brain Res. 1975 Jul 25;93(1):152–157. doi: 10.1016/0006-8993(75)90294-2. [DOI] [PubMed] [Google Scholar]
- Sherman S. M., Wilson J. R., Kaas J. H., Webb S. V. X- and Y-cells in the dorsal lateral geniculate nucleus of the owl monkey (Aotus trivirgatus). Science. 1976 Apr 30;192(4238):475–477. doi: 10.1126/science.816006. [DOI] [PubMed] [Google Scholar]
- Stone J., Fukuda Y. Properties of cat retinal ganglion cells: a comparison of W-cells with X- and Y-cells. J Neurophysiol. 1974 Jul;37(4):722–748. doi: 10.1152/jn.1974.37.4.722. [DOI] [PubMed] [Google Scholar]
- Stone J., Hoffmann K. P. Very slow-conducting ganglion cells in the cat's retina: a major, new functional type? Brain Res. 1972 Aug 25;43(2):610–616. doi: 10.1016/0006-8993(72)90416-7. [DOI] [PubMed] [Google Scholar]
- Werblin F. S. Lateral interactions at inner plexiform layer of vertebrate retina: antagonistic responses to change. Science. 1972 Mar 3;175(4025):1008–1010. doi: 10.1126/science.175.4025.1008. [DOI] [PubMed] [Google Scholar]
- West R. W. Light and electron microscopy of the ground squirrel retina: functional considerations. J Comp Neurol. 1976 Aug 1;168(3):355–377. doi: 10.1002/cne.901680304. [DOI] [PubMed] [Google Scholar]
- Wilson P. D., Rowe M. H., Stone J. Properties of relay cells in cat's lateral geniculate nucleus: a comparison of W-cells with X- and Y-cells. J Neurophysiol. 1976 Nov;39(6):1193–1209. doi: 10.1152/jn.1976.39.6.1193. [DOI] [PubMed] [Google Scholar]
- Wyatt H. J., Daw N. W. Directionally sensitive ganglion cells in the rabbit retina: specificity for stimulus direction, size, and speed. J Neurophysiol. 1975 May;38(3):613–626. doi: 10.1152/jn.1975.38.3.613. [DOI] [PubMed] [Google Scholar]
- Wyatt H. J., Day N. W. Specific effects of neurotransmitter antagonists on ganglion cells in rabbit retina. Science. 1976 Jan 16;191(4223):204–205. doi: 10.1126/science.1857. [DOI] [PubMed] [Google Scholar]


