Abstract
By correlating immunofluorescence light microscopy with electron microscope studies and with kallikrein concentrations under various conditions, we have made the following observations and conclusions about kallikrein in the submandibular and other salivary glands.
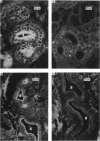
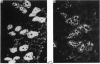
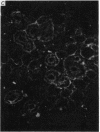
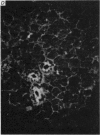
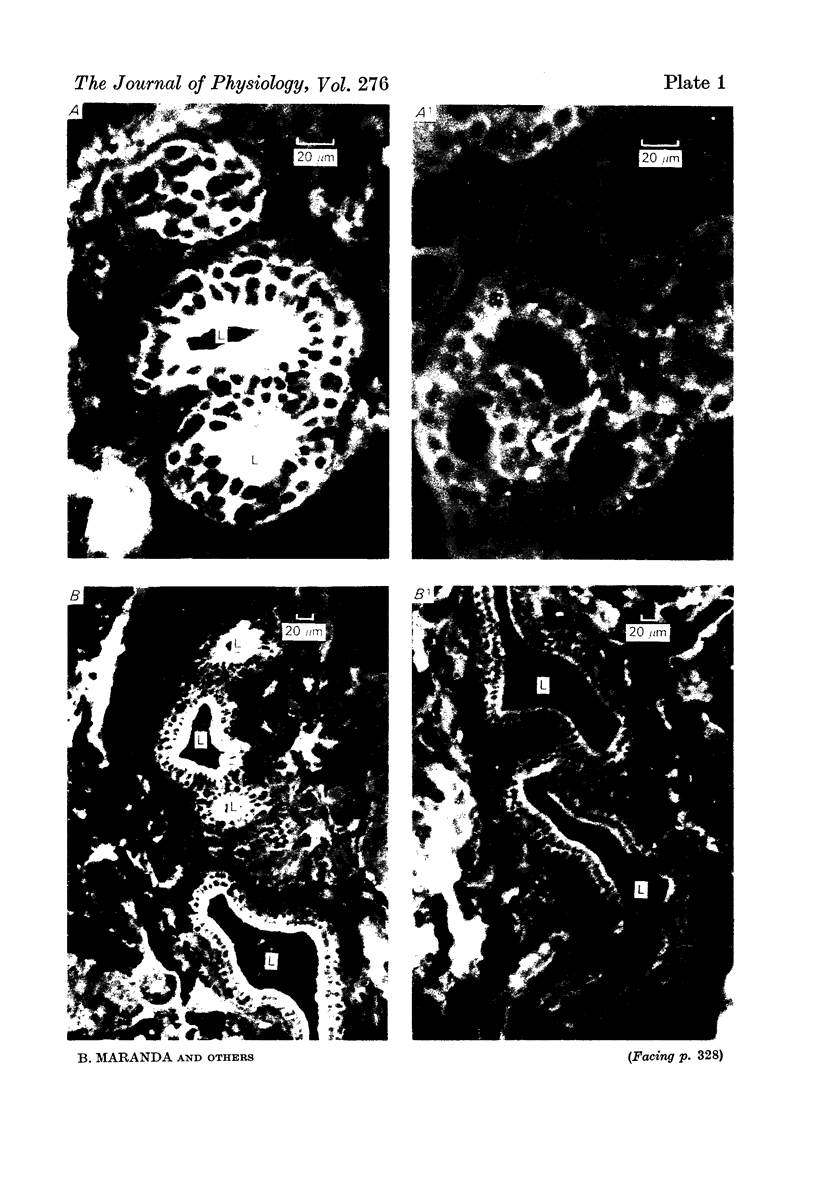
1. In the submandibular gland, specific immunofluorescence to kallikrein was observed in the luminal region of the striated ducts particularly, but also in the outer epithelial cells of the stratified epithelial collecting ducts. Sympathetic nerve stimulation resulted in a reduction in intensity of specific fluorescence and in its increased localization towards the lumen. The nearly complete elimination of kallikrein from the gland by duct obstruction for four days resulted in complete disappearance of specific fluorescence in the gland. Prolonged parasympathetic nerve stimulation at frequencies which did not reduce the kallikrein concentration of the gland failed to alter the specific immunofluorescence despite copious secretion of saliva. Our results failed to reveal evidence of secretion of kallikrein either into or towards the interstitium of the gland.
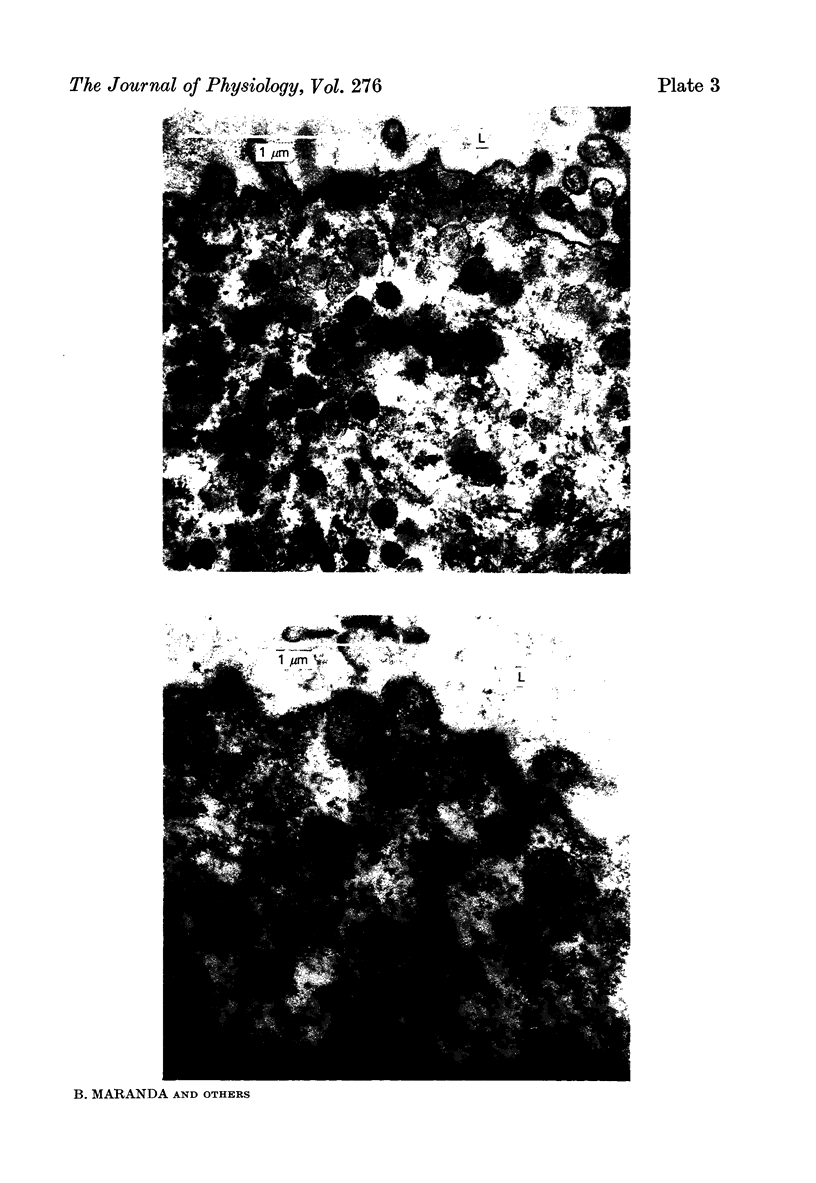
The luminal layer of stratified epithelial cells in the collecting ducts contained small secretory granules closely resembling those in the striated ducts. Our results are not conclusive, but do suggest that kallikrein is located in these granules whence it is secreted into the lumen of the duct.
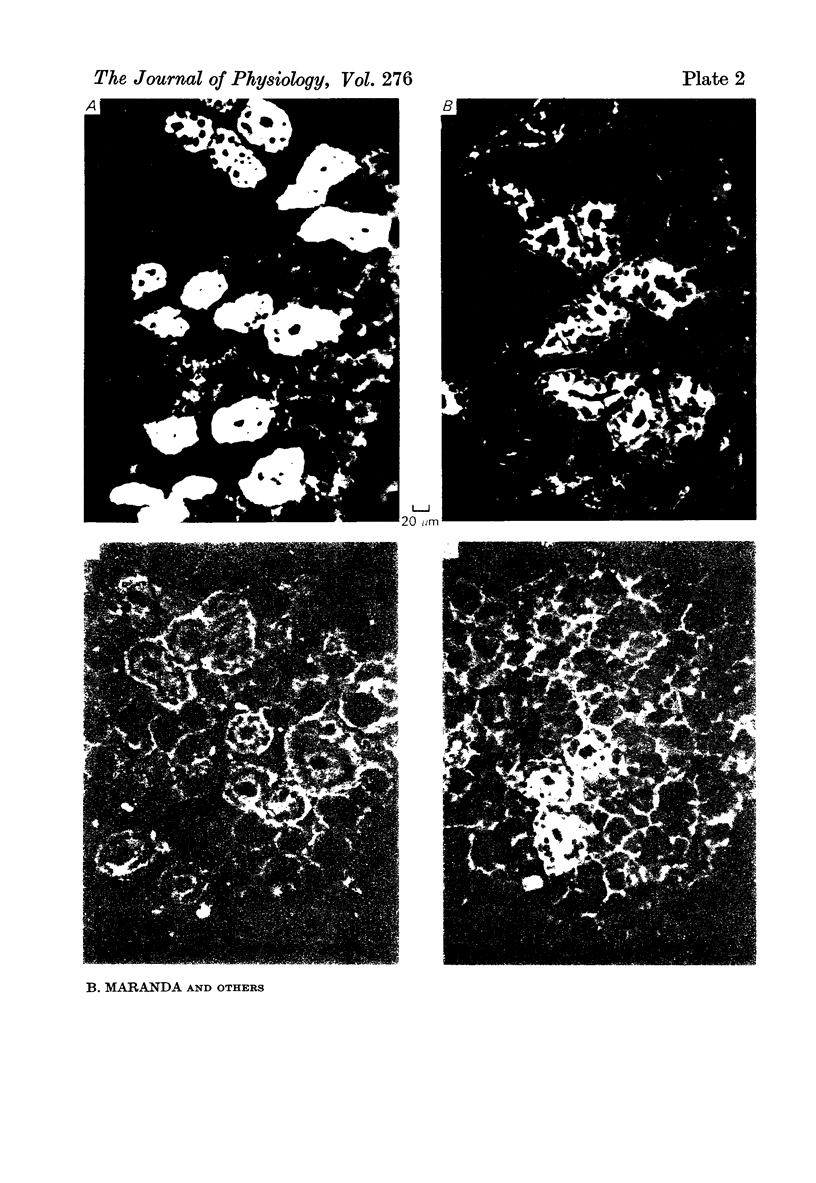
2. The parotid gland was found to contain much lower concentrations of kallikrein than the submandibular gland. This finding was associated with the presence of far fewer striated ducts in the parotid gland. Otherwise, specific fluorescence and the response to sympathetic nerve stimulation was like that of the submandibular gland. Small secretory granules in the striated and collecting ducts resembled those of the submandibular gland.
3. The sublingual gland, like the parotid, had a low concentration of kallikrein and very few striated ducts. These ducts were unevenly distributed and were concentrated in only a few lobules of the gland. Specific immunofluorescence was seen only in sections containing striated ducts.
4. The possible physiological role of kallikrein in the salivary glands is discussed.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barton S., Sanders E. J., Schachter M., Uddin M. Autonomic nerve stimulation, kallikrein content anc acinar cell granules of the cat's submandibular gland. J Physiol. 1975 Oct;251(2):363–369. doi: 10.1113/jphysiol.1975.sp011097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beilenson S., Schachter M., Smaje L. H. Secretion of kallikrein and its role in vasodilatation in the submaxillary gland. J Physiol. 1968 Dec;199(2):303–317. doi: 10.1113/jphysiol.1968.sp008655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandtzaeg P., Gautvik K. M., Nustad K., Pierce J. V. Rat submandibular gland kallikreins: purification and cellular localization. Br J Pharmacol. 1976 Feb;56(2):155–167. doi: 10.1111/j.1476-5381.1976.tb07438.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cozzari C., Angeletti P. U., Lazar J., Orth H., Gross F. Separation of isorenin activity from nerve growth factor (NGF) activity in mouse submaxillary gland extracts. Biochem Pharmacol. 1973 Jun 1;22(11):1321–1327. doi: 10.1016/0006-2952(73)90306-7. [DOI] [PubMed] [Google Scholar]
- Ekfors T. O., Hopsu-Havu V. K. Immunofluorescent localization of trypsin-like esteropeptidases in the mouse submandibular gland. Histochem J. 1971 Nov;3(6):415–420. doi: 10.1007/BF01014779. [DOI] [PubMed] [Google Scholar]
- Garrett J. R., Kidd A. Effects of nerve stimulation and denervation on secretory material in submandibular striated duct cells of cats, and the possible role of these cells in the secretion of salivary kallikrein. Cell Tissue Res. 1975 Aug 1;161(1):71–84. doi: 10.1007/BF00222115. [DOI] [PubMed] [Google Scholar]
- Hojima Y., Maranda B., Moriwaki C., Schachter M. Direct evidence for the location of kallikrein in the striated ducts of the cat's submandibular gland by the use of specific antibody. J Physiol. 1977 Jul;268(3):793–801. doi: 10.1113/jphysiol.1977.sp011882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraus F. W., Mestecky J. Immunohistochemical localization of amylase, lysozyme and immunoglobulins in the human parotid gland. Arch Oral Biol. 1971 Jul;16(7):781–789. doi: 10.1016/0003-9969(71)90122-1. [DOI] [PubMed] [Google Scholar]
- Pasquini F., Petris A., Sbaraglia G., Scopelliti R., Cenci G., Frati L. Biological activities in the granules isolated from the mouse submaxillary gland. Exp Cell Res. 1974 Jun;86(2):233–236. doi: 10.1016/0014-4827(74)90708-3. [DOI] [PubMed] [Google Scholar]
- Schachter M., Barton S., Uddin M., Karpinski E., Sanders E. J. Effect of nerve stimulation, denervation, and duct ligation, on kallikrein content and duct cell granules of the cat's submandibular gland. Experientia. 1977 Jun 15;33(6):746–748. doi: 10.1007/BF01944167. [DOI] [PubMed] [Google Scholar]
- Stroud R. M. A family of protein-cutting proteins. Sci Am. 1974 Jul;231(1):74–88. doi: 10.1038/scientificamerican0774-74. [DOI] [PubMed] [Google Scholar]
- Tandler B., Poulsen J. H. Ultrastructure of the cat sublingual gland. Anat Rec. 1977 Feb;187(2):153–171. doi: 10.1002/ar.1091870204. [DOI] [PubMed] [Google Scholar]
- Yasuda K., Coons A. H. Localization by immunofluroescence of amylase, trypsinogen and chymotrypsinogen in the acinar cells of the pig pancreas. J Histochem Cytochem. 1966 Apr;14(4):303–313. doi: 10.1177/14.4.303. [DOI] [PubMed] [Google Scholar]