Abstract
Bacterial lipopolysaccharides (LPS) activate cells of innate immunity, such as macrophages, by stimulating signaling through toll-like receptor 4 (TLR4). We and others have hypothesized that LPS derived from different bacterial species may function through TLR4-independent mechanisms. To test this hypothesis, we have generated using a nonviral transformation procedure a bone marrow-derived macrophage cell line called 10ScNCr/23 from mouse strain C57BL/10ScNCr. This mouse strain has a deletion of the TLR4 locus, causing the mouse strain to be nonresponsive to stimulation by LPS from Escherichia coli while responding normally to other bacterial substrates, such as lipoteichoic acid (LTA) from Staphylococcus aureus, which signal TLR4 independently. Stimulation with LTA induces five- and sixfold increases in 10ScNCr/23 cell line tumor necrosis factor alpha and macrophage inflammatory protein-2 (MIP-2) secretion, but no increases in either cytokine were found when cells were stimulated with E. coli LPS. Bacteroides fragilis-derived LPS, however, can effectively stimulate MIP-2 expression in the absence of functional TLR4 in the 10ScNCr/23 cell line. This gives rise to the notion that LPS from some bacterial species will utilize alternative receptors to stimulate the innate immune response.
Macrophages and the macrophage-mediated innate immune response have received increased attention recently through the cloning and functional characterization of the Toll protein family of transmembrane receptors (18). Macrophages are considered the first line of defense against bacterial pathogens, where the toll-like receptors (TLR) have been implicated as the major receptors for bacterial pathogen binding on the cell membrane (23). The Toll family consists of a minimum of 10 different members (21), each binding to a specific group of pathogens. TLR4 is the best characterized receptor of the family and was shown to be the signaling receptor for endotoxically active lipopolysaccharide (LPS) of gram-negative bacteria (15), while TLR5 binds bacteria expressing the flagellin protein (8). TLR2, in combination with TLR6, is thought to bind gram-positive bacteria, yeast, mycoplasma, and viruses (7). TLR9 more recently has been shown to be involved in initiating an immune response in response to CpG DNA (3), while TLR3 has been identified as the receptor for double-stranded RNA (1). Recent evidence has shown that the lines defining the specificity of the Toll receptor-pathogen interaction are not as clear as originally thought. Certain bacteria, such as spirochetes, can signal through TLR2 as well as TLR4 (19), and heat shock protein was shown to signal through both TLR2 and TLR4 (2), which can synergize (5). These findings generate the need for a simple assay to determine whether ligands signal through TLRs and whether signaling can occur through more than one TLR.
In addition, recent evidence has indicated that even though all TLRs are believed to signal through an MyD88-initiated signal transduction cascade, different downstream proteins, such as kinases, are involved in the signal transduction events initiated by TLR2 and TLR4 in monocytes. Even though this is the first evidence of TLR-specific signaling and has only been shown in one cell type so far, it is very likely that different TLRs initiate different signal transduction cascades. Most likely this differential signaling is true for additional cell types besides monocytes.
The cell line described in this paper is derived from mouse strain C57BL/10ScNCr. This mouse strain has a deletion of the TLR4 gene (17) but is wild type for the interleukin-12 (IL-12) gene, in contrast to its close relative C57BL/10ScCr (16). The macrophages from this mouse strain are nonresponsive to stimulation by LPS. This makes the novel cell line 10ScNCr/23 an important tool to define the TLR specificity of novel bacterial substrates as well as a useful tool to dissect the differential cytokine expression pattern in response to TLR-mediated signal transduction.
MATERIALS AND METHODS
Generation of cell lines.
The cell line 10ScNCr/23 was generated using the previously described methods of Monner and Walker with minor modifications (12, 22). The cell line was propagated using microbeads until a sufficient number and density were reached to cryopreserve the clonal isolates. The PCR analysis (carried out by M. A. Freudenberg, Max-Planck Institute for Immunobiology, Freiburg, Germany) revealed that the cell line 10ScNCr/23 carried a deletion of the TLR4 locus, identical to that observed in C57BL/10ScCr mice, but the point mutation in the IL-12Rβ2 gene, characteristic for C57BL/10ScCr mice, was absent (16).
Cell culture medium and maintenance.
Once immortalized, cells were grown in a conditioned medium, prepared fresh on a weekly basis. The conditioned medium contained 10% heat-inactivated fetal calf serum, 1% l-glutamine, and 20% LADMAC (catalog no. CRL 2420; American Type Culture Collection) supernatant in minimal essential medium (22). This medium provides the isolated macrophages with colony-stimulating factor-1. To eliminate the potential for contamination as a source for unusual cytokine secretion profiles, it was verified that the cell lines tested negative for mycoplasma.
Immune stimuli.
Lipoteichoic acid (LTA) was prepared from Staphylococcus aureus by butanol extraction as described elsewhere (13). LPS from Escherichia coli 0111:B4 and LPS from E. coli 026:B6 were purchased as lyophilized powder from Sigma, solubilized in Hank's buffer, and tested for endotoxin content as described previously (10). LPS from E. coli 0111:B4 does not signal through TLR2, as determined previously (11). The Bacteroides fragilis LPS was a gift of Ribi, Inc. The endotoxin preparations for in vitro testing using the cell line were selected based on their failure to induce IL-1 production from whole human blood at concentrations comparable to that of E. coli-derived LPS (T. Hartung, unpublished data).
ELISAs.
All cytokine assays were done in duplicate based on 50 μl of cell culture supernatant, including a standard curve for normalization in between different experiments. Enzyme-linked immunosorbent assay (ELISA) kits were purchased from R&D Biosystems and used according to the manufacturer's instructions. ELISA plates were read on a microplate reader (Powerwave; Biotek Systems) with attached software (KCjunior; Biotek Systems) that allowed a transformation of the absorbance reading into concentration curves (in picograms per milliliter). The lowest limits of detection for the cytokines when using this method were 5.1 pg/ml for tumor necrosis factor alpha (TNF-α) and 7.8 pg/ml for macrophage inflammatory protein-2 (MIP-2).
Flow cytometry.
Cells were labeled with fluorescein-conjugated antibodies to F4/80 (mature macrophages), CD11b (bone marrow-derived macrophages), and C11a (a general macrophage marker) (12). Isotype control antibodies were rat anti-mouse immunoglobulin G2a (IgG2a) for CD11a and rat anti-mouse IgG2b for F4/80 as well as CD11b. All antibodies were purchased from CalTag Laboratories (Burlingame, Calif.) and were used according to the manufacturer's specifications. Analysis was performed on a Coulter Epics XL flow cytometer (Beckman-Coulter, Fullerton, Calif.), and data were analyzed using CellQuest software (Becton-Dickinson, Mountain View, Calif.).
Statistics.
All statistical analyses were performed using the SPSS version 9.0 software package. Statistical significance was determined using a cutoff of 0.05.
RESULTS
The C57BL/10ScNCr-derived cell line exhibits a macrophage-like phenotype.
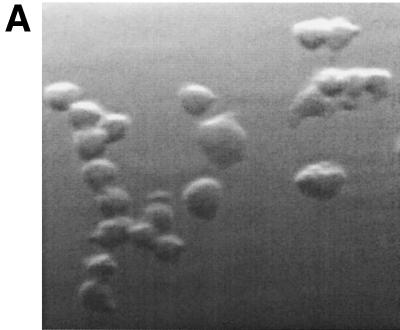
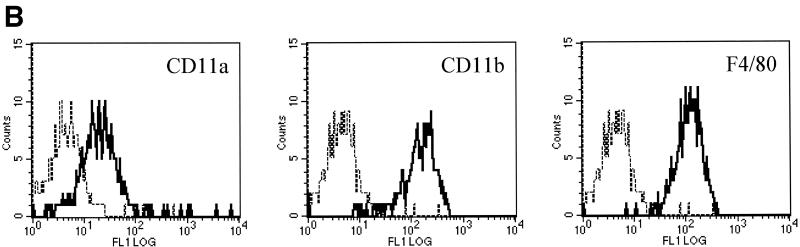
To determine whether the resulting cell line, 10ScNCr/23, was a clonal derivative of the primary C57BL/10ScNCr-derived macrophages, we determined the expression level of macrophage-specific surface markers by flow cytometry. Light microscopy had shown that the cell line 10ScNCr/23 exhibited macrophage-like morphology. The resulting cells were rounded in shape, adherent to tissue culture plates, and sensitive to trypsin detachment (Fig. 1A). Flow cytometric analysis of the cell line isolated in this study showed that all three surface markers were expressed at robust levels in cell line 10ScNCr/23, verifying the bone marrow-derived macrophage phenotype of the cells (Fig. 1B).
FIG. 1.
The cell line 10ScNCr/23 is derived from mouse bone marrow macrophages. (A) Light micrograph of cells derived from the cell line 10ScNCr/23, indicating a macrophage-like appearance and adherent phenotype. (B) Flow cytometry analysis to determine expression levels of surface markers CD11a, CD11b, and F4/80, indicating the bone marrow macrophage origin of 10ScNCr/23.
The murine macrophage cell line is unresponsive to LPS but responsive to LTA.
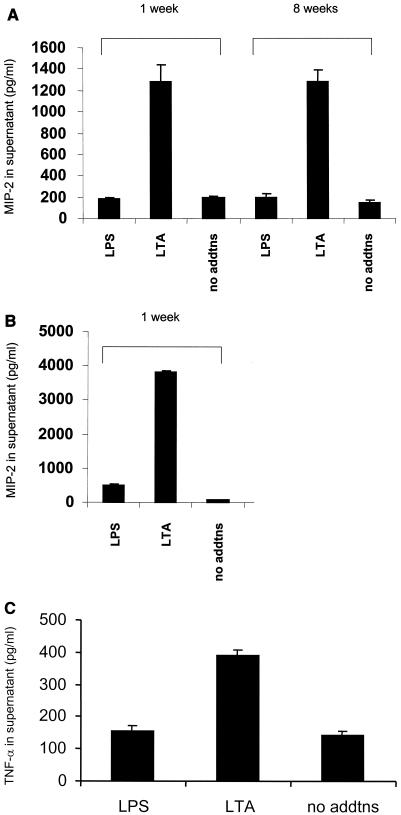
Mouse strain C57BL/10ScNCr was originally identified because of its hyporesponsive phenotype to LPS stimulation. Since LTA signals through the TLR2 receptor (14) rather than the TLR4 receptor, which is deleted in mouse strain C57BL/10ScNCr, we determined whether functional TLR2 signaling was present in the mouse macrophage cell lines by adding endotoxin-free LTA. A total of 100,000 macrophages were plated per well in a 12-well dish and stimulated with 100 ng of LPS/ml or 1.5 μg of LTA/ml. Unstimulated cells were included as a negative or baseline control. Supernatants were collected on a weekly basis for 8 weeks to determine whether the macrophage cell line had a stable LPS-unresponsive phenotype while being responsive to LTA. As a control, primary peritoneal macrophages from mouse strain C57BL/10ScNCr were stimulated in parallel with LPS or LTA. Supernatants were used in ELISAs to determine whether MIP-2 or TNF-α secretion was induced by either LTA or LPS. Figure 2 shows that the cell line has a response profile similar to that of the primary mouse macrophages. Neither cell type is responsive to LPS, but both demonstrate strong cytokine induction in response to LTA.
FIG. 2.
The cell line 10ScNCr/23 is identical to primary macrophages from C57BL/10ScNCr mice in terms of cytokine secretion. (A) MIP-2 expression levels (in picograms per milliliter) in the cellular supernatants following culture for 1 or 8 weeks. Stimulants used included E. coli LPS 0111:B4 (100 ng/ml) and S. aureus LTA (1.5 μg/ml) for 4 h. A no-addition negative control was included as well. (B) MIP-2 secretion (in picograms per milliliter) of cellular supernatants from primary C57BL/10ScNCr bone marrow macrophages following a 4-h stimulation with E. coli 0111:B4 LPS at 100 ng/ml and S. aureus LTA (1.5 μg/ml). The primary macrophages were maintained in an identical medium as for the cell line for the duration of the experiment. A no-addition negative control was included as well. (C) TNF-α expression levels in the cellular supernatant of the macrophage cell line, maintained in culture for 1 week, following a 4-h stimulation with LPS or LTA. All experiments were repeated a minimum of three times and assayed in duplicate.
Certain LPS preparations can signal independently of TLR4. ****
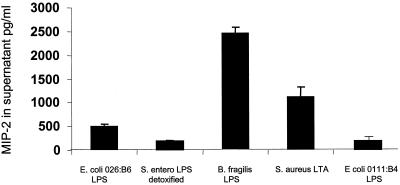
To initially determine the TLR specificity of various LPS preparations, we stimulated the TLR4−/− macrophage cell lines with a variety of LPS in vitro. Using S. aureus LTA as a positive control and detoxified LPS from Salmonella enterica serovar Enteritidis as a negative control, we performed in vitro testing of several endotoxin preparations. These endotoxin preparations were selected based on the lower induction of IL-1 secretion in human whole blood. We therefore hypothesized that these endotoxins are either nonfunctional or they signal through alternative receptors which do not result in IL-1 production. Since B. fragilis LPS and E. coli 026:B6 LPS are structurally different from typical E. coli and Salmonella endotoxins, they may be able to signal in the absence of TLR4. As can be seen in Fig. 3, B. fragilis LPS shows strong MIP-2 induction in the absence of TLR4, while E. coli 026:B6 LPS has some signal activity but significantly less than S. aureus LTA, the positive control. This result proves the applicability of the TLR4−/− macrophage cell line for determining the TLR specificity of endotoxins and additional bacterial compounds. In addition, this experiment identifies two novel endotoxin isolates that potentially signal in a TLR4-independent manner.
FIG. 3.
B. fragilis LPS signals in the absence of TLR4. The MIP-2 secretion levels (in picograms per milliliter) of cellular supernatant following the stimulation of the cell line 10ScNCr/23 with E. coli 026:B6 LPS (100 ng/ml), S. aureus LTA (1.5 μg/ml), and B. fragilis LPS (100 ng/ml) for 4 h are shown. Detoxified LPS derived from S. enterica serovar Enteritidis (S. entero; 100 ng/ml) and LPS from E. coli 0111:B4 were included as negative controls. All experiments were repeated a minimum of three times and assayed in triplicate.
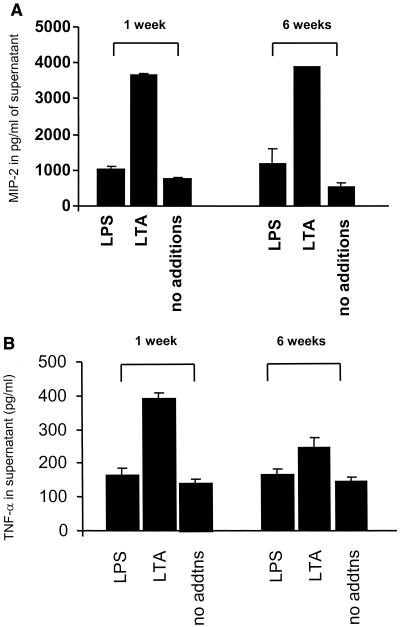
In the absence of TLR4, chronic LPS exposure does not lead to activation of alternative LPS response pathways.
Since TLR4 has been identified as the major LPS receptor in mammals, we hypothesized that in the absence of TLR4 and continuous LPS stimulation, potentially novel receptors could be activated to respond to LPS. We therefore stimulated the TLR4-deficient macrophage cell line for 6 weeks with LPS at a concentration of 100 ng/ml and collected supernatant on a weekly basis. ELISAs for MIP-2 and TNF-α from supernatants collected at 1 and 6 weeks did not show any altered levels of cytokine secretion (Fig. 4). This result supports TLR4 as the only receptor for E. coli 0111:B4 LPS, directing cytokine secretion in macrophages following LPS stimulation at physiological levels.
FIG. 4.
Chronic exposure to E. coli 0111:B4 LPS does not induce alternative receptors in the absence of functional TLR4. (A) MIP-2 secretion by the 10ScNCr/23 cell line (in picograms per milliliter) following 6 weeks of continuous LPS exposure. LPS was added at 100 ng/ml, LTA was added at 1.5 μg/ml, and a no-addition negative control was included as well. (B) TNF-α readout for the identical experimental conditions. All experiments were repeated twice and assayed in triplicate.
Interestingly, in contrast to MIP-2 production, the level of TNF-α secretion induced by chronic LTA stimulation did decrease, potentially indicating different pathways for regulating cytokine gene expression in response to LTA.
DISCUSSION
We have isolated a novel murine macrophage cell line, 10ScNCr/23, derived from mouse strain C57BL/10ScNCr. This mouse was originally isolated as an LPS-nonresponsive mouse strain because of a deletion of the TLR4 genomic region (17). In order to determine the specific role of TLR4 in signal transduction events initiated by bacterial pathogens, we set out to develop an in vitro model that would lack specifically TLR4. Rather than selecting mouse strain C3H/HeJ, which has a point mutation in TLR4 (Pro712His) that functions as a dominant-negative mutation, we selected a deletion strain for TLR4. The major drawback of using a cell line from a dominant-negative TLR4 mouse strain is the fact that binding of LPS to TLR4 is still occurring at normal levels. Since His712Pro is located in the intracellular domain of TLR4, this most likely leaves the LPS binding properties of TLR4 unaffected and will result in titrating out LPS from the culture medium. While other mammalian cell lines lacking TLR4, such as 293T cells, have been widely used, we wanted to develop an assay system that would specifically lack TLR4 while leaving the remaining constituents of the LPS signaling pathway, such as MD-2 and MyD88, intact. While mouse strain C57BL/10ScNCr has a deletion that is not limited to TLR4, the chromosomal region affected by the deletion has been mapped (17), and no other genes in the vicinity of TLR4 have been implicated in the response to LPS. We therefore selected C57BL/10ScNCr as the mouse strain for generating the TLR4-deficient cell lines.
Previously, differences in the signal transduction between rough and smooth endotoxin preparations as well as the ability of C3H/HeJ-derived macrophages to respond to certain LPS preparations were described (6). While this ability of macrophages expressing a dominant-negative TLR4 mutant protein to respond to endotoxin could have been interpreted as evidence for a TLR4-alternative receptor in endotoxin signaling (20), the structural diversity of the bacterial endotoxins was a more obvious reason for the C3H/HeJ endotoxin responsiveness. Since one of our aims was the detection of endotoxins signaling in a TLR4-independent fashion, we selected LPS preparations which required unusually high threshold concentrations to induce cytokine release in human whole blood (data not shown). We suspected that because of the structural diversity, some bacterial endotoxin could employ non-TLR4 signal pathways. Indeed, two of three LPS preparations, namely, the B. fragilis LPS and E. coli 026:B6 LPS, were capable of stimulating the TLR4-deficient cell line 10ScNCR/23. Since these two LPS preparations induce IL-1 secretion in whole blood only at highly elevated concentrations compared to E. coli and Salmonella-derived LPS preparations, it is possible that they contain a signaling property unusual to most common LPS preparations. A second possibility is contamination with primary compounds, such as lipopeptides (9).
The experiments presented in the paper indicate that a macrophage cell line derived from mouse strain C57BL/10ScNCr is refractory to E. coli 0111:B4 LPS stimulation as measured by cytokine secretion. Since this phenotype is maintained over a time span of several weeks and with chronic endotoxin exposure, the results in this paper support TLR4 as the sole conduit for E. coli 0111:B4 LPS (4). Furthermore, the cell line maintains the responsiveness to bacterial pathogen signaling through alternative TLRs, such as TLR2, as shown by S. aureus LTA stimulation. This cell line should prove an excellent resource for chronic or long-term studies, which were previously not possible in TLR4-deficient macrophages. An immortal cell line makes such cells permanently available without preparation artifacts and further dedifferentiation during cultivation. The alternative approach, i.e., transfection of cell lines with selected TLR cDNAs, carries the risk of nonphysiological overexpression and compartmentalization within the cells. This might be an explanation for some of the controversies in the roles of TLR2 and TLR4 in LPS and LTA signaling.
In summary, this cell line should prove an excellent resource for the biochemical and genetic dissection of the Toll receptor pathway, since it allows the culture of large numbers of cells for protein and RNA extraction as well as reconstitution experiments using recombinant adenovirus expressing various mouse TLR4 isoforms.
Acknowledgments
This study was supported by grants from the Department of Veterans Affairs, the National Institute of Environmental Health Sciences (ES06537, ES07498, ES09607, and U19 ES11375-01), and the National Heart, Lung, and Blood Institute (HL62628). E.L. was the recipient of an NHRSA and a VA merit entry level award.
We thank Marina Freudenberg (Max-Planck Institute for Immunobiology, Freiburg, Germany) for technical assistance and helpful discussions.
T. Hartung and D. A. Schwartz contributed equally to the paper.
Editor: R. N. Moore
REFERENCES
- 1.Alexopolou, L., A. C. Holt, R. Medzhitov, and R. A. Flavell. 2001. Recognition of double-stranded RNA and activation of NF-κB by toll-like receptor 3. Nature 413:732-738. [DOI] [PubMed] [Google Scholar]
- 2.Asea, A., M. Rehli, E. Kabingu, J. Boch, O. Bare, P. Auron, M. Stevenson, and S. Calderwood. 2002. Novel signal transduction pathway utilized by extracellular HSP70: role of TLR2 and TLR4. J. Biol. Chem. 277:15028-15034. [DOI] [PubMed] [Google Scholar]
- 3.Bauer, S., C. J. Kirschning, H. Haecker, V. Redecke, S. Hausmann, S. Akira, H. Wagner, and G. B. Lipford. 2001. Human TLR9 confers responsiveness to bacterial DNA via species-specific CpG motif recognition. Proc. Natl. Acad. Sci. USA 98:9237-9242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beutler, B., X. Du, and A. Poltorak. 2001. Identification of Toll-like receptor 4 (Tlr4) as the sole conduit for LPS signal transduction: genetic and evolutionary studies. J. Endotoxin Res. 7:277-280. [PubMed] [Google Scholar]
- 5.Beutler, E., T. B. Gelbart, and C. West. 2001. Synergy between TLR2 and TLR4: a safety mechanism. Blood Cells Mol. Dis. 27:728-730. [DOI] [PubMed] [Google Scholar]
- 6.Flebbe, L. M., S. K. Chapes, and D. C. Morrison. 1990. Activation of C3H/HeJ macrophage tumoricidal activity and cytokine release by R-chemotype lipopolysaccharide preparations. J. Immunol. 145:1505-1511. [PubMed] [Google Scholar]
- 7.Hajjar, A. M., D. S. O'Mahony, A. Ozinsky, D. M. Underhill, A. Aderem, S. J. Klebanoff, and C. B. Wilson. 2001. Cutting edge: functional interactions between Toll-like receptor (TLR) 2 and TLR1 or TLR6 in response to phenol-soluble modulin. J. Immunol. 166:15-19. [DOI] [PubMed] [Google Scholar]
- 8.Hayashi, F., K. D. Smith, A. Ozinsky, T. R. Hawn, E. C. Yi, D. R. Goodlett, J. K. Eng, S. Akira, D. M. Underhill, and A. Aderem. 2001. The innate immunity response to bacterial flagellin is mediated by Toll-like receptor 5. Nature 410:1099-1102. [DOI] [PubMed] [Google Scholar]
- 9.Hirschfeld, M., Y. Ma, J. H. Weis, S. N. Vogel, and J. J. Weis. 2000. Cutting edge: repurification of lipopolysaccharide eliminates signaling through both human and murine toll-like receptor 2. J. Immunol. 264:618-622. [DOI] [PubMed] [Google Scholar]
- 10.Lorenz, E., M. Jones, C. Wohlford-Lenane, N. C. Meyer, K. L. Frees, N. C. Arbour, and D. A. Schwartz. 2001. Genes, other than TLR4, are involved in the response to LPS. Am. J. Physiol. 281:L1106-L1114. [DOI] [PubMed] [Google Scholar]
- 11.Lorenz, E., J.-P. Mira, K. L. Cornish, N. C. Arbour, and D. A. Schwartz. 2000. A novel polymorphism in the Toll-like receptor 2 gene and its potential association with staphylococcal infection. Infect. Immun. 68:6398-6401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Monner, D. A., and B. Denker. 1997. Characterization of clonally derived, spontaneously transformed bone marrow macrophage cell lines from lipopolysaccharide hyporesponsiveness LPS(d) and normal LPS(n) mice. J. Leukoc. Biol. 61:469-480. [DOI] [PubMed] [Google Scholar]
- 13.Morath, S., A. Geyer, and T. Hartung. 2001. Structure-function relationship of cytokine induction by lipotechoic acid from Staphylococcus aureus. J. Exp. Med. 193:393-397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Opitz, B., N. W. Schroeder, I. Spreitzer, K. S. Michelsen, C. J. Kirschning, W. Hallatscheck, U. Zaehringer, T. Hartung, U. B. Goebel, and R. R. Schumann. 2001. Toll-like receptor-2 mediates Treponema glycolipid and lipoteichoic acid-induced NF-κB translocation. J. Biol. Chem. 276:22041-22047. [DOI] [PubMed] [Google Scholar]
- 15.Poltorak, A., X. He, I. Smirnova, M.-Y. Liu, C. Van Huffel, X. Du, D. Birdwell, E. Alejos, M. Silva, C. Galanos, M. Freudenberg, P. Ricciardi-Castagnoli, B. Layton, and B. Beutler. 1998. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science 282:2085-2088. [DOI] [PubMed] [Google Scholar]
- 16.Poltorak, A., T. Merlin, P. J. Nielsen, O. Sandra, I. Smirnova, S. I., T. Boehm, C. Galanos, and M. A. Freudenberg. 2001. A point mutation in the IL-12Rβ2 gene underlies the IL-12 unresponsiveness of LPS-defective C57BL/10ScCr mice. J. Immunol. Methods 167:2106-2111. [DOI] [PubMed] [Google Scholar]
- 17.Poltorak, A., I. Smirnova, R. Clisch, and B. Beutler. 2000. Limits of a deletion spanning TLR4 in C57BL/10ScCr mice. J. Endotoxin Res. 6:51-56. [DOI] [PubMed] [Google Scholar]
- 18.Rock, F. L., G. Hardiman, J. C. Timans, R. A. Kastelein, and J. F. Bazan. 1998. A family of human receptors structurally related to Drosophila Toll. Proc. Natl. Acad. Sci. USA 95:588-593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schroeder, N. W., B. Optiz, N. Lamping, K. S. Michelsen, U. Zaehringer, U. B. Goebel, and R. R. Schumann. 2000. Involvement of lipopolysaccharide binding protein, CD14, and toll-like receptors in the initiation of innate immune responses by Treponema glycolipids. J. Immunol. 165:2683-2693. [DOI] [PubMed] [Google Scholar]
- 20.Schwartz, D. A. 2001. Inhaled endotoxin, a risk for airway disease in some people. Respir. Physiol. 128:47-55. [DOI] [PubMed] [Google Scholar]
- 21.Takeuchi, O., and S. Akira. 2001. Toll-like receptor; their physiological role and signal transduction system. Int. Immunopharmacol. 1:625-635. [DOI] [PubMed] [Google Scholar]
- 22.Walker, W. S. 1994. Establishment of mononuclear phagocyte cell lines. J. Immunol. Methods 174:25-31. [DOI] [PubMed] [Google Scholar]
- 23.Wright, S. D. 1999. Toll, a new piece in the puzzle of innate immunity. J. Exp. Med. 189:605-609. [DOI] [PMC free article] [PubMed] [Google Scholar]