Abstract
Objective
CC chemokines and their receptors play a fundamental role in trafficking and activation of leukocytes at sites of inflammation, contributing to joint damage in rheumatoid arthritis. Met-RANTES, an amino-terminal–modified methionylated form of RANTES (CCL5), antagonizes the binding of the chemokines RANTES and macrophage inflammatory protein 1α (MIP-1α; CCL3) to their receptors CCR1 and CCR5, respectively. The aim of this study was to investigate whether Met-RANTES could ameliorate adjuvant-induced arthritis (AIA) in the rat.
Methods
Using immunohistochemistry, enzyme-linked immunosorbent assay, real-time reverse transcription–polymerase chain reaction, Western blot analysis, adoptive transfer, and chemotaxis, we defined joint inflammation, bony destruction, neutrophil and macrophage migration, Met-RANTES binding affinity to rat receptors, proinflammatory cytokine and bone marker levels, CCR1 and CCR5 expression and activation, and macrophage homing into joints with AIA.
Results
Administration of Met-RANTES as a preventative reduced the severity of joint inflammation. Administration of Met-RANTES to ankles with AIA showed decreases in inflammation, radiographic soft tissue swelling, and bone erosion. Met-RANTES significantly reduced the number of neutrophils and macrophages at the peak of arthritis compared with saline-injected controls. Competitive chemotaxis in peripheral blood mononuclear cells demonstrated that Met-RANTES inhibited MIP-1α and MIP-1β at 50% inhibition concentrations of 5 nM and 2 nM, respectively. Furthermore, levels of tumor necrosis factor α, interleukin-1β, macrophage colony-stimulating factor, and RANKL were decreased in joints with AIA in the Met-RANTES group compared with the control group. Interestingly, the expression and activation of CCR1 and CCR5 in the joint were down-regulated in the Met-RANTES group compared with the control group. Functionally, Met-RANTES administration decreased adoptively transferred peritoneal macrophage homing into the joint.
Conclusion
The data suggest that the targeting of Th1-associated chemokine receptors reduce joint inflammation, bone destruction, and cell recruitment into joints with AIA.
Rheumatoid arthritis (RA) is a chronic inflammatory disease characterized by infiltration of monocytes, T cells, and polymorphonuclear cells into the joint. Leukocytes and other cells in the RA synovial tissue produce mediators of inflammation, including cytokines, chemokines and matrix-degrading enzymes, among others (1). Chemokines are chemotactic cytokines that participate in immune and inflammatory responses by facilitating chemoattraction and the migration of leukocytes from the circulation to sites of inflammation. Chemokines are divided into 4 subfamilies (CXC, CC, C, and CX3C) based on the arrangement of their amino-terminal cysteine residues (2).
Macrophage inflammatory protein 1α (MIP-1α; CCL3) and RANTES (CCL5), which are ligands for CCR1 and CCR5, respectively, are chemotactic for monocytes, T cells, natural killer cells, eosinophils, and basophils (3,4). RA synovial fibroblasts produce MIP-1α and RANTES upon stimulation with tumor necrosis factor α (TNFα) or interleukin-1 (IL-1) (5,6). Evidence from our laboratory indicates that CCR1 is expressed in normal and RA peripheral blood monocytes and on some synovial fluid monocytes, whereas CCR5 expression on monocytes and T memory cells from RA synovial fluid is significantly higher than the expression on monocytes and memory T cells from RA and normal peripheral blood. In RA synovial tissue, CCR1 and CCR5 immunoreactivity has been detected in areas in which there are CD68+ macrophages (7).
We recently showed that CCR1, CCR5, and MIP-1β/CCL4 messenger RNA (mRNA) expression is up-regulated on day 14 (early arthritis) after adjuvant injection and peaks on day 18 after adjuvant injection, which is coincident with maximum inflammation in the joints of rats with AIA (8). In addition, increases in receptor tyrosine phosphorylation of CCR1 (days 14, 18, 21, and 24 after adjuvant injection) and CCR5 (days 14, 18, and 21 after adjuvant injection) were detected in rats with AIA compared with nonarthritic control rats. CCR1 and CCR5 mRNA up-regulation was simultaneous with phosphorylation of these 2 receptors on day 14 after adjuvant injection in rat ankles with AIA. Phosphorylated CCR1 and CCR5 subsequently associate with JAK-1/STAT-1/STAT-3 at different stages of disease; however, their signaling activation overlaps during the peak of arthritis in AIA (8). Based on these observations, we treated AIA in the rat with an amino-terminal–modified methionylated RANTES (Met-RANTES).
Met-RANTES antagonizes the binding of RANTES to CCR1 and CCR5 with nanomolar potency (9). Met-RANTES blocks calcium mobilization and T cell and monocyte chemotaxis, and induces only weak internalization of CCR1 and CCR5 (10). In this study, we present evidence that Met-RANTES blocks receptors in the rat with high affinity. Met-RANTES has previously been shown to reduce symptoms of inflammation in Th1-mediated disorders, such as allograft nephropathy (11) and colitis (12), in the rat, as well as in a rat model of organ transplant rejection (13).
In this study, we found that the incidence and severity of arthritis in rats with AIA were reduced by the administration of Met-RANTES. This was assessed by measuring clinical indicators of inflammation, macrophage and neutrophil infiltration, and bone destruction. Met-RANTES blocked the binding of human MIP-1α and MIP-1β to rat peripheral blood mononuclear cells (PBMCs) and decreased levels of proinflammatory cytokines, markers of bone destruction, and CCR expression and activation in the joints of rats. We also demonstrated that the mechanism of amelioration of AIA by Met-RANTES is by down-regulating the migration of macrophages into the joint.
MATERIALS AND METHODS
Isolation and chemotaxis of rat PBMCs
Rat blood was purchased from Harlan Sera-Lab (Loughborough, UK), and PBMCs were isolated using OptiPrep according to the manufacturer’s instructions (Accurate, Westbury, NY). Chemotaxis was performed using a method previously described (14), with the following modifications. The pore size used for PBMC migration was 3 μm. Rat PBMC chemotaxis was performed with different doses (from 10 pM to 10 μM) of human MIP-1α and MIP-1β. To test inhibition by Met-RANTES, a constant concentration of 1 nM of the agonists MIP-1α and MIP-1β was placed in the lower chambers, with the various concentrations of Met-RANTES (from 10 pM to 10 μM) in the upper chambers.
Induction of AIA in rats and treatment with Met-RANTES
Five-week-old female Lewis rats weighing 100 gm were injected subcutaneously at the base of the tail with 300 μl (5 mg/ml) of lyophilized Mycobacterium butyricum (Difco, Detroit, MI) in sterile mineral oil on day 0. On day 5 after adjuvant injection, Met-RANTES was diluted with 0.9% NaCl, and rats were given a daily intraperitoneal (IP) injection of 100 μg of Met-RANTES until day 18 after adjuvant injection (total of 14 days). We chose the dosage of 100 μg/day in order to be in the middle range of the doses in previous studies (11–13). Control rats received injections of 0.9% NaCl from day 5 to day 18 after adjuvant injection. The experimental and the control groups contained 8 rats each. Rats with AIA that received saline showed signs of inflammation around days 10–14 after adjuvant injection, and on day 18, most of the rats had severe arthritis.
Clinical measurements
The clinical parameters measured included body weight, an articular index (AI) score, ankle circumference, and paw volume. AI scores were recorded for each ankle joint by the same observer (SS), who was blinded to the treatment the animal received. Scoring for arthritis was performed using a 0–4 scale, where 0 = no swelling or erythema, 1 = slight swelling and/or erythema, 2 = low-to-moderate edema, 3 = pronounced edema with limited use of the joint, and 4 = excessive edema with joint rigidity. Ankle circumferences were determined by measuring 2 perpendicular diameters, the laterolateral diameter and the anteroposterior diameter, using Lange calipers (Cambridge Scientific, Cambridge, MA). Circumference was determined using the following formula:
where a and b represent the measured diameters. Evaluations of body weight, AI score, and ankle circumference were performed on days 0, 3, 7, 10, 14, 16, 18, 23, 25, and 29. Hind ankle volume was determined using a paw volume plethysmometer (Kent Scientific, Litchfield, CT) on days 0, 16 and 29. Rats were killed on days 0, 14, 18, and 29, and the blood was removed and stored for laboratory testing.
Antibodies and immunohistochemistry
Staining was performed as previously described (15,16). Macrophages were identified with mouse anti-rat CD68 monoclonal antibody (mAb) (1:50 dilution; Serotec, Oxford, UK). Isotype-matched control IgG (mouse) was used as a negative control. Neutrophils were distinguished by staining with hematoxylin and eosin.
Microscopic analysis
Macrophages were distinguished based on immunostaining for CD68. A 4-point scale was used to score both vascularity (1 = marked decrease in vessel density, 2 = normal vessel density, 3 = increased vessel density, and 4 = marked increase in vessel density, resembling granulation tissue) and inflammation (1 = normal, 2 = increased number of inflammatory cells, arrayed as individual cells, 3 = increased number of inflammatory cells including distinct clusters (aggregates), and 4 = marked diffuse infiltrate of inflammatory cells). A 5-point scale was used to score neutrophils (0 = none, 1 = rare, 2 = scattered, 3 = many, and 4 = abscess-like). The score data were pooled, and the mean ± SEM was calculated in each group of data.
Protein extracts
In order to perform enzyme-linked immunosorbent assay (ELISA) and Western blotting analyses, protein was extracted from ankles as previously described (8).
ELISA
Cytokine levels in ankle homogenates were determined using commercially available ELISA kits that specifically recognize the rat cytokines TNFα, IL-1β, and RANTES (BioSource International, Camarillo, CA) (17,18). Assays were performed according to the manufacturer’s instructions.
Ankle radiographs and radiographic scoring
On day 29 after adjuvant injection, rats were killed, ankles were removed and placed on ice, and radiographs were taken. Radiographs were scored for the degree of bony destruction/erosions, assigning 1 point for an erosion in the tibia, the calcaneus, the talus, and the metatarsals (considered together) (total score 0–4). For example, the maximum score an ankle could receive was 4 if erosion was present in the tibia, calcaneus, talus, and any one or more of the metatarsals. Soft tissue swelling was scored on a scale of 0–3, where 0 = mild or no swelling, 2 = moderate swelling, and 3 = severe swelling (maximum score of 3 for a single ankle). Radiographs were scored by 2 observers (CCP and RMP), who were blinded to the experimental groups.
TaqMan real-time reverse transcription–polymerase chain reaction (RT-PCR)
Preparation and reverse transcription of mRNA were performed as described previously (8). The PCR primer and the TaqMan fluorogenic probe were designed using the Primer Express program (version 1.01; Perkin-Elmer Applied Biosystems, Foster City, CA). The primer and probe sequences were chosen from GenBank, as follows: for macrophage colony-stimulating factor (M-CSF), NM_023981; for RANKL, NM_057149OPG, and for osteoprotegerin (OPG), U94330. The primer and probe sets used for CCR1 and CCR5 were as previously described (8). The TaqMan probe carries a 5′ FAM reporter dye and a 3′ TAMRA quencher dye (Mega Bases, Chicago, IL). The quantity of complementary DNA (cDNA) for the gene of interest was directly related to the fluorescence detection of FAM after 40 cycles.
The amount of cDNA was calculated using a comparative threshold (Ct) method and standard curve method as described in Perkin Elmer ABI Prism 7700 User Bulletin 2, 1997, and by Favy et al (19). In both methods, the estimated amount of the gene of interest was normalized against the amount of GAPDH to compensate for variations in quantity as well as for differences in reverse transcription efficiency, as previously described (8). The primer and probe sequence for M-CSF, RANKL, and OPG are shown in Table 1.
Table 1.
Primer and probe sequences for markers of bone destruction*
| Forward primer | TaqMan probe | Reverse primer | |
|---|---|---|---|
| M-CSF | CTTGGCTTGGGATGATTCTCA | AGGACAGAGGGAAGCTCCCTCTTGCC | TCTATGCGAAGAGGCAGATCACT |
| RANKL | TGAGGCTCAGCCGTTTGC | CACCTCACCATCAATGCTGCCGA | ATGGGAACCCGATGGGAT |
| OPG | AGCTGGCACACGAGTGATGA | TGCGTGTACTGCAGCCCCGTG | TTTCACGGTCTGCAGTTCCTT |
M-CSF = macrophage colony-stimulating factor; OPG = osteoprotegerin.
Immunoprecipitation
Immunoprecipitation was performed according to the instructions of Roche (Indianapolis, IN). The reagents used were 50 μl of protein A–agarose (Roche) and 5 μl of either CCR1 (SC-7934; Santa Cruz Biotechnology, Santa Cruz, CA) or CCR5 (obtained from BD PharMingen, San Diego, CA, through the Acquired Immunodeficiency Syndrome Research and Reference Reagent Program, National Institute of Allergy and Immune Diseases, National Institutes of Health, Bethesda, MD) as described previously (8).
Western blot analysis
Equal amounts of each sample were loaded on a 10% sodium dodecyl sulfate–polyacrylamide gel electrophoresis gel and transferred to nitrocellulose membranes using a semidry transblotting apparatus (Bio-Rad, Hercules, CA). Nitrocellulose membranes were blocked for 60 minutes at room temperature with 5% nonfat milk in Tris buffered saline–Tween (TBST) buffer (20 mM Tris, 137 mM NaCl, pH 7.6, with 0.1% Tween 20). Blots were incubated for 60 minutes with anti-CCR1, CCR5 antibody (pan–antibody) (Santa Cruz Biotechnology), or antiphosphotyrosine (Cell Signaling Technology, Beverly, MA) at a dilution of 1:1,000 in TBST containing 5% nonfat milk (8).
Western blotting was performed identically for all antibodies and samples used. In this study, all 4 polyacrylamide minigels were run and blotted simultaneously in a semidry transfer apparatus. In addition, all 4 membranes (pCCR1/control day 14, pCCR1/Met-RANTES day 14, pCCR1/control day 18, and pCCR1/Met-RANTES day 18) were placed in the same cassette and exposed to radiographic film for 2 minutes. The same was done for the pCCR5 membranes. Thereafter, the membranes were stripped and rehybridized with anti-CCR1 antibody or with anti-CCR5 antibody. The 4 membranes of each group were then placed in the same cassette and simultaneously exposed to radiographic film for 2 minutes. The blots were scanned, and the pCCR1 and pCCR5 band intensities were measured and normalized against those of CCR1 and CCR5, respectively.
Adoptive transfer of dye-tagged resident peritoneal macrophages
On day 0, AIA was induced in female Lewis rats. In the experimental group (n = 8), each rat received a daily IP injection of 100 μg of Met-RANTES from day 1 to day 14 after adjuvant injection (total of 14 days). The control rats (n = 8) received 0.9% NaCl from day 1 to day 15 after adjuvant injection. Rats were then killed, and 20 ml of Hanks’ balanced salt solution (HBSS) containing 2 mM EDTA (pH 8.0) was injected into the abdominal cavity. After massaging the abdomen for 10–15 minutes, the abdominal cavity was opened, and the intraperitoneal fluid was obtained. The harvested cells were resuspended in HBSS, and the number and purity of the cells were evaluated. Freshly isolated resident rat peritoneal macrophages from female Lewis rats (n = 10) were dye-tagged with PKH26 (Sigma, St. Louis, MO) according to the manufacturer’s instructions. Successful labeling of the cells was monitored with a fluorescence microscope after performing a cytospin.
Labeled cells (2 × 106/rat) were injected intravenously into the tail vein of rats with AIA, and 48 hours later (day 18 after adjuvant injection), the animals were killed, and the ankles were removed and frozen. Cryosections (10 μm thick) were cut, and the sections were examined using a fluorescence microscope. The number of fluorescent macrophages that migrated was assessed by counting four 100× fields from 20 sections (10 sections in duplicate) obtained from the right and left ankles of 5 rats (total of 10 ankles). The number of cells per high-power field (100×) were counted blindly in ankle joint sections from each treatment group (n = 10 ankles per group).
Statistical analysis
Clinical data were analyzed using Wilcoxon’s 2-sample test or rank sum test and Student’s 2-sample t-test assuming equal variance. The remainder of the data were analyzed using Student’s t-test. P values less than 0.05 were considered significant.
RESULTS
High-affinity binding of Met-RANTES to rat receptors
Rat mononuclear cell chemotaxis was performed with different concentrations of MIP-1α and MIP-1β. Using 1 nM MIP-1α and MIP-1β, a competitive chemotaxis was performed with different concentrations of Met-RANTES in rat PBMCs (Figures 1B and D). The results showed that Met-RANTES inhibited MIP-1α with a 50% inhibition concentration (IC50) of 5 nM and inhibited MIP-1β at an IC50 of 2 nM. Since both MIP-1α and MIP-1β bind to CCR1 and CCR5, these data support the high-affinity binding of Met-RANTES to these rat receptors.
Figure 1.
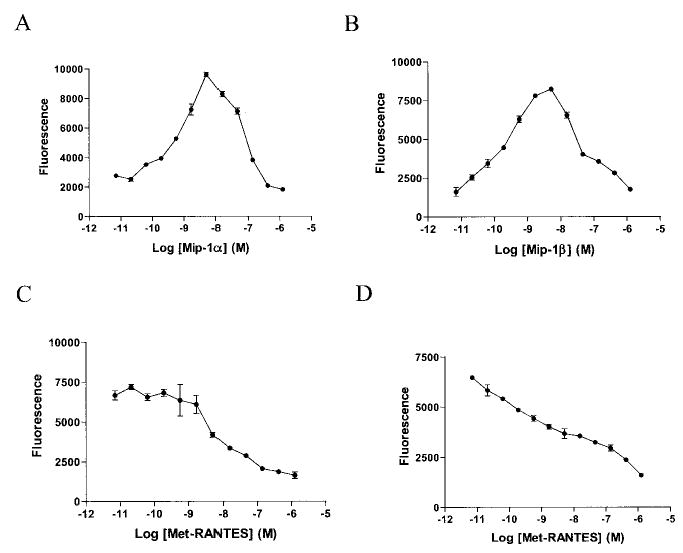
Inhibition of the binding of human macrophage inflammatory protein 1α (MIP-1α) and MIP-1β to rat mononuclear cells by Met-RANTES in the nanomolar range. A, Chemotaxis of rat peripheral blood mononuclear cells (PBMCs) was induced by adding different doses of human MIP-1α. B, Dose-response curve of human MIP-1β chemotaxis performed using rat PBMCs. C, Met-RANTES inhibited human MIP-1α–induced chemo-taxis in rat PBMCs. D, Met-RANTES displaced human MIP-1β–induced chemotaxis in rat PBMCs. Values are the mean ± SEM of 2 rats per group.
Reduction of clinical features of AIA in the rat by Met-RANTES administration
Met-RANTES was administered IP from day 5 to day 18 after adjuvant injection (100 μg/day per rat; n = 8 rats). Control rats received sterile saline. Scores on the AI (0–4 for each ankle) were calculated as the sum of the left and right ankles. The mean AI scores for control ankles show that AIA-induced swelling began around day 10, increased through day 23, and plateaued thereafter (until day 29). In comparison, in the Met-RANTES–treated animals, only a mild increase in the mean AI scores was observed from day 10 until day 16 after adjuvant injection and then leveled off through day 29. From day 18 through day 29, the mean AI score in Met-RANTES–injected animals was significantly lower (44–58% decrease; P < 0.05) than that in the control rats (Figure 2A).
Figure 2.
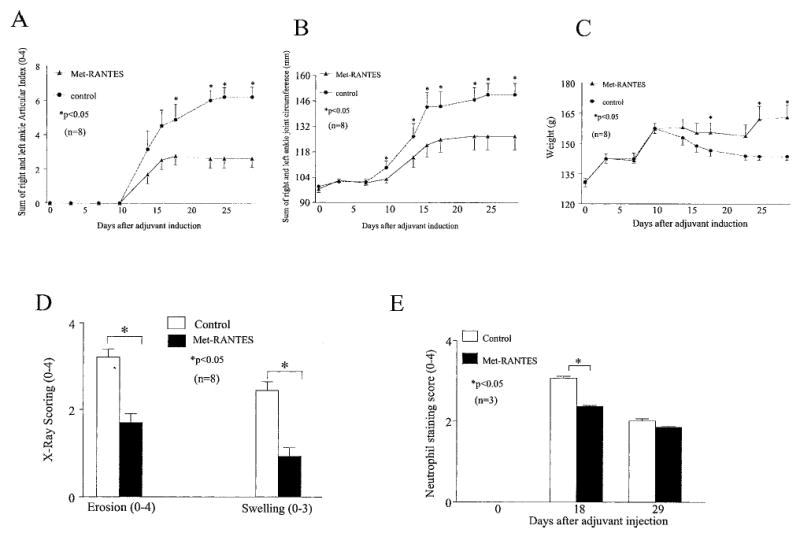
Amelioration and reduction of the severity of joint inflammation, bony destruction, and neutrophil recruitment in the ankles of rats with adjuvant-induced arthritis (AIA) by Met-RANTES administered as a preventative. Changes in A, the sum of articular indices on the right and left sides, B, the sum of the ankle joint circumferences on the right and left sides, C, the body weights, D, radiographic scores, and E, neutrophil staining scores of rats in the Met-RANTES and control groups over the 29-day course of study of AIA. Ankles were radiographed on day 29 after adjuvant injection and were scored for bony erosion and soft tissue swelling. Values were significantly lower in the Met-RANTES–treated group compared with the control group at the time points indicated (scale ends on day 29). Values are the mean and SEM of 8 rats per group, except for neutrophil staining, which represents 3 rats per group.
The mean ankle circumference in the Met-RANTES–treated group demonstrated significantly smaller ankles (P < 0.05) on days 10 (6% difference), 14 (10%), 16 (15%), 18 (13%), 23 (14%), 25 (16%), and 29 (16%) after adjuvant injection compared with the control group (Figure 2B). Plethysmometry showed that mean paw volume for the Met-RANTES–treated group with AIA was significantly lower (P < 0.05) on days 16 (45%) and 29 (65%) after adjuvant injection as compared with the control group (data not shown). These results, as well as the consistency of results for the 3 methods of measuring inflammation in the rat paws, indicate that Met-RANTES reduced the inflammation of AIA in the rat.
Furthermore, the mean body weight of control rats was lower on day 10 after adjuvant injection and declined gradually throughout the study. In contrast, the mean body weights of the Met-RANTES–treated rats remained fairly constant from day 10 until day 23 after adjuvant injection and then increased for the remainder of the study. Met-RANTES–treated rats had significantly higher mean body weights on days 18 (6% difference), 25 (12%), and 29 (13%) compared with the control group (Figure 2C).
Radiographs of all ankles harvested on day 29 after adjuvant injection were scored for bony erosion and soft tissue swelling. Consistent with the physical examination findings, Met-RANTES treatment resulted in less radiographic swelling and significantly reduced joint erosion by almost 50% (Figure 2D). Our results demonstrate that Met-RANTES treatment decreased neutrophil influx by 23% on day 18 after adjuvant injection compared with the control group. However, on day 29 after adjuvant injection, there was no significant difference in neutrophil recruitment between the 2 treatment groups (Figure 2E). Therefore, Met-RANTES administration not only reduced signs of inflammation and joint destruction, but also decreased the numbers of neutrophils, which are important in early phases of arthritis.
Reduction of inflammation and CD68+ macrophage detection in synovial tissues of rats with AIA by Met-RANTES administration
Ankles from rats with AIA were obtained on day 18 after adjuvant injection and were immunostained with anti-CD68+ antibody (Figure 3). Consistent with the clinical data, the Met-RANTES–treated animals had significantly lower scores for inflammation and vascularity than did the controls (P < 0.05). Staining for macrophages in the synovial lining and sublining was also down-regulated in the Met-RANTES group (by 46% and 54%, respectively) compared with the control group (P < 0.05). These observations suggest that decreased inflammation and macrophage infiltration were responsible for the effects of Met-RANTES.
Figure 3.
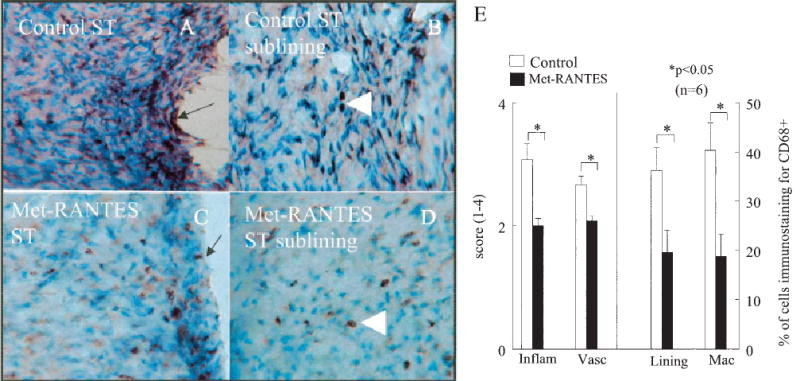
Decrease in CD68+ macrophage immunostaining in Met-RANTES–treated rats with adjuvant-induced arthritis (AIA) compared with control rats with AIA on day 18. Shown is staining of A, synovial tissue (ST) from a control rat, B, synovial tissue sublining from a control rat, C, synovial tissue from a Met-RANTES–treated rat, and D, synovial tissue sublining from a Met-RANTES–treated rat. Arrows indicate the lining cell layer; arrowheads indicate subsynovial macrophages. (Original magnification × 200 in A; × 400 in B–D.) E, Quantification of CD68+ immunoreactivity in synovial tissue from Met-RANTES–treated and control rats with AIA. Inflam = inflammation; vasc = vascularity; lining = synovial lining; Mac = macrophages. Values are the mean and SEM percentage of cells (n = 6 ankles per group).
Down-regulation of proinflammatory cytokine levels in the joint and markers of bone destruction in rats with AIA by Met-RANTES administration
Met-RANTES treatment significantly reduced joint levels of TNFα (normalized to protein content) on days 14 (71%), 18 (61%), and 29 (86%) after adjuvant injection compared with the control group (P < 0.05) (Figure 4A). IL-1β levels were also down-regulated by Met-RANTES administration on days 18 (76%) and 29 (71%) after adjuvant injection in rat ankles with AIA (Figure 4B). The mean level of RANTES in the joints of Met-RANTES–treated rats was significantly lower on days 14 (78%) and 29 (80%) after adjuvant injection compared with control rats. However, Met-RANTES treatment had no effect on RANTES secretion on day 18 after adjuvant injection compared with the control group (data not shown). This may be because there was some ongoing inflammation in the Met-RANTES–treated AIA group, although it was still significantly lower than that in the control group. As seen in Figure 4A, joint levels of TNFα were also increased at that time point, so it is not unlikely that there were higher levels of RANTES. Based on our data from competitive chemotaxis studies, Met-RANTES may theoretically have a better affinity for replacing MIP-1α and MIP-1β binding to CCR1 and CCR5. The variability in RANTES response may also be due to the fact that it is produced by several cell types, namely, monocyte/macrophages, T cells, and RA synovial fibroblasts.
Figure 4.
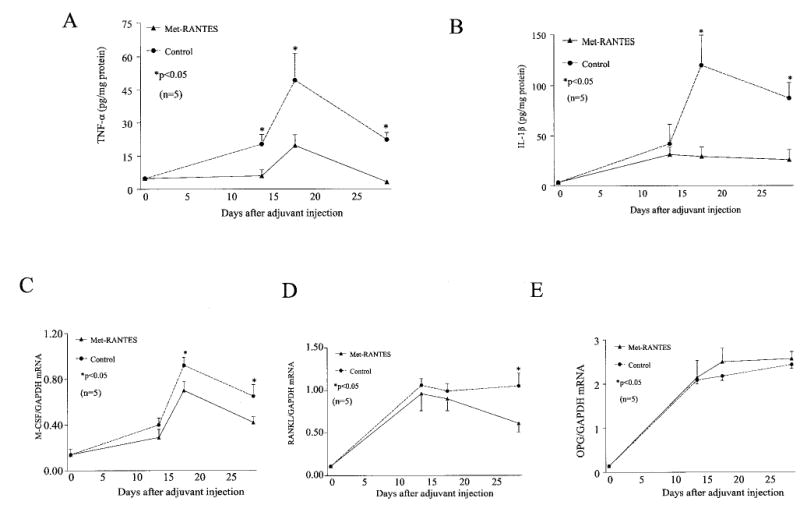
Lower levels of proinflammatory cytokines and markers of bone destruction in rats with adjuvant-induced arthritis (AIA) given Met-RANTES as a preventative compared with control rats with AIA. Changes in levels of A, tumor necrosis factor α (TNFα), B, interleukin-1β (IL-1β), C, macrophage colony-stimulating factor (M-CSF) messenger RNA (mRNA), D, RANKL mRNA, and E, osteoprotegerin (OPG) mRNA. Cytokine levels were measured in ankle homogenates by enzyme-linked immunosorbent assay and were normalized against the protein concentration. Levels of mRNA for M-CSF, RANKL and OPG were normalized against mRNA for GAPDH. Values were significantly lower in the Met-RANTES–treated group compared with the control group at the time points indicated (scale ends on day 29). Met-RANTES–treated rats had significantly lower levels of M-CSF after the peak of arthritis. There was no significant difference in OPG levels between the 2 groups at any time point. Values are the mean and SEM of 5 rats per group.
M-CSF and RANKL are 2 essential factors that induce the production of osteoclasts from hemopoietic progenitors. OPG is a soluble nonsignaling receptor for RANKL and interferes with RANK activation. The mean levels of M-CSF mRNA on days 18 and 29 after adjuvant injection in the Met-RANTES group were 24% and 36% below those in the control group at the same time points (Figure 4C). The mean level of RANKL mRNA was significantly lower (39%) on day 29 after adjuvant injection in the treatment group than in the control group, whereas there were no differences in the RANKL mRNA levels in the 2 groups on days 14 and 18 after adjuvant injection (Figure 4D). In contrast, Met-RANTES treatment had little or no effect on mean OPG mRNA levels (Figure 4E). These results indicate that Met-RANTES significantly decreased M-CSF mRNA levels on day 18 after adjuvant injection, prior to the reduction of RANKL on day 29.
Reduction of the expression and activation of CCR1 and CCR5 in ankles with AIA by Met-RANTES administration
We quantified CCR1 and CCR5 mRNA levels using real-time RT-PCR at different time points. Our results indicated that the CCR1 mRNA levels were significantly lower (P < 0.05) in the joints of Met-RANTES–treated animals on days 14 (55%), 18 (57%), and 29 (40%) after adjuvant injection as compared with the controls (Figure 5A). Similarly, Met-RANTES administration in rats with AIA suppressed CCR5 mRNA levels on days 14 (50%), 18 (40%), and 29 (50%) after adjuvant injection as compared with the controls (Figure 5B).
Figure 5.
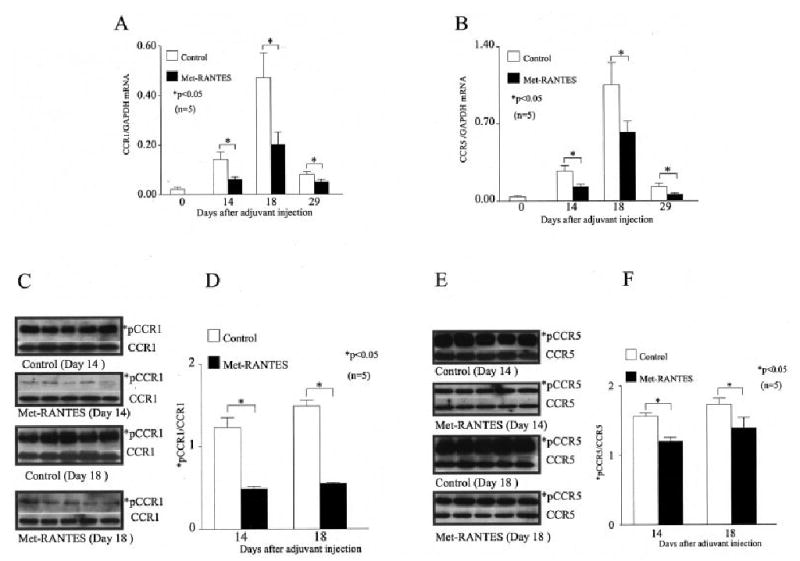
Significant decreases in joint levels of CCR1 and CCR5 messenger RNA (mRNA) and in tyrosine phosphorylation of CCR1 (pCCR1) and CCR5 (pCCR5) in Met-RANTES–treated rats with adjuvant-induced arthritis (AIA) compared with the control rats with AIA. Expression of A, CCR1 mRNA and B, CCR5 mRNA in the ankles of Met-RANTES–treated and control rats over the course of AIA. Values were normalized against mRNA for GAPDH. On days 14 and 18 after adjuvant injection, ankle homogenates from the 2 groups were immunoprecipitated with C, anti-CCR1 antibody and E, anti-CCR5 antibody and then Western blotted with antiphosphotyrosine antibody. Levels of *pCCR1 and *pCCR5 were normalized against the total CCR1 and CCR5 values, respectively. Quantification of D, *pCCR1/CCR1 and F, *pCCR5/CCR5 was obtained by the Western blot analyses performed in C and E, respectively. The intensity of the Western blot bands was quantified using Un-Scan-It software (version 5.1; Silk Scientific, Orem, UT). Values in A, B, D, and F are the mean and SEM of 5 animals per group; in C and E, each lane represents a different animal.
Phosphorylation of CCR1 and CCR5 was studied on days 14 and 18 after adjuvant injection since we previously showed that these 2 chemokine receptors were phosphorylated at these times (8). Phosphorylation of both CCR1 and CCR5 was significantly down-regulated (P < 0.05) by Met-RANTES administration on day 14 (60% for CCR1; 24% for CCR5) and on day 18 (63% for CCR1; 20% for CCR5) as compared with the controls (Figures 5C–F). The reduced activation of the receptors was not due to their diminished expression, since the intensities of pCCR1 or pCCR5 were normalized by the total CCR1 or CCR5 values, respectively. Taken together, the findings show that Met-RANTES reduced the levels of CCR1 and CCR5, as well as the level of receptor activation, in ankles with AIA.
Decreased migration of resident peritoneal macrophages into rat synovial tissue by Met-RANTES administration
To determine whether Met-RANTES administration could functionally decrease adoptively transferred peritoneal macrophage homing into the joint, an in vivo cell migration assay was used. Results from this assay demonstrated that Met-RANTES treatment decreased resident peritoneal macrophage recruitment into the rat synovial tissue by almost 50% (Figure 6). Together with the other data, this observation suggests that Met-RANTES suppressed inflammation and joint destruction by inhibiting the migration of macrophages into the joints.
Figure 6.
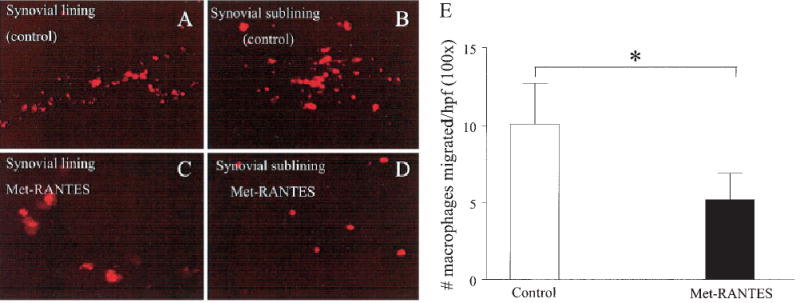
Significant reductions in the numbers of adoptively transferred peritoneal macrophages migrating into the ankle joints of rats with adjuvant-induced arthritis (AIA) treated with Met-RANTES as compared with controls. Macrophages were dye-tagged with PKH26. Shown are macrophages that migrated to A, the synovial lining layer of a control ankle, B, the synovial sublining layer of a control ankle, C, the synovial lining layer of a Met-RANTES–treated ankle, and D, the synovial sublining layer of a Met-RANTES–treated ankle. (Original magnification × 200 in A and B; × 400 in C and D.) E, Quantification of the number of macrophages per high-power field (hpf; 100×) that migrated into the synovial tissue of Met-RANTES–treated and control rats with AIA. Cells in 4 high-power fields from 20 ankle joint sections (10 sections in duplicate) obtained from the right and left ankles of 5 rats (total of 10 ankles) were counted blindly. Values are the mean and SEM. Color figure can be viewed in the online issue, which is available at http://www.arthritisrheum.org.
DISCUSSION
Met-RANTES has previously been shown to be effective in other animal models of inflammation, including a collagen-induced arthritis model (20). However, little is known about the mechanism by which Met-RANTES exerts its effect in animal models of RA; therefore, we studied the mechanisms of Met-RANTES amelioration in AIA in rats. The pharmacokinetics of Met-RANTES has not been elucidated in rats. In determining the appropriate dosage of Met-RANTES, we examined other studies that used Met-RANTES in rodent models of disease (11–13). In a mouse model of atherosclerosis, Veillard et al (21) show that Met-RANTES could be rapidly detected in sera 2 hours after an initial injection of 100 μg. Those investigators administered a total of 400 mg of Met-RANTES to 12-week-old male mice (weighing ~25 gm) over a 2-week period (21). In our rat model, we administered a total of 1,400 mg of Met-RANTES to 5-week-old rats (weighing ~100 gm) over a 2-week period. Since Met-RANTES antagonizes the binding of human MIP-1α and MIP-1β to rat mononuclear cells (see Figure 1) at a similar range as its binding to THP-1 cells (IC50 of 6 nM) (9), the dosage used in our study would be comparable to that in previous studies.
In the Met-RANTES–treated animals, only a mild increase in the mean AI scores, paw volume, and joint circumference was observed until day 16 after adjuvant injection and then plateaued thereafter, whereas the same clinical parameters were elevated for a longer period in the control group before leveling off (Figure 2). In general, Met-RANTES significantly ameliorated levels of the clinical parameters beyond day 18 after adjuvant injection. This could be attributed to the fact that Met-RANTES treatment may modify the composition of leukocyte infiltrates by selective blockade of CCR1 and CCR5.
Consistent with the findings in our study, a previous study showed that polyclonal antibody against RANTES ameliorates inflammation and lymphocyte infiltration (22). Interestingly, the same group of investigators showed that anti–MIP-1α antibody had no effect on AIA, and they further hypothesized that the effect of RANTES in the AIA model may be through an un-cloned chemokine receptor or through a distinct signaling pathway. However, in the present study, we found evidence that Met-RANTES significantly decreases CCR1 and CCR5 mRNA and tyrosine phosphorylation, which as we have previously shown, signal through the JAK-1/STAT-1/STAT-3 pathway. Moreover, it would be expected that blockade of RANTES alone may differ from blockade of CCR1/CCR5, in which binding of a number of chemokines may be affected. A recent study showed that the collagen-induced arthritis phenotype in CCR5-deficient mice was similar to that in wild-type mice (23). We therefore speculate that the protective role of Met-RANTES in AIA may be attributed to the blockage of both CCR1 and CCR5.
Met-RANTES treatment decreased joint neutrophil influx on day 18 after adjuvant injection compared with control non–Met-RANTES–treated rats. It has been reported that neutrophils express CCR1 and CCR3 (24) and are responsive to MIP-1α (25) in in vivo migration assays. We previously showed that the mRNA and protein levels of MIP-1α increased in the initial and peak stages of arthritis in rat AIA. Thus, Met-RANTES might decrease neutrophil migration directly by blocking CCR1 or indirectly by down-regulating monocyte infiltration and secretion of cytokine-induced neutrophil chemoattractant (26). Consistent with our findings, Ajuebor et al (12) showed that Met-RANTES administration significantly reduces neutrophil infiltration in a rat model of colitis.
CD68+ immunostaining of ankles from the Met-RANTES group demonstrated reduced inflammation and synovial tissue lining cell and macrophage staining during peak inflammation (Figure 2). Consistent with our findings, other investigators have shown that Met-RANTES treatment in animal models decreases mononuclear cell recruitment to sites of inflammation (11,13,21). Kaufmann et al (27) showed that as peripheral blood monocytes mature to macrophages, they show higher levels of transcript and cell surface CCR1 and CCR5, and lower levels of CCR2. We have also previously shown that the vast majority of normal peripheral blood monocytes express CCR1 and CCR2 (87% and 84%, respectively); however, little cell surface CCR5 was detected (7). Since macrophages recruited into the AIA joint coexpress CCR1 and CCR5, the blocking of both receptors could result in decreased leukocyte adhesion and transmigration into the joint. CCR2 may be important in AIA, as described in a recent preliminary report (28) of a study showing that treatment with a CCR2 antagonist in AIA resulted in significant inhibition of inflammation and macrophage migration. Since Met-RANTES could not completely abrogate inflammation, receptors such as CCR2 and CXCR3 could account for the residual cell attraction.
Met-RANTES decreased the secretion of the proinflammatory cytokines TNFα and IL-1β in rat joints with AIA (Figures 4A and B). It is known that RA synovial fibroblasts produce RANTES, MIP-1α, and MIP-1β upon stimulation with TNFα and IL-1β (5,6). Interestingly, RANTES has been reported to induce TNFα and IL-1β production in murine astrocytes (29) and bone marrow–derived dendritic cells (30). There may thus be several possible mechanisms that could account for the action of Met-RANTES. It may be that Met-RANTES inhibits RANTES induction of proinflammatory cytokines in macrophages. Given that the proinflammatory cytokines enhance the production of RANTES through fibroblasts, reductions in levels of TNFα and IL-1β could affect RANTES secretion in AIA. In contrast to our findings, Veillard and coworkers (21) showed that Met-RANTES treatment had no effect on RANTES expression in the mouse model of hyper-cholesterolemia. However, the levels of MIP-1α, MIP-1β, and MIP-2 mRNA were down-regulated within vascular atherosclerotic tissue (21). We have previously shown that the maximum increase in the number of macrophages in AIA synovial tissue occurs between days 11 and 29 after adjuvant injection (15). Since Met-RANTES treatment in AIA down-regulates macrophage infiltration during this period, this may be another reason why TNFα and IL-1β production was found to be suppressed.
RANKL, M-CSF, and OPG are considered to be key regulators of osteoclastogenesis (31,32). Osteoclast precursors express RANK, which recognizes RANKL through cell-to-cell interaction, and this binding is inhibited by OPG (31,32). Osteoclast differentiation is M-CSF dependent and is either induced by RANKL–RANK interaction or through a TNFα-mediated pathway (31,33–36). IL-1 and RANKL regulate the activation of the osteoclast subsequent to its differentiation (36,37).
OPG treatment of rat AIA had no effect on inflammation but completely blocked bone loss (38). However, our data indicate that Met-RANTES treatment did not have any effect on the levels of joint OPG (Figure 4D). OPG production is increased by treatment with bone morphogenetic protein, IL-1, TNF, and estrogen, among other factors (39). Theoretically, since levels of TNFα and IL-1β were suppressed by Met-RANTES, OPG levels were not significantly increased to competitively block RANKL binding to its receptor.
TNFα activates M-CSF production (40), whereas levels of RANKL can be enhanced by IL-1 in T cells (39). Interestingly, in the present study, we demonstrated that M-CSF down-regulation occurred subsequent to TNFα reduction in rat AIA, whereas levels of IL-1β were reduced preceding the reduction of RANKL (Figure 4). To our knowledge, this is the first demonstration that a chemokine antagonist suppresses markers of bone destruction. It is noteworthy that TNFα stimulates osteoclast differentiation, and levels of TNFα were significantly decreased prior to those of IL-1β, which mediates osteoclast activation (Figure 2).
The down-regulation of markers of bony destruction is consistent with the findings of radiographic analysis of Met-RANTES–treated animals, which demonstrated 50% less bony erosion compared with controls (Figure 2C). It is possible that Met-RANTES reduces bone destruction by inhibiting bone resorption through its actions on osteoclast regulators and proinflammatory cytokines.
Met-RANTES treatment not only decreased the number of both receptors, but it also suppressed receptor activation. The maximum reduction in CCR1 (57%) was on day 18; however, the levels of CCR5 mRNA were almost equally reduced at the different time points examined (Figures 5A and B). Met-RANTES administration in rats with AIA resulted in lower pCCR1 levels compared with those of pCCR5 on days 14 and 18 (Figures 5C and D). These results suggest that the up-regulation and activation of CCR1 and CCR5 during peak arthritis, as previously shown by us (8), is probably due to an influx of receptor-expressing cells rather than an induction of CCR1+/CCR5+ cells present in the AIA joint.
To test this hypothesis, we adoptively transferred peritoneal macrophages into the tail vein of rats with AIA subsequent to Met-RANTES treatment. Previous studies have shown that adoptively transferred macrophages stay in the peripheral circulation only for a matter of hours. Specifically, the number of circulating peritoneal macrophages following intravenous injection is highest after 1 hour, declines significantly thereafter, and is detectable for 6 hours in the blood (41). After initial arrest in the lungs, peritoneal macrophages rapidly disseminate to the liver, and within 4 hours postinjection, they can be detected in the spleen (42). Hence, it would be difficult to account for the adoptively transferred macrophages that did not migrate to the joint. Our data demonstrate that Met-RANTES decreased almost by half the homing of peritoneal macrophages into the synovial tissue lining and sublining (Figure 6). This indicates that Met-RANTES participates functionally in the inhibition of the inflammatory process of arthritis by interfering with leukocyte migration into the joint lesion. Moreover, this observation supports the idea that suppression of leukocyte migration can control the AIA inflammatory response.
In conclusion, Met-RANTES reduces joint inflammation and neutrophil and macrophage migration, prevents bony destruction, has high-affinity binding for rat receptors, down-regulates levels of proinflammatory cytokines in the joint, lowers the levels of markers of bone destruction, reduces the levels of expression and activation of CCR1 and CCR5, and functionally decreases macrophage recruitment into AIA joints. These findings highlight the contributions of CCR1 and CCR5 in AIA.
Acknowledgments
We thank the staff of Dr. Steven M. Wolinsky’s laboratory (Northwestern University, Chicago, IL) for allowing us to use the TaqMan apparatus.
Footnotes
Supported by the NIH (grants AR-049353, AR-48267, HL-58695, AI-40987, AR-048269, and AR-049217), the Gallagher Professorship for Arthritis Research, the Frederick G. L. Huetwell and William D. Robinson, MD, endowed professorship, and the Veterans Administration Research Service.
References
- 1.Firestein GS. Evolving concepts of rheumatoid arthritis. Nature. 2003;423:356–61. doi: 10.1038/nature01661. [DOI] [PubMed] [Google Scholar]
- 2.Luster AD. Chemokines: chemotactic cytokines that mediate inflammation. N Engl J Med. 1998;338:436–45. doi: 10.1056/NEJM199802123380706. [DOI] [PubMed] [Google Scholar]
- 3.Sallusto F, Lanzavecchia A, Mackay CR. Chemokines and chemokine receptors in T-cell priming and Th1/Th2-mediated responses. Immunol Today. 1998;19:568–74. doi: 10.1016/s0167-5699(98)01346-2. [DOI] [PubMed] [Google Scholar]
- 4.Volin MV, Shah MR, Tokuhira M, Haines GK, Woods JM, Koch AE. RANTES expression and contribution to monocyte chemotaxis in arthritis. Clin Immunol Immunopathol. 1998;89:44–53. doi: 10.1006/clin.1998.4590. [DOI] [PubMed] [Google Scholar]
- 5.Hosaka S, Akahoshi T, Wada C, Kondo H. Expression of the chemokine superfamily in rheumatoid arthritis. Clin Exp Immunol. 1994;97:451–7. doi: 10.1111/j.1365-2249.1994.tb06109.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Koch AE, Kunkel SL, Harlow LA, Mazarakis DD, Haines GK, Burdick MD, et al. Macrophage inflammatory protein-1α: a novel chemotactic cytokine for macrophages in rheumatoid arthritis. J Clin Invest. 1994;93:921–8. doi: 10.1172/JCI117097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Katschke KJ, Jr, Rottman JB, Ruth JH, Qin S, Wu L, LaRosa G, et al. Differential expression of chemokine receptors on peripheral blood, synovial fluid, and synovial tissue monocytes/macrophages in rheumatoid arthritis. Arthritis Rheum. 2001;44:1022–32. doi: 10.1002/1529-0131(200105)44:5<1022::AID-ANR181>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 8.Shahrara S, Amin MA, Woods JM, Haines GK, Koch AE. Chemokine receptor expression and in vivo signaling pathways in the joints of rats with adjuvant-induced arthritis. Arthritis Rheum. 2003;48:3568–83. doi: 10.1002/art.11344. [DOI] [PubMed] [Google Scholar]
- 9.Proudfoot AE, Power CA, Hoogewerf AJ, Montjovent MO, Borlat F, Offord RE, et al. Extension of recombinant human RANTES by the retention of the initiating methionine produces a potent antagonist. J Biol Chem. 1996;271:2599–603. doi: 10.1074/jbc.271.5.2599. [DOI] [PubMed] [Google Scholar]
- 10.Oppermann M, Mack M, Proudfoot AE, Olbrich H. Differential effects of CC chemokines on CC chemokine receptor 5 (CCR5) phosphorylation and identification of phosphorylation sites on the CCR5 carboxyl terminus. J Biol Chem. 1999;274:8875–85. doi: 10.1074/jbc.274.13.8875. [DOI] [PubMed] [Google Scholar]
- 11.Song E, Zou H, Yao Y, Proudfoot A, Antus B, Liu S, et al. Early application of Met-RANTES ameliorates chronic allograft nephropathy. Kidney Int. 2002;61:676–85. doi: 10.1046/j.1523-1755.2002.00148.x. [DOI] [PubMed] [Google Scholar]
- 12.Ajuebor MN, Hogaboam CM, Kunkel SL, Proudfoot AE, Wallace JL. The chemokine RANTES is a crucial mediator of the progression from acute to chronic colitis in the rat. J Immunol. 2001;166:552–8. doi: 10.4049/jimmunol.166.1.552. [DOI] [PubMed] [Google Scholar]
- 13.Grone HJ, Weber C, Weber KS, Grone EF, Rabelink T, Klier CM, et al. Met-RANTES reduces vascular and tubular damage during acute renal transplant rejection: blocking monocyte arrest and recruitment. FASEB J. 1999;13:1371–83. [PubMed] [Google Scholar]
- 14.Proudfoot AE, Handel TM, Johnson Z, Lau EK, LiWang P, Clark-Lewis I, et al. Glycosaminoglycan binding and oligomerization are essential for the in vivo activity of certain chemokines. Proc Natl Acad Sci U S A. 2003;100:1885–90. doi: 10.1073/pnas.0334864100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Szekanecz Z, Haines GK, Harlow LA, Shah MR, Fong TW, Fu R, et al. Increased synovial expression of transforming growth factor (TGF)-β receptor endoglin and TGF-β1 in rheumatoid arthritis: possible interactions in the pathogenesis of the disease. Clin Immunol Immunopathol. 1995;76:187–94. doi: 10.1006/clin.1995.1114. [DOI] [PubMed] [Google Scholar]
- 16.Halloran MM, Szekanecz Z, Barquin N, Haines GK, Koch AE. Cellular adhesion molecules in rat adjuvant arthritis. Arthritis Rheum. 1996;39:810–9. doi: 10.1002/art.1780390514. [DOI] [PubMed] [Google Scholar]
- 17.Woods JM, Katschke KJ, Volin MV, Ruth JH, Woodruff DC, Amin MA, et al. IL-4 adenoviral gene therapy reduces inflammation, proinflammatory cytokines, vascularization, and bony destruction in rat adjuvant-induced arthritis. J Immunol. 2001;166:1214–22. doi: 10.4049/jimmunol.166.2.1214. [DOI] [PubMed] [Google Scholar]
- 18.Woods JM, Amin MA, Katschke KJ, Jr, Volin MV, Ruth JH, Connors MA, et al. Interleukin-13 gene therapy reduces inflammation, vascularization, and bony destruction in rat adjuvant-induced arthritis. Hum Gene Ther. 2002;13:381–93. doi: 10.1089/10430340252792512. [DOI] [PubMed] [Google Scholar]
- 19.Favy DA, Lafarge S, Rio P, Vissac C, Bignon YJ, Bernard-Gallon D. Real-time PCR quantification of full-length and exon 11 spliced BRCA1 transcripts in human breast cancer cell lines. Biochem Biophys Res Commun. 2000;274:73–8. doi: 10.1006/bbrc.2000.3100. [DOI] [PubMed] [Google Scholar]
- 20.Plater-Zyberk C, Hoogewerf AJ, Proudfoot AE, Power CA, Wells TN. Effect of a CC chemokine receptor antagonist on collagen induced arthritis in DBA/1 mice. Immunol Lett. 1997;57:117–20. doi: 10.1016/s0165-2478(97)00075-8. [DOI] [PubMed] [Google Scholar]
- 21.Veillard NR, Kwak B, Pelli G, Mulhaupt F, James RW, Proudfoot AE, et al. Antagonism of RANTES receptors reduces atherosclerotic plaque formation in mice. Circ Res. 2004;94:253–61. doi: 10.1161/01.RES.0000109793.17591.4E. [DOI] [PubMed] [Google Scholar]
- 22.Barnes DA, Tse J, Kaufhold M, Owen M, Hesselgesser J, Strieter R, et al. Polyclonal antibody directed against human RANTES ameliorates disease in the Lewis rat adjuvant-induced arthritis model. J Clin Invest. 1998;101:2910–9. doi: 10.1172/JCI2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Quinones MP, Ahuja SK, Jimenez F, Schaefer J, Garavito E, Rao A, et al. Experimental arthritis in CC chemokine receptor 2-null mice closely mimics severe human rheumatoid arthritis. J Clin Invest. 2004;113:856–66. doi: 10.1172/JCI20126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cheng SS, Lai JJ, Lukacs NW, Kunkel SL. Granulocyte-macrophage colony stimulating factor up-regulates CCR1 in human neutrophils. J Immunol. 2001;166:1178–84. doi: 10.4049/jimmunol.166.2.1178. [DOI] [PubMed] [Google Scholar]
- 25.Lee SC, Brummet ME, Shahabuddin S, Woodworth TG, Georas SN, Leiferman KM, et al. Cutaneous injection of human subjects with macrophage inflammatory protein-1α induces significant recruitment of neutrophils and monocytes. J Immunol. 2000;164:3392–401. doi: 10.4049/jimmunol.164.6.3392. [DOI] [PubMed] [Google Scholar]
- 26.Watanabe K, Koizumi F, Kurashige Y, Tsurufuji S, Nakagawa H. Rat CINC, a member of the interleukin-8 family, is a neutrophil-specific chemoattractant in vivo. Exp Mol Pathol. 1991;55:30–7. doi: 10.1016/0014-4800(91)90016-q. [DOI] [PubMed] [Google Scholar]
- 27.Kaufmann A, Salentin R, Gemsa D, Sprenger H. Increase of CCR1 and CCR5 expression and enhanced functional response to MIP-1α during differentiation of human monocytes to macrophages. J Leukoc Biol. 2001;69:248–52. [PubMed] [Google Scholar]
- 28.Brodmerkel CM, Collins R, Hall L, Hertel D, Stewart R, Wang A, et al. Evaluation of a small molecule CCR2 antagonist in a rat model of rheumatoid arthritis [abstract] Arthritis Rheum. 2004;50 (Suppl 9):S369. [Google Scholar]
- 29.Luo Y, Berman MA, Zhai Q, Fischer FR, Abromson-Leeman SR, Zhang Y, et al. RANTES stimulates inflammatory cascades and receptor modulation in murine astrocytes. Glia. 2002;39:19–30. doi: 10.1002/glia.10079. [DOI] [PubMed] [Google Scholar]
- 30.Fischer FR, Luo Y, Luo M, Santambrogio L, Dorf ME. RANTES-induced chemokine cascade in dendritic cells. J Immunol. 2001;167:1637–43. doi: 10.4049/jimmunol.167.3.1637. [DOI] [PubMed] [Google Scholar]
- 31.Yasuda H, Shima N, Nakagawa N, Yamaguchi K, Kinosaki M, Mochizuki S, et al. Osteoclast differentiation factor is a ligand for osteoprotegerin/osteoclastogenesis-inhibitory factor and is identical to TRANCE/RANKL. Proc Natl Acad Sci U S A. 1998;95:3597–602. doi: 10.1073/pnas.95.7.3597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lacey DL, Timms E, Tan HL, Kelley MJ, Dunstan CR, Burgess T, et al. Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell. 1998;93:165–76. doi: 10.1016/s0092-8674(00)81569-x. [DOI] [PubMed] [Google Scholar]
- 33.Kong YY, Yoshida H, Sarosi I, Tan HL, Timms E, Capparelli C, et al. OPGL is a key regulator of osteoclastogenesis, lymphocyte development and lymph-node organogenesis. Nature. 1999;397:315–23. doi: 10.1038/16852. [DOI] [PubMed] [Google Scholar]
- 34.Matsuzaki K, Udagawa N, Takahashi N, Yamaguchi K, Yasuda H, Shima N, et al. Osteoclast differentiation factor (ODF) induces osteoclast-like cell formation in human peripheral blood mono-nuclear cell cultures. Biochem Biophys Res Commun. 1998;246:199–204. doi: 10.1006/bbrc.1998.8586. [DOI] [PubMed] [Google Scholar]
- 35.Quinn JM, Elliott J, Gillespie MT, Martin TJ. A combination of osteoclast differentiation factor and macrophage-colony stimulating factor is sufficient for both human and mouse osteoclast formation in vitro. Endocrinology. 1998;139:4424–7. doi: 10.1210/endo.139.10.6331. [DOI] [PubMed] [Google Scholar]
- 36.Kobayashi K, Takahashi N, Jimi E, Udagawa N, Takami M, Kotake S, et al. Tumor necrosis factor α stimulates osteoclast differentiation by a mechanism independent of the ODF/RANKL-RANK interaction. J Exp Med. 2000;191:275–86. doi: 10.1084/jem.191.2.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jimi E, Nakamura I, Duong LT, Ikebe T, Takahashi N, Rodan GA, et al. Interleukin 1 induces multinucleation and bone-resorbing activity of osteoclasts in the absence of osteoblasts/stromal cells. Exp Cell Res. 1999;247:84–93. doi: 10.1006/excr.1998.4320. [DOI] [PubMed] [Google Scholar]
- 38.Kong YY, Feige U, Sarosi I, Bolon B, Tafuri A, Morony S, et al. Activated T cells regulate bone loss and joint destruction in adjuvant arthritis through osteoprotegerin ligand. Nature. 1999;402:304–9. doi: 10.1038/46303. [DOI] [PubMed] [Google Scholar]
- 39.Horowitz MC, Xi Y, Wilson K, Kacena MA. Control of osteoclastogenesis and bone resorption by members of the TNF family of receptors and ligands. Cytokine Growth Factor Rev. 2001;12:9–18. doi: 10.1016/s1359-6101(00)00030-7. [DOI] [PubMed] [Google Scholar]
- 40.Cenci S, Weitzmann MN, Roggia C, Namba N, Novack D, Woodring J, et al. Estrogen deficiency induces bone loss by enhancing T-cell production of TNF-α. J Clin Invest. 2000;106:1229–37. doi: 10.1172/JCI11066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rosen H, Gordon S. Adoptive transfer of fluorescence-labeled cells shows that resident peritoneal macrophages are able to migrate into specialized lymphoid organs and inflammatory sites in the mouse. Eur J Immunol. 1990;20:1251–8. doi: 10.1002/eji.1830200609. [DOI] [PubMed] [Google Scholar]
- 42.Wiltrout RH, Brunda MJ, Gorelik E, Peterson ES, Dunn JJ, Leonhardt J, et al. Distribution of peritoneal macrophage populations after intravenous injection in mice: differential effects of eliciting and activating agents. J Reticuloendothel Soc. 1983;34:253–69. [PubMed] [Google Scholar]


