Abstract
Objective:
To compare the performance of community- vs university-based clinical centers in 3 multicenter randomized clinical trials of intraocular surgery.
Methods:
Each Submacular Surgery Trials clinical center was classified as a university-based center, if the contract to perform as a center was signed by a university official, or as a community-based center. The 2 groups of centers were compared on performance, assessed cumulatively by the Submacular Surgery Trials Quality Assurance and Monitoring Subcommittee.
Outcome Measures:
Patient accrual, completion of scheduled examinations, completion of masked vision examinations 2 years after enrollment (the designated primary study end point evaluation time), timeliness of submission of retinal photographs required by protocol to the Photograph Reading Center, completion of health- and vision-related quality-of-life interviews, and timeliness of submission of the primary outcome data to the Coordinating Center after completion of the examination.
Results:
Almost all centers performed at a very high (good) level, although there was a trend for some community-based centers to be at the lower end of most distributions.
Conclusions:
Most community- and university-based centers performed well in these multicenter clinical trials. Monitoring performance and periodically providing feedback to clinical center investigators may encourage excellent performance in areas critical to the success of clinical trials, regardless of whether the center is community or university based.
Monitoring the performance of centers participating in multicenter clinical trials is critical to the success of the trials. This type of supervision, for example, allows the study organization to determine whether (1) accrual of study participants is occurring at an acceptable rate, (2) participants enrolled meet eligibility criteria, (3) procedures are followed per protocol, and (4) loss to follow-up is minimized. A study1 from the oncology field published in 1981 based on monitoring suggested that an important indicator of the quality of a clinical center in multicenter clinical trials was the number of study participants enrolled at that center. The authors suggested that an institution should not participate in multicenter trials unless some predetermined minimal number of study participants can be enrolled by investigators at the institution within a specific time.1 The study generated letters agreeing2,3 and disagreeing4,5 with this conclusion, including an analysis from the Eastern Cooperative Oncology Group4, which found that the quality of participation of institutions with few patients entered was similar to that of institutions with many patients entered when aspects of performance were considered. That same oncology group6 subsequently published another study that compared the performance of university hospitals or major treatment centers with that of community hospitals. They concluded that including community hospitals in their clinical trials did not reduce the quality of the data or compromise the therapeutic outcomes.6 Another oncology group7 also compared the quality of participation of community affiliates with that of universities and found that the performance of the community affiliates equaled or surpassed that of the university centers in most measures.
These results in the oncology field may or may not apply to performance in multicenter clinical trials in ophthalmology for a variety of reasons. For example, ophthalmic problems usually are not life threatening and often may be managed in an out-patient setting. To determine whether there was a relationship between rate of patient enrollment and quality of clinical center performance in ophthalmology, performance in 2 multicenter clinical trials (the Macular Photocoagulation Study and the Early Treatment Diabetic Retinopathy Study) was analyzed.8 This evaluation led to the conclusion that clinical centers enrolling fewer patients in these trials performed in some areas as well as or better than centers enrolling larger number of patients.8 However, the evaluation did not compare the performance of community-based clinical centers with that of university-based centers.
The purpose of this current analysis is to compare the performance of community-based clinical centers with that of university-based clinical centers in 3 multicenter clinical trials of intraocular surgery. In addition, this study comments on issues that should be considered when identifying and selecting community- and university-based practices to participate in multicenter randomized clinical trials and suggests strategies designed to keep performance levels high.
METHODS
STUDY DESIGN
The Submacular Surgery Trials (SST) includes 3 randomized clinical trials designed to evaluate whether surgical removal of choroidal neovascularization and associated blood increases the chance of achieving stable or improved vision compared with no surgery in selected patients with subfoveal choroidal neovascularization due to age-related macular degeneration, ocular histoplasmosis syndrome, or idiopathic causes.9 These trials are sponsored by the National Eye Institute of the National Institutes of Health, US Department of Health and Human Services, Bethesda, Md. The SST design and methods and the activities of the SST resource centers were approved by an institutional review board of The Johns Hopkins University School of Medicine, Baltimore, Md. The SST protocol, detailed in the SST Manual of Procedures,10 was approved by the SST Executive Committee (responsible for the scientific direction and leadership of the SST); the Data and Safety Monitoring Committee (an independent committee appointed by the director of the National Eye Institute); and the institutional review board of each participating clinical center before enrollment of patients began at that center.
SELECTION OF CLINICAL CENTERS
After the proposing principal investigators of 3 SST resource centers (the Chairman’s Office, Coordinating Center, and Photograph Reading Center) were notified that funding had been awarded to conduct the first trial of the SST, invitations to participate in the SST were sent first to ophthalmologists at 12 clinical centers that had performed well with respect to enrollment, follow-up, and protocol adherence among 18 centers that had participated in an earlier pilot study.11 Four additional centers were invited to participate because they had an ophthalmologist qualified to be a principal investigator who had expressed interest in participating and had documented the ability to accrue eligible patients. These 16 centers (11 community based and 5 university based) were chosen without regard to whether the center was community based and without completion of a formal application. When funding was awarded to conduct 2 additional trials, it was recognized that additional clinical centers would be required to meet accrual goals. A letter was sent by the SST leadership to approximately 1200 ophthalmologists in the United States identified by the American Academy of Ophthalmology as specializing in retinal diseases and to US members of 3 retina subspecialty societies (the Macula Society, the Retina Society, and the Vitreous Society) requesting an application package by a specified date to indicate an interest in organizing a participating clinical center in the SST.
Applications received by the specified deadline were reviewed by the SST Executive Committee. Items considered in the review included documentation of the proposing investigator’s surgical experience, evidence of successful participation in other clinical trials of similar conditions, availability of key personnel (eg, clinical center coordinators and retinal photographers) with experience following clinical trial protocols, and documentation of an adequate number of patients diagnosed each year as having the target eye conditions and of the number who would have met the eligibility criteria for the trials. Based on this information, 11 centers were selected to participate in the SST. No consideration was given to whether the proposing center was community based or university based.
STUDY TIMETABLE AND DATA COLLECTION METHODS
The first patient was enrolled in the first trial initiated in April 1997; accrual ended in all 3 trials in September 2001. All patients were examined at baseline; 3, 6, and 12 months after enrollment; and at annual intervals thereafter for up to 4 years. Additional data were collected 1 and 9 months after enrollment and at the midpoint between annual examinations by telephone contact or during an in-person examination at the SST clinical center or by another eye care provider. Scheduled protocol examinations typically included examination by an SST-certified ophthalmologist, vision testing, retinal photography, and a centrally administered telephone interview concerning health- and vision-related quality of life.12–15 The details are provided in the SST Manual of Procedures.10 All patients were followed through the 4-year examination, or through the 2-, 3-, or 4-year annual examination scheduled between October 2002 and September 2003, or, in a few cases, until death, whichever came first.
Data from examinations were recorded on paper forms that were faxed to the Coordinating Center. Clinical center personnel were instructed to submit each form as soon as possible after data were recorded, ideally on the same day. Photographs were expected to arrive at the Photograph Reading Center within 28 days of photography to allow time for processing before submission.
PERFORMANCE MONITORING OF CLINICAL CENTERS
Personnel at all clinical centers completed SST training and certification to perform their study roles before initiating accrual of patients to participate in the SST. The SST Quality Assurance and Monitoring Subcommittee was appointed by the Executive Committee in 1997 to monitor clinical center and resource center performance and the performance of individual study personnel with respect to adherence to the study protocol and to goals set by the Executive Committee. Selected performance data were discussed in detail as part of each annual meeting of the SST Research Group. An initial site visit was made to each clinical center to review operations and, in the case of centers that already had initiated patient enrollment and surgery, to compare selected data in each patient’s clinical chart with the information reported to the SST Coordinating Center. A follow-up site visit for quality-monitoring purposes was scheduled near the end of the patient follow-up examinations and data collection at each site. Priorities for site visits were established by the Executive Committee on the basis of the overall contribution of the center to the study database, perceptions of problems that prompted an early site visit, changes in key personnel, and resources available for site visits.
At the November 17, 2000, meeting, the Executive Committee recommended that each principal investigator be sent a report for his or her center that summarized performance as reported during a Quality Assurance and Monitoring Subcommittee meeting the previous day. The specific measures of performance to be included in the summary report were identified. Subsequently, the resource center principal investigators sent individual clinical center summary reports with a letter to each principal investigator and clinic coordinator. The summary showed for each measure of performance the statistic for all centers combined, the range of values across all centers, the statistic for the center, and the rank of the individual center on that measure. In addition, an overall rank among SST centers was determined for each center, combining all of the performance measures reported. A follow-up teleconference was conducted with each principal investigator and clinic coordinator, typically by 2 members of the Operations Committee (including the principal investigator of the Coordinating Center) or 1 member of the Operations Committee and a coinvestigator of the Coordinating Center. The aim of the teleconference would be to review the summary report and to discuss possible methods of improving performance. This process was repeated after the Quality Assurance and Monitoring Subcommittee and Executive Committee meetings in October 2001 and October 2002.
DATA ANALYSIS
The performance measures included in the individual summary reports varied depending on the issues of particular concern each year. However, certain performance issues were addressed in each report:
Completion of scheduled examinations, expressed as the percentage of those expected that had been completed overall.
Completion of masked vision examinations 2 years after enrollment (the examination designated for assessing the primary outcome in each trial).
Completion of health- and vision-related quality-of-life interviews.
Timeliness of submission of the visual acuity data to the Coordinating Center after completion of the examination, expressed as a percentage of all outcome examinations completed.
Timeliness of submission of retinal photographs to the Photograph Reading Center.
The first summary report considered cumulative performance to that date; subsequent reports considered performance during the year just completed. For comparison of community-and university-based centers in this analysis, a special cumulative analysis of performance measures through June 30, 2002, the cutoff date for the most recent report, was considered.
Each clinical center was classified as community based or university based depending on the organization with which funding for that center was contracted. A university-based center was defined as a site where the contract to perform as an SST clinical center was signed by a university official. A community-based clinical center was defined as one not university based. Classifications were reviewed and approved by the Executive Committee.
Because of the small number of centers in each category (community based and university based), only descriptive statistics are reported. No formal statistical tests were used to compare the 2 groups of centers. Individual center data are displayed graphically using a method proposed by Canner and colleagues.16
RESULTS
Based on a review of all applications submitted by the deadline, 27 centers were selected (17 community-based centers and 10 university-based centers). The total number of patients enrolled at each center is shown in Figure 1. By 1 year after initiating enrollment, 6 centers (4 community based and 2 university based) had failed to accrue study participants at a rate judged sufficient to meet accrual goals, resulting in a recommendation to halt further accrual of patients at these centers but to continue scheduled follow-up examinations of patients already enrolled. Performance data for these 6 centers are not included in the remaining data displays.
Figure 1.
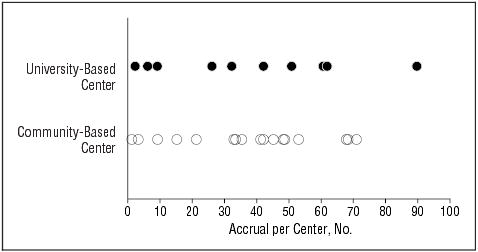
The total number of patients accrued by each center.
Distributions of the percentage of scheduled examinations completed, the percentage of 2-year masked examinations completed, and the percentage of quality-of-life interviews completed (Figure 2A-C) show that most community-and university-based centers performed at a high level. However, all the centers in the lower portion of these distributions were community-based centers. Nevertheless, all but 2 centers had values greater than 70%, and most had values greater than 80%, for all 3 measures of performance. When the percentage of scheduled 2-year examinations completed was evaluated, distributions were similar to the percentage of all scheduled examinations completed. With respect to timeliness of submission of materials, more variability was observed among centers. For the percentage of vision examination data received within 3 days of vision measurements, 1 outlier clinic achieved this goal for only 27% of vision examinations; otherwise, the distribution for community-based centers was similar to that of university-based centers (Figure 2D). With respect to receipt of retinal photographs within 28 days of photography, 2 outlier centers—1 community based and 1 university based—were apparent, with only 27% and 16% of photographs received at the Photograph Reading Center by 4 weeks after they were taken, respectively (Figure 2E). However, the distributions otherwise were similar for the 2 groups of clinics. Medians for these performance measures for 21 clinical centers by type of center are given in the Table and are similar for most measures when comparing community- vs university-based centers.
Figure 2.
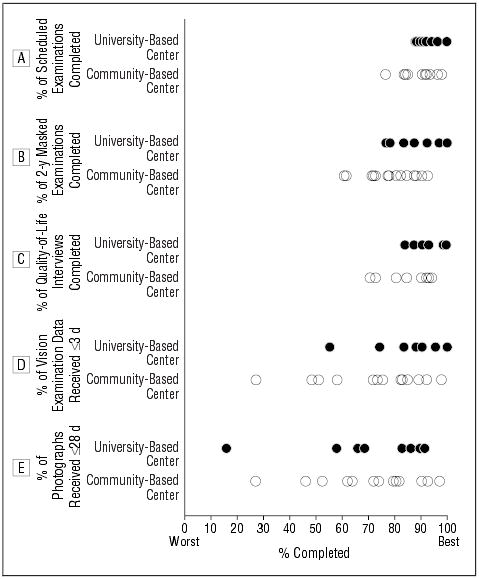
A-C, Percentage of scheduled examinations, 2-year masked examinations, and quality-of-life telephone interviews completed by each center. D, Percentage of vision examination data received within 3 days of vision measurements by each center. E, Percentage of retinal photographs received within 28 days by each center.
Performance Measures for 21 Clinical Centers by Type of Center*.
| Performance Measure | Community-Based Center (n = 13) | University-Based Center (n = 8) |
|---|---|---|
| Patient accrual, No. | 45 | 46.5 |
| Scheduled examinations completed, % | 91 | 94 |
| 2-y Masked vision examinations completed, % | 81 | 88 |
| Scheduled health-related quality-of-life telephone interviews† completed, % | 81 | 90 |
| Vision examination data transmitted promptly, % | 76 | 86 |
| Retina photographs arrived promptly, % | 74 | 76 |
Data are given as medians.
Centrally administered health-related quality-of-life telephone interviews were undertaken to assess patient-centered outcomes. Interview instruments included the National Eye Institute Visual Function Questionnaire,12 the 36-Item Short-Form Health Survey,13 the Hospital Anxiety and Depression Scale,14 and the SST Vision Preference Value Scale.15
COMMENT
Overall, community- and university-based centers participating in the SST performed at a high (good) level, although there was a trend for some community-based centers to perform at a lower level than university-based centers. In addition, 6 centers (2 university based and 4 community based) chosen to participate did not meet accrual expectations early in the trials, prompting cessation of accrual at these centers. The overall high quality of performance may have resulted from judicious selection of centers, central training of study personnel at all centers initially, or ongoing monitoring of performance. However, we observed study-wide improvement over time in most of the areas of performance measured, suggesting that monitoring and feedback regarding performance at annual meetings and through individual “report cards” contributed to good performance at both community- and university-based centers.
Although there was a trend for some community-based centers not to perform as well in some areas compared with university-based centers, most community-based centers performed as well as university-based centers on most measures. Conclusions are limited by the number of centers and by the small number of performance measures evaluated. Nevertheless, these results suggest that organizers of multicenter clinical trials in ophthalmology should not assume that community-based centers will perform either better or worse than university-based centers.
Multicenter clinical trials typically include 1 or more resource centers in the scientific and administrative organization, each with a different role. Performance monitoring and comparisons of resource centers located in different settings are outside the scope of this analysis. Also, the performance measures chosen for this analysis were judged to be the most important objective measures evaluated by the SST Quality Assurance and Monitoring Subcommittee with respect to the goals of these trials. Other objective measures, such as number of query rates and missing value rates, were not judged to be as important as the metrics displayed in Figures 1 and 2. Subjective performance measures, such as the quality of photographs as judged by Photograph Reading Center personnel, although important to study goals, were not included in this analysis. Such information might have modified the conclusions.
The ability to generalize these findings is unknown; they may apply only to ophthalmic trials or only to trials in which surgery is evaluated. Evaluation of performance in “large, simple” randomized clinical trials, such as the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT),17 showed no difference in recruitment performance among the 623 centers used to enroll at least 40000 study participants when comparing sites with limited or no previous research experience with those with more experienced sites. The ALLHAT17 included university-affiliated centers (11.4% of sites),“private solo or group practices” (54.7% of sites), “community health centers” (8.0% of sites), health maintenance organizations (2.9% of sites), and others. However, these evaluations did not compare performance in community-vs university-based centers. Other evaluations of clinical performance by the AIDS Clinical Trials Group18 or visual field testing in the Optic Neuritis Treatment Trial19 also did not compare performance in community-based centers with that in university-based centers.
Unlike previously published studies in other multicenter clinical trials evaluating performance, surgical trials have unique challenges that could affect the performance measures evaluated. These challenges include financial incentives not to enroll patients to avoid losing surgical fees for patients assigned to observation or some nonsurgical control arm; patient care costs extending beyond 1 provider (possibly including surgical fees, anesthesia fees, and hospital charges); and logistical issues, such as scheduling operating room time that would be needed promptly for study participants assigned to surgery but cancelled when participants were assigned to observation or some other nonsurgical intervention.
In an attempt to minimize the impact of such issues on performance, open discussions with the SST Research Group (investigators, clinic coordinators, and central resource personnel) were undertaken before and during accrual. Research Group members agreed that financial incentives should not be allowed to affect decisions regarding patient care or participation in these clinical trials. In addition, members agreed that if surgery should prove beneficial over observation, evidence from randomized clinical trials likely would provide third-party payers with justification to cover surgical costs in the future that otherwise might not be covered as budgetary constraints limit future reimbursement. The SST Research Group worked closely with surgical scheduling coordinators at each clinical center so that these employees, who usually were not part of the research team, understood the rationale and logistics of scheduling potential SST study participants before randomization so that if patients were assigned to surgery, operations could be undertaken soon after randomization. Close communication with surgical scheduling coordinators also helped them understand why surgical slots on the operating room schedule might be cancelled suddenly when patients were assigned to observation.
Other challenges that could have affected performance included the need to identify an appropriate institutional review board for some community-based centers. Also, 2 community-based centers and 2 university-based centers had principal investigators leave their respective clinical centers to work in another practice either in the same city or elsewhere. Although turnover of personnel in community- and university-based centers cannot be prevented, loss of a principal investigator in a small practice, whether community based or university based, can have a negative impact on continuity of patient care and follow-up. Investigators were encouraged to communicate anticipated moves and other personnel changes promptly to provide adequate time to train replacement personnel. With commitment to the success of the SST by all investigators (principal investigators and coinvestigators) at the onset of the trials and ongoing communication with investigators to manage such scenarios, these potential problems were minimized. Success is reflected in the excellent performance regarding completion of follow-up examinations that otherwise might be difficult for the age groups (averaging in the mid-70s to early 80s) participating in 2 of these trials.
Experiences in the SST parallel the oncology experience6,7; both community- and university-based centers performed well. As competition increases to identify patients with targeted ophthalmic conditions, such as macular edema from diabetes mellitus or choroidal neovascularization from age-related macular degeneration, to participate in clinical trials, it is important to recognize that community- and university-based centers can achieve good to excellent performance in multicenter clinical trials.
Members of the Submacular Surgery Trials Research Group: April 1997 Through December 2002
Clinical Centers and Personnel Who Contributed Data
Centers are listed in alphabetical order by city. Asterisk indicates community-based center. The number of patients enrolled is given in parentheses after the center location. Personnel listed are principal investigators and other personnel who had performed 5 or more examinations or procedures that had been reported by December 31, 2002.
Emory University Eye Center, Atlanta, Ga: (n=62) Principal Investigators: G. Baker Hubbard III, MD; Paul Sternberg, Jr, MD (1999–2002); Antonio Capone, Jr, MD (1998–1999). Ophthalmologist: Thomas M. Aaberg, Jr, MD. SST Coordinator: Jayne M. Brown. Vision Examiners: Lindy G. Dubois, COMT; Judy Johnson, COMT; Natalie I. Schmitz. Photographers: James Gilman, CRA; Robert A. Myles, CRA; Ray Swords, CRA.
The Wilmer Ophthalmological Institute, Baltimore, Md: (n=90) Principal Investigator: Julia A. Haller, MD. Ophthalmologists: Peter A. Campochiaro, MD; Mark Humayun, MD; Eugene de Juan, Jr, MD; Dante J. Pieramici, MD; Ingrid Zimmer-Galler, MD. SST Coordinators and Vision Examiners: Michaele Hartnett, COT; Patricia L. Hawse, COMT; Tracey L. Porter, COT; Ann Eager Youngblood. Photographers: Judith E. Belt; Dennis Cain, CRA; David Emmert; Rachel E. Falk; Terry George; Mark Herring; Jacquelyn McDonald.
Massachusetts Eye and Ear Infirmary, Boston, Mass: (n=2) Principal Investigator: Jorge Arroyo, MD.
Northwestern University Medical School, Chicago, Ill: (n=6) Principal Investigator: David V. Weinberg, MD. Ophthalmologist: Robert Schroeder, MD. SST Coordinator: Jill Koecher. Vision Examiner: Zuzanna Strugala, OMA. Photographers: Marsha Apushkin; Alexander Habib; Jim Yuhr, CRA.
Cole Eye Institute, Cleveland, Ohio*: (n=45) Principal Investigator: Hilel Lewis, MD. Ophthalmologist: Peter K. Kaiser, MD. SST Coordinators: Laura Holody, COA; Larissa S. Schaaf, RN. Vision Examiners: Ginny Ambrose; Joyce Conway; Anthony Fattori; Helene Siegel. Photographers: Gloria M. Bartram; Stephanie L. Burke; Nicole Drozda; Tami Fecko; Deborah J. Ross, CRA.
Retina Associates of Cleveland*: (n=42) Principal Investigator: Lawrence J. Singerman, MD. Ophthalmologists: Michael A. Novak, MD; Scott Pendergast, MD. SST Coordinators and Vision Examiners: Lori M. Campana; Kim Tilocco DuBois, COA; Susan C. Rath, PA-C; Vivien Tanner, COT. Photographers: John DuBois, CRA; Greg Greanoff; David Lehnhardt, COA; Sheila Smith-Brewer, COT; Kimberley Spagnoletta.
Ohio State University, Columbus: (n=26) Principal Investigator: Frederick H. Davidorf, MD. Ophthalmologist: Robert Chambers, DO. SST Coordinator: Cynthia Taylor. Vision Examiners: Jill Milliron, COA; Jerilyn Perry, COT. Photographer: Scott Savage, EMT-A.
Texas Retina Associates, Dallas*: (n=21) Principal Investigator: David G. Callanan, MD. Ophthalmologist: Gary Edd Fish, MD. SST Coordinators: Jodi R. Creighton, COA; Jeff L. Harris, COA; Nancy Resmini; Rubye Rollins. Vision Examiner: Marilyn Andrews. Photographers: Hank Aguado, CRA; Bob Boleman; Penny Ellenich.
Duke University Eye Center, Durham, NC: (n=42) Principal Investigator: Cynthia Toth, MD. Ophthalmologists: Glenn Jaffe, MD; Brooks McCuen, MD. SST Coordinators and Vision Examiners: Malcolm W. Anderson, PA-C, COT; Jennifer V. Caldwell; Photographers: Teresa Jackson Hawks; Gregory Hoffmeyer; Jeff Napoli.
Illinois Retina Associates, Harvey and Chicago*: (n=33) Principal Investigator: Mathew W. MacCumber, MD, PhD. Ophthalmologists: Joseph M. Civantos, MD; Kirk H. Packo, MD. SST Coordinators: Marguerita DeAlba, ST; Bruce L. Gaynes, OD; Laurie Rago, COA; Carrie Violetto. Vision Examiners: Michelle M. Franzyck, COT; Chris Morrison. Photographers: Douglas A. Bryant, CRA; Donald S. Doherty; Frank Morini.
Retina Associates of Hawaii, Honolulu*: (n=9) Principal Investigator: Neal H. Atebara, MD. SST Coordinator: Susan Pelke. Vision Examiner and Photographer: Deborah J. Nobler.
Midwest Eye Institute, Indianapolis, Ind*: (n=3) Principal Investigator: John T. Minturn, MD. SST Coordinator: Donna Agugliaro, RN. Vision Examiner: Shelly Cohen. Photographer: Carolyn Lamb.
University of Iowa, Iowa City: (n=9) Principal Investigator: James C. Folk, MD. SST Coordinators and Vision Examiners: Betty Folmer; Connie Fountain, COT; Steven A. Wallace. Photographers: Ed Hefron; Carolyn Vogel.
Mid-America Retina Consultants, Kansas City, Mo*: (n=15) Principal Investigator: William N. Rosenthal, MD. Ophthalmologist: David S. Dyer, MD. SST Coordinators and Vision Examiners: Denise Moore, RN; Barbara Petro, COT; Dalton J. Thibodeaux. Photographer: R. Scott Varner.
Southeastern Retina Associates, Knoxville, Tenn*: (n=68) Principal Investigator: John C. Hoskins, MD. Ophthalmologist: Joseph M. Googe, MD. SST Coordinators: Katie E. Carter, COA; Stephanie M. Evans; Tina Thibodeaux Higdon; Jennifer L. Holton. Vision Examiner: Bruce D. Gilliland, OD. Photographers: Paul Andrew Blais; Philip Michael Jacobus.
Retina and Vitreous Associates of Kentucky, Lexington*: (n=53) Principal Investigator: William J. Wood, MD. Ophthalmologist: Rick Isernhagen, MD. SST Coordinators: Michelle L. Buck, COA; J. Lynn Cruz, COT; Joni D. James, RN; Tammy L. Jordan, PA-C; Jenny L. Wolfe, RN. Vision Examiners: Christine Brown, COT; Wanda Heath, COT; Catherine Millett, COA. Photographers: Marty Reid, COA; Edward Slade, CRA, COA.
Jules Stein Eye Institute, Los Angeles, Calif: (n=61) Principal Investigator: Steven D. Schwartz, MD. Ophthalmologists: Robert Engstrom, MD; Kent Small, MD. SST Coordinators: Jo Eure; Jessica Hsu; Rosaleen Ostrick, MPH, MA; Dai Tran; Tina Wong. Vision Examiners: Lisa Barnhart; Janine Chen; Melissa Chun, OD; Larissa Johnson; Jennie Y. Kageyama, OD. Photographers: Mirella Tetreault; Dennis Thayer; Bret Trump.
California Vitreoretinal Associates, Menlo Park*: (n=1) Principal Investigator: Mark S. Blumenkranz, MD. SST Coordinator: Patricia Mattio.
Vitreoretinal Surgery PA, Minneapolis, Minn*: (n=35) Principal Investigator: David F. Williams, MD. Ophthalmologists: Sundeep Dev, MD; Robert A. Mittra, MD. SST Coordinators and Vision Examiners: Julianne Enloe; Scott D. Marella; Neal Oestrich. Photographer: Holly N. Cheshier.
McGee Eye Institute, Oklahoma City, Okla: (n=32) Principal Investigators: Reagan H. Bradford, MD; Sumit K. Nanda, MD (1998–2002). SST Coordinators and Vision Examiners: Angela Monlux; Lisa M. Ogilbee; Photographer: Russ Burris, COT, CRA.
Retinal Consultants of Arizona, Phoenix*: (n=68) Principal Investigator: Jack O. Sipperley, MD. Ophthalmologist: Scott R. Sneed, MD. SST Coordinators: Jaclin J. Jacobsen, CRA, COA; Eleonora Tysiac. Vision Examiners: Denise Freistroffer; Nickie Perez; Pearl Rosas; Debra Tomaszewski. Photographers: John J. Bucci; Sharon H. Kosecki; John V. Martin.
Retina Vitreous Consultants, Pittsburgh, Pa*: (n=48) Principal Investigator: Robert L. Bergren, MD. Ophthalmologist: Bernard Doft, MD. SST Coordinators: Donna J. Metz, RN; Kathryn Sedory, RN; Christina Trombetta, CST. Vision Examiners: Grace Rigoni; Lynn Wellman; Linda Wilcox, COA. Photographers: Alan Campbell, CRA; David Steinberg, CRA; Gary Vagstad, CRA.
Oregon Health Sciences University, Portland: (n=51) Principal Investigator: David J. Wilson, MD. SST Coordinator and Vision Examiner: Susan Pope. Photographers: Ellen Redenbo; Peter Steinkamp; Patrick Wallace.
Associated Retinal Consultants, Royal Oak, Mich*: (n=41) Principal Investigator: George A. Williams, MD. Ophthalmologists: Antonio Capone, MD; Bruce R. Garretson, MD; Alan Ruby, MD. SST Coordinators and Vision Examiners: Kristi L. Cumming, RN, MSN; Bobbie Lewis, RN; Patricia Manatrey; Mary Zajechewski. Photographers: Craig Bridges; Patricia Steasick; Lynette Szydlowski.
Barnes Retina Institute, St Louis, Mo*: (n=71) Principal Investigator: Nancy Melberg Holekamp, MD. Ophthalmologists: Daniel P. Joseph, MD; Matthew A. Thomas, MD. SST Coordinators and Vision Examiners: Julie Binning, COT; Lynda Boyd, COT; Janel Gualdoni, COT; Virginia Nobel, COT. Photographers: Rhonda Allen; Bryan Barts; Pamela K. Bauer; Jon Dahl; Timothy S. Holle; Deborah Kaiser, RN, COA; Ella Ort; Matt Raeber; John Mark Rogers.
West Coast Retina Medical Group Inc, San Francisco, Calif*: (n=33) Principal Investigator: H. Richard McDonald, MD. Ophthalmologist: Robert N. Johnson, MD. SST Coordinators: Margaret Stolarczuk, OD; Pat Wood, LVN. Vision Examiner: Kevan E. Curren. Photographers: Kelly Ann DeBoer; Sarah M. Huggans; Jeremy R. Miller; John Uy.
St Vincent Mercy Medical Center, Retina Vitreous Associates, Toledo, Ohio*: (n=48) Principal Investigator: Samuel R. Pesin, MD. Ophthalmologists: Charles K. Dabbs, MD; Nicholas J. Leonardy, MD. SST Coordinator and Vision Examiner: James M. Haener, COT. Photographers: Lauren M. Cedoz, CRA; Dawn DeFalco; Richard D. Hill.
Resource Centers: The Wilmer Ophthalmological Institute, Baltimore
Chairman’s Office, Retinal Vascular Center: Principal Investigator and SST Chairperson: Neil M. Bressler, MD. Traveling Vision Examiners: Kristi L. Cumming, RN, MSN; James M. Haener, COT; Michael Hartnett, COT; Patricia L. Hawse, COMT; Peggy R. Orr, MPH, COMT. Vision Testing Coordinator; Economic Analyst: Eric B. Bass, MD, MPH. Other Personnel: Dawn A. Childs; Connie Lawson; Irene L. Felicetti (1997–2002); Patricia Staflin (1997–2001).
Coordinating Center, Wilmer Clinical Trials and Biometry: Principal Investigator: Barbara S. Hawkins, PhD. Biostatisticians: Ashley L. Childs, MS; Li Ming Dong, PhD; Marta J. Marsh, MS. Epidemiologist: Päivi H. Miskala, PhD. Quality of Life Advisor: Carol M. Mangione, MD, MSPH (participation supported by a contract between the David Geffen School of Medicine, University of California, Los Angeles, and The Johns Hopkins University). Data Coordination and Telephone Interviews: Rob G. Casper, MS; Alice D. Keith; Lee D. McCaffrey, MA; Dawn K. Smith. Systems Management and Programming: Kurt Dreger; Harris A. Jaffee, PhD; M. Marvin Newhouse; Stephen C. Grubb, MS (1997–1999). Other Personnel: Patricia A. James; Lisa Lassiter; Christine B. Alden (2000–2002); Takisha R. Kiah (1998–2002); Nancy A. Prusakowski, MS (1998–2000).
Photograph Reading Center, Wilmer Photograph Reading Center: Principal Investigator: Susan B. Bressler, MD. Ophthalmologists: Dante J. Pieramici, MD (1998–2000); Srinivas R. Sadda, MD (2000–2001); Oliver D. Schein, MD. Consultant: Sharon D. Solomon, MD. Administrative Directors: Judith Alexander (1997–1998); Kelly S. Manos, MAS (1998–2002). SST Coordinators: LaKaye Mbah; Reva W. Strozykowski; Isabel Mills (1999–2002). Photograph Graders: Kelly Ann Davies; Rita L. Denbow, MLA; Michael P. Minotti; Deborah A. Phillips; Yan Tian.
Pathology Center, Emory University Eye Center, Atlanta: Principal Investigator: Hans E. Grossniklaus, MD. SST Coordinator: Pingbo Liu. Consultant Pathologist: W. Richard Green, MD.
Sponsor: The National Eye Institute, National Institutes of Health: Directors: Paul A. Sieving, MD, PhD; Carl Kupfer, MD (1998–2000). Deputy Director: Jack A. McLaughlin, PhD. Program Directors: Maryann Redford, DDS, MPH; Mary Frances Cotch, PhD (1997–2001).
Committees and Members
Executive Committee: (reviewed and approved this manuscript) Ex Officio Members: Neil M. Bressler, MD (chairperson); Eric B. Bass, MD, MPH; Susan B. Bressler, MD; Hans E. Grossniklaus, MD; Julia A. Haller, MD; Barbara S. Hawkins, PhD; Carol M. Mangione, MD, MSPH; Peggy R. Orr, MPH, COMT; Maryann Redford, DDS, MPH; Paul Sternberg, Jr, MD; Matthew A. Thomas, MD. Rotating Members: Jayne Brown; Nancy M. Holekamp, MD; Samuel R. Pesin, MD; David J. Wilson, MD.
Quality Assurance and Monitoring Subcommittee: Barbara S. Hawkins, PhD (chairperson); Susan B. Bressler, MD (vice chairperson); Judith E. Belt; Li Ming Dong, PhD; Julia A. Haller, MD; Mike Hartnett, COT; Harris A. Jaffee, PhD; Carol M. Mangione, MD, MSPH; Marta J. Marsh, MS; Lee D. McCaffrey, MA; Päivi H. Miskala, PhD; Peggy R. Orr, MPH, COMT; Kelly S. Manos, MAS (1998–2002).
Vision Testing Subcommittee: Peggy R. Orr, MPH, COMT (chairperson); Mike Hartnett, COT; Patricia L. Hawse, COMT; Marta J. Marsh, MS; Lee D. McCaffrey, MA.
Surgery Subcommittee: Matthew A. Thomas, MD (chairperson); Julia A. Haller, MD (vice chairperson); Eugene de Juan, Jr, MD; Paul Sternberg, Jr, MD.
Patient-Centered Outcomes Subcommittee: Carol M. Mangione, MD, MSPH (chairperson); Eric B. Bass, MD, MPH (vice chairperson); Neil M. Bressler, MD; Ashley L. Childs, MS; Li Ming Dong, PhD; Barbara S. Hawkins, PhD; Harris A. Jaffee, PhD; Marta J. Marsh, MS; Lee D. McCaffrey, MA; Päivi H. Miskala, PhD.
Operations Committee: Neil M. Bressler, MD (SST chairperson); Paul Sternberg, Jr, MD, and Matthew A. Thomas, MD (SST vice chairpersons); Susan B. Bressler, MD; Barbara S. Hawkins, PhD; Maryann Redford, DDS, MPH.
Data and Safety Monitoring Committee: Voting Members (Appointed): Argye I. Hillis, PhD (chairperson); Gary W. Abrams, MD; John E. Connett, PhD; Christine Grady, RN, PhD; Earl G. Harrison, LLD; Lee M. Jampol, MD. Nonvoting Members (Ex Officio): Neil M. Bressler, MD; Li Ming Dong, PhD; Barbara S. Hawkins, PhD; Marta J. Marsh, MS; Maryann Redford, DDS, MPH; Mary Frances Cotch, PhD (1997–2000).
Adverse Event Review Committee: Voting Members: Julia A. Haller, MD, (chairperson); Gary W. Abrams, MD; Lee M. Jampol, MD. Ex Officio Member: Barbara S. Hawkins, PhD.
Writing Committee for Submacular Surgery Trials Report No. 2: Neil M. Bressler, MD (chairperson); Barbara S. Hawkins, PhD; Susan B. Bressler, MD; Päivi H. Miskala, PhD; Marta J. Marsh, MS. The Submacular Surgery Trials Report No. 2 Writing Committee members assume authorship responsibility and had complete access to the raw data necessary for this study. Each SST clinical center and resource center principal investigator approved this study for publication.
References
- 1.Sylvester RJ, Pinedo HM, De Pauw M, et al. Quality of institutional participation in multicenter clinical trials. N Engl J Med. 1981;305:852–855. doi: 10.1056/NEJM198110083051503. [DOI] [PubMed] [Google Scholar]
- 2.Thomas P, Novak JW, Knowlton A, Holyoke ED. Quality of institutional participation in multicenter clinical trials [letter] N Engl J Med. 1982;306:814. [Google Scholar]
- 3.Palva IP Finnish Leukaemia Group. . Quality of institutional participation in multicenter clinical trials [letter] N Engl J Med. 1982;306:813–814. doi: 10.1056/NEJM198204013061320. [DOI] [PubMed] [Google Scholar]
- 4.Begg CB, Elson PJ, McFadden E, Zelen M, Carbone PP. Quality of institutional participation in multicenter clinical trials [letter] N Engl J Med. 1982;306:813. [Google Scholar]
- 5.De Dombal FT. Quality of institutional participation in multicenter clinical trials [letter] N Engl J Med. 1982;306:813. doi: 10.1056/NEJM198204013061320. [DOI] [PubMed] [Google Scholar]
- 6.Begg CB, Carbone PP, Elson PJ, Zelen M. Participation of community hospitals in clinical trials: analysis of five years of experience in the Eastern Cooperative Oncology Group. N Engl J Med. 1982;306:1076–1080. doi: 10.1056/NEJM198205063061803. [DOI] [PubMed] [Google Scholar]
- 7.Koretz MM, Jackson PM, Torti FM, Carter SK. A comparison of the quality of participation of community affiliates and that of universities in the Northern California Oncology Group. J Clin Oncol. 1983;1:640–644. doi: 10.1200/JCO.1983.1.10.640. [DOI] [PubMed] [Google Scholar]
- 8.Hawkins BS, Prior MJ, Fisher MR, Blackhurst DW. Relationship between rate of patient enrollment and quality of clinical center performance in two multicenter trials in ophthalmology. Control Clin Trials. 1990;11:374–394. doi: 10.1016/0197-2456(90)90177-4. [DOI] [PubMed] [Google Scholar]
- 9.Bressler NM. Submacular surgery: are randomized trials necessary? Arch Ophthalmol. 1995;113:1557–1560. doi: 10.1001/archopht.1995.01100120087016. [DOI] [PubMed] [Google Scholar]
- 10.SST Manual of Procedures. Springfield, Va: National Technical Information Services, US Dept of Commerce; June 1998. Accession No. PB98-166648.
- 11.Submacular Surgery Trials Pilot Study Investigators. . Submacular Surgery Trials randomized pilot trial of laser photocoagulation versus surgery for recurrent choroidal neovascularization secondary to age-related macular degeneration, I: ophthalmic outcomes, SST Pilot Study Report No 1. Am J Ophthalmol. 2000;130:387–407. doi: 10.1016/s0002-9394(00)00729-7. [DOI] [PubMed] [Google Scholar]
- 12.Mangione CM, Lee PP, Pitts J, et al. Psychometric properties of the National Eye Institute Visual Function Questionnaire (NEI-VFQ) Arch Ophthalmol. 1998;116:1496–1504. doi: 10.1001/archopht.116.11.1496. [DOI] [PubMed] [Google Scholar]
- 13.Ware JE, Snow KK, Kosinski M, Gaskel B. SF-36 Health Survey Manual and Interpretation Guide. Boston, Mass: Health Institute, New England Medical Center; 1993.
- 14.Zigmond AS, Snaith RP. The Hospital Anxiety and Depression Scale. Acta Psychiatr Scand. 1983;67:361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 15.Submacular Surgery Trials Research Group. Patients’ perceptions of the value of current vision: assessment of preference values among patients with subfoveal choroidal neovascularization: the Submacular Surgery Trials Vision Preference Value (SST-VPV) Scale: SST Report No. 6. Arch Ophthalmol. In press. [DOI] [PMC free article] [PubMed]
- 16.Canner PL, Huang YB, Meinert CL. On the detection of outlier clinics in medical and surgical trials, I: practical considerations. Control Clin Trials. 1981;2:231–240. doi: 10.1016/0197-2456(81)90013-1. [DOI] [PubMed] [Google Scholar]
- 17.Wright JT, Jr, Cushman WC, Davis BR, et al. The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT): clinical center recruitment experience. Control Clin Trials. 2001;22:659–673. doi: 10.1016/s0197-2456(01)00176-3. [DOI] [PubMed] [Google Scholar]
- 18.Rosendorf LL, Dafni U, Amato DA, et al. Performance evaluation in multicenter clinical trials: development of a model by the AIDS Clinical Trials Group. Control Clin Trials. 1993;14:523–537. doi: 10.1016/0197-2456(93)90032-9. [DOI] [PubMed] [Google Scholar]
- 19.Keltner JL, Johnson CA, Beck RW, Cleary PA, Spurr JO Optic Neuritis Study Group. . Quality control functions of the Visual Field Reading Center (VFRC) for the Optic Neuritis Treatment Trial (ONTT) Control Clin Trials. 1993;14:143–159. doi: 10.1016/0197-2456(93)90016-7. [DOI] [PubMed] [Google Scholar]


