Abstract
Most vaccines are still given parenterally. Mucosal vaccination would offer different advantages over parenteral immunization, including blocking of the pathogens at the portal of entry. In this paper, nontoxic Escherichia coli heat-labile enterotoxin (LT) mutants and Supramolecular Biovector systems (SMBV) were evaluated in mice as mucosal adjuvants and delivery systems, respectively, for intranasal immunization with the conjugated group C meningococcal vaccine. The conjugated vaccine formulated together with the LT mutants and the SMBV induced very high titers of serum and mucosal antibodies specific for the group C meningococcal polysaccharide. This vaccination strategy also induced high titers of antibodies with bactericidal activity, which is known to correlate with efficacy. Importantly, the mucosal vaccination, but not the conventional parenteral vaccination, induced bactericidal antibodies at the mucosal level. These data strongly support the feasibility of development of intranasal vaccines with an enhanced protective efficacy against meningococci and possibly against other encapsulated bacteria.
The vast majority of existing vaccines are still given by systemic injection. Although widely accepted, parenteral vaccination is not devoid of potential drawbacks. Most of the pathogens invade their human hosts at the level of the mucosal surfaces, which represent the very first antimicrobial barrier through nonspecific (anatomical) and specific (immune) defense mechanisms. Mucosal vaccination would offer several advantages over the parenteral route of vaccination. First, by inducing local microbial-specific immune responses, it would block pathogens at the portal of entry, thus increasing the general efficacy of the vaccine. Second, by avoiding the traumatic procedure of injection, it would increase the compliance and consequently the coverage. Third, it would facilitate vaccine delivery, especially in poorer countries. Last, but not least, mucosal immunization would significantly decrease the risk of unwanted spread of infectious agents via contaminated syringes (9, 17), especially in areas with a high incidence of viral hepatitis and human immunodeficiency virus infections.
Multiple strategies are currently pursued for the development of systems aimed at enhancing the immunogenicity of mucosally delivered vaccines. Most antigens are not immunogenic when delivered mucosally and require the use of strong adjuvants. Cholera toxin (CT) and Escherichia coli heat-labile enterotoxin (LT) are the strongest mucosal adjuvants known so far (26). However, their use in humans is hampered by their very high toxicity. Indeed, 5 μg of CT is able to induce 1 to 6 liters of diarrheal feces (19). During the past few years, site-directed mutagenesis has permitted the generation of LT and CT mutants devoid of toxic activity, while retaining their strong mucosal adjuvanticity (26). Among these are the LTK63 and the LTR72 mutants (mutation in the A subunit Ser63→Lys and Ala72→Arg, respectively) (12, 26). The mucosal adjuvanticity of these mutants has now been tested in various systems with viral, bacterial, and parasitic models. After oral or intranasal (i.n.) delivery, vaccination with these mutants as adjuvants induced appropriate immune effector mechanisms (antibodies, CD4+ helper T cells, and CD8+ cytotoxic T cells) able to confer protection against the relevant infectious challenge (23).
Other systems aimed at enhancing immunogenicity of vaccines after i.n. delivery include the so-called “Supramolecular Biovectors” (SMBV), which are nanoparticles made of cationic cross-linked polysaccharides surrounded by a lipidic bilayer (8, 22). These nanoparticles are known to act as carriers for antigens and adjuvants (25, 28), to facilitate the delivery of antigens to antigen-presenting cells, and to induce strong antigen-specific immune responses after nasal delivery (2, 4, 25).
In the present work, we compared the mucosal adjuvanticity of one LT mutant (LTK63) with that of the SMBV system and evaluated the eventual enhancing effect of combining these adjuvant-delivery systems. To this end, we decided to use as a model a conjugated vaccine against group C Neisseria meningitidis, because it induces bactericidal antibodies, the titers of which are well known to correlate with protection. This vaccine consists of the group C capsular oligosaccharide from N. meningitidis conjugated to the nontoxic mutant of diphtheria toxin, CRM197 (CRM-MenC) (6); it has been shown to be immunogenic and efficacious in several clinical trials (18, 20, 21) and is now commercially available in several countries.
MATERIALS AND METHODS
Conjugated CRM-MenC vaccine, LTK63 mutant, and nanoparticles.
The conjugated CRM-MenC vaccine was prepared as previously described in detail elsewhere (6). The alum-adjuvanted vaccine (which is currently used in several countries [18]) was used at a 1:4 dilution for subcutaneous (s.c.) immunization of mice. The equivalent bulk before adsorption with alum was used for formulation with the LTK63 mutant and/or with the SMBV.
The fully nontoxic LTK63 mutant of the E. coli LT was produced by site-directed mutagenesis of the wild-type toxin and purified as previously described (12).
SMBV nanoparticles were prepared from maltodextrin as described previously (3). Briefly, maltodextrin was dissolved in 2 N sodium hydroxide under magnetic stirring at room temperature, reticulated with epichlorohydrin, and derivatized with a cationic ligand (glycidyltrimethylammonium chloride). After 20 h, the gel obtained was neutralized with acetic acid and finally sheared under high pressure in a Minilab homogenizer (Rannie, APV Baker, Evreux, France). The 60-nm-diameter polysaccharidic nanoparticles obtained were untrafiltered to remove low-molecular-weight reagents and salts. SMBV nanoparticles were prepared by mixing polysaccharidic nanoparticles, dipalmitoyl phosphatidyl choline (DPPC), and cholesterol at 80°C, allowing the adsorption of the lipids onto the nanoparticles (3, 8). The mean diameter of the SMBV, determined by laser light scattering with the N4MD Coulter nanoparticle analyzer (Coultronics, Margency, France), was 60 ± 15 nm. SMBV were composed of polysaccharides (77%) and a mixture of DPPC and cholesterol (70:30 [wt/wt]). Phospholipid, cholesterol, and polysaccharide concentrations were determined according to established methods (1, 10). Conjugated CRM-MenC vaccine and the LTK63 mucosal adjuvant were formulated together with the SMBV nanoparticles by simple mixing.
Mice and immunizations.
Groups of six to eight female 6- to 8-week-old BALB/c mice (Charles River, Calco, Italy) were used in all of the experiments. In all groups, irrespective of the route of immunization, the mice received doses of the CRM-MenC vaccine, which contained 2.5 μg of the MenC oligosaccharide and 5 μg of the CRM197 carrier protein (corresponding to a 1:4 human dose). Whenever present, the mice received at each dose 1 μg of the LTK63 mutant and/or 100 μg of the Biovector system. For i.n. immunizations, unanesthetized mice received 20 μl of the vaccine formulation (10 μl in each nostril). Immunizations, either s.c. or i.n., were carried out on days 0, 21, and 35. Serum samples were taken on days 0 (pre), 20 (post-1), 34 (post-2), and 45 (post-3). At day 45, mice were sacrificed, and nasal washes (1 ml) were also collected from single mice according to the procedure already described (7, 12). Individual serum samples and nasal washes were frozen at −20°C until use.
Quantitation of MenC-specific and LT-specific antibodies.
Titration of Men-C- and LT-specific immunoglobulin G (IgG) and IgA antibodies was performed with sera and nasal washes from each mouse according to the assays already described. Briefly, for the titration of MenC-specific antibodies, enzyme-linked immunosorbent assay (ELISA) microwell plates were coated with purified group C N. meningitidis capsular polysaccharide in the presence of methylated human albumin (31). For anti-LT antibodies, microwell plates were first coated with purified GM1 ganglioside (Sigma Chemical Co., St. Louis, Mo.) followed by incubation with wild-type LT, as described in detail elsewhere (7, 12). Antigen-specific IgG and IgA were titrated by using alkaline phosphatase-conjugated goat anti-mouse IgG or biotin-conjugated goat anti-mouse IgA antibody, respectively (anti-IgG antibody from Sigma Chemical Co. and anti-IgA antibody from Kierkegaard and Perry Laboratories, Gaithersburg, Md.). Antibody titers were defined as the serum or nasal wash dilution giving an optical density (OD) value higher than the mean plus 5 standard deviations of the values obtained with the preimmunization samples at the first dilution. Preimmunization samples consistently gave an OD value below 0.1. IgA antibody levels in nasal washes were normalized by dividing the antigen-specific IgA titers by the total amount of IgA measured by ELISA in the nasal washes from each mouse.
Titration of bactericidal antibodies.
Bactericidal antibodies were titrated as already described (14, 24). Briefly, group C N. meningitidis strain 2996 was grown overnight at 37°C on chocolate agar plates (starting from a frozen stock) with 5% CO2. Colonies were collected and used to inoculate 7 ml of Mueller-Hinton broth containing 0.25% glucose to reach an OD at 620 nm (OD620) of 0.05 to 0.08. The culture was incubated for approximately 1.5 h at 37°C with shaking until the OD620 reached 0.23 to 0.24. Bacteria were diluted in 50 mM phosphate buffer (pH 7.2) containing 10 mM MgCl2, 10 mM CaCl2, and 0.5% (wt/vol) bovine serum albumin (BSA) (assay buffer) at the working dilution of 105 CFU/ml. The total volume of the final reaction mixture was 50 μl, with 25 μl of the serial twofold dilution of test serum (individual or pooled samples) or nasal wash (pooled samples), 12.5 μl of bacteria at the working dilution, and 12.5 μl of baby rabbit complement (final concentration, 25%). Controls included bacteria incubated with complement serum and immune sera incubated with bacteria and with complement inactivated by heating at 56°C for 30 min. Immediately after the addition of the baby rabbit complement, 10 μl of the controls was plated on Mueller-Hinton agar plates by the tilt method (time 0). The 96-well plate was incubated for 1 h at 37°C with rotation. Seven microliters of each sample was plated on Mueller-Hinton agar plates as spots, whereas 10 μl of the controls was plated on Mueller-Hinton agar plates by the tilt method (time 1). Agar plates were incubated for 18 h at 37°C, and the colonies corresponding to time 0 and time 1 were counted.
RESULTS AND DISCUSSION
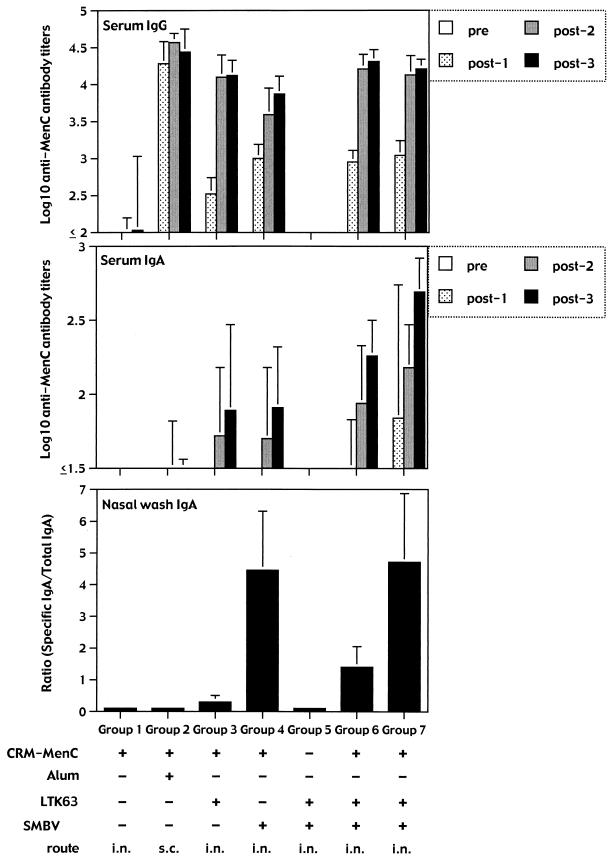
A series of formulations were prepared (Table 1) in order to evaluate the immunogenicity of the conjugated CRM-MenC vaccine prepared with LTK63 mutants and/or with the SMBV and delivered i.n. to groups of eight BALB/c mice. As a control, groups of mice received the conventional CRM-MenC vaccine formulated with aluminum hydroxide (alum) and given s.c. As shown in Fig. 1, after three immunizations either i.n. or s.c., all groups of animals produced high titers of anti-MenC-specific serum IgG antibodies. The vaccine given s.c. already induced the strongest response after the first immunization. This response remained significantly higher than those of all of the other groups as well as after the second immunization. However, after the third dose, the anti-MenC IgG antibody titers in the serum samples from mice immunized with the two formulations containing both the LTK63 and the SMBV were similar to those obtained after s.c. immunization (P > 0.05 for both groups 6 and 7). On the contrary, the anti-MenC IgG antibody titers were significantly higher than those found in mice immunized (group 4) with the vaccine formulated with the SMBV alone (P < 0.01). The use of the LTK63 mutant and/or of the SMBV did not influence the production of MenC-specific IgG subclasses, in that equal amounts of anti-MenC IgG1 and IgG2a isotypes were detected in all groups of mice (data not shown)
TABLE 1.
Groups of mice and immunization treatments
| Mouse group no. | Immunization treatment
|
Route | |||
|---|---|---|---|---|---|
| CRM-MenC | Alum | LTK63 | SMBV | ||
| 1 | Yes | No | No | No | i.n. |
| 2 | Yes | Yes | No | No | s.c. |
| 3 | Yes | No | Yes | No | i.n. |
| 4 | Yes | No | No | Yes | i.n. |
| 5 | No | No | Yes | Yes | i.n. |
| 6 | Yes | No | Yes | Yes | i.n. |
| 7 | Yesa | No | Yesa | Yes | i.n. |
In group 7, conjugated CRM-MenC vaccine and the LTK63 mutant were in the same particles, whereas in group 6, they were in different particles.
FIG. 1.
Serum and mucosal anti-MenC antibody responses. Groups of six to eight BALB/c mice were immunized three times with the different formulations shown, containing the conjugated CRM-MenC vaccine (2.5 μg of oligosaccharide per dose) and the LTK63 mutant (1 μg per dose) with or without the SMBV (100 μg per dose). In group 7, conjugated CRM-MenC vaccine and the LTK63 mutant were in the same particles, whereas in group 6, they were in different particles. Serum samples were taken before (pre) and after (post-1, -2, and -3) each immunization and tested individually to quantitate MenC-specific IgG and IgA antibody titers. Nasal washes were taken only after the third immunization at the moment of sacrifice. Each column represents the mean of each group at each time point plus 1 standard deviation.
The data clearly show that the nontoxic LTK63 mutant and the SMBV have a synergistic (or at least additive) effect on the immunogenicity of the conjugated CRM-MenC vaccine given i.n. In fact, intranasal immunization with the conjugated CRM-MenC vaccine formulated with both the nontoxic LTK63 mutant and the SMBV induces specific serum IgG antibody titers indistinguishable from those induced by the same vaccine given s.c. It is interesting that this strong effect of i.n. immunization was achieved with doses of as little as 2.5 μg of MenC and 1 μg of LTK63, much lower than those used in previous reports showing the i.n. adjuvanticity of the LTK63 mutant for this vaccine (31).
The strong immunogenicity of the MenC vaccine given i.n. after formulation with both the LT mutant and the SMBV is further supported by the data representing the anti-MenC IgA antibody titers in the sera and in the nasal washes. Serum IgA titers to MenC were significantly higher in the groups of mice immunized with the vaccine in the presence of both adjuvant systems than in those receiving the vaccine with either one of the systems (Fig. 1). It is noteworthy that a higher serum IgA antibody response was evident already after the second immunizing dose. Furthermore, anti-MenC IgA antibody titers were detectable as well in the nasal washes taken after the third immunization. Interestingly, the highest IgA antibody titers in the serum were observed in the groups of mice that received the conjugated MenC vaccine in association with the SMBV (Fig. 1). In the nasal washes, the highest anti-MenC IgA antibody titers were observed in the groups of mice receiving the vaccine together with the SMBV (groups 4, 6, and 7). It is interesting that anti-MenC-specific IgG antibodies were consistently not detectable in the nasal washes of all groups of mice (data not shown).
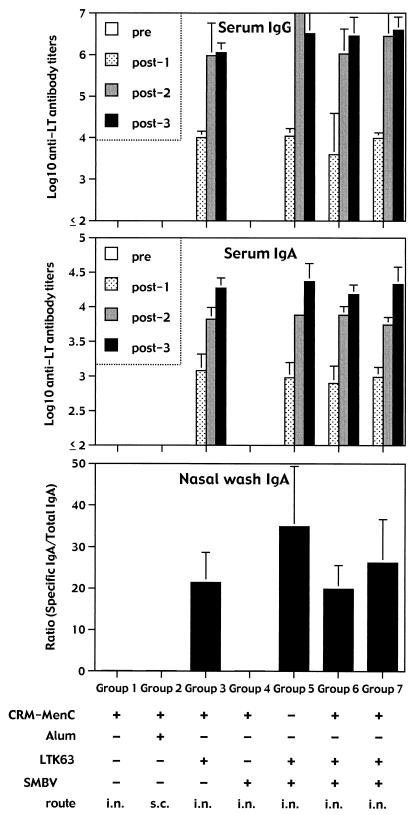
As expected by the already known high immunogenicity of these mutants (26), all groups of mice immunized with vaccine formulations containing the LTK63 mutant had already mounted a strong anti-LT antibody response after the first immunization (Fig. 2). High titers of IgA antibodies were also detected in the serum samples. In addition, nasal washes contained very high titers of both IgA anti-LT antibodies, unlike the mucosal MenC-specific antibody response, which was characterized by IgA only. Anti-LT IgG antibodies were also detected in the nasal washes of all groups receiving the vaccine plus the LTK63 mutants (data not shown).
FIG. 2.
Serum and mucosal anti-LT antibody responses. Groups of six to eight BALB/c mice were immunized three times with the different formulations shown, containing the conjugated CRM-MenC vaccine (2.5 μg of oligosaccharide per dose) and the LTK63 mutant (1 μg per dose) with or without the SMBV (100 μg per dose). In group 7, conjugated CRM-MenC vaccine and the LTK63 mutant were in the same particles, whereas in group 6, they were in different particles. Serum samples were taken before (pre) and after (post-1, -2, and -3) each immunization, and tested individually to quantitate LT-specific IgG and IgA antibody titers. Nasal washes were taken only after the third immunization at the moment of sacrifice. Each column represents the mean of each group at each time point plus 1 standard deviation.
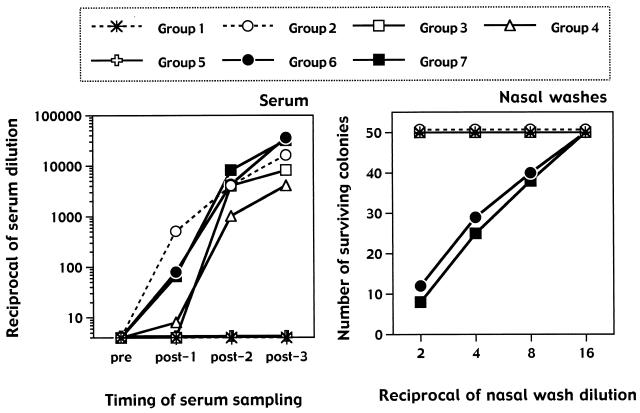
Vaccine-induced protection against N. meningitidis is mediated by anticapsular antibodies able to induce lysis of the bacteria in the presence of complement (13). It is well accepted that bactericidal antibody titers correlate with the protective efficacy of vaccines consisting of capsular oligosaccharides alone or conjugated to carrier proteins such as the CRM. We then asked whether i.n. immunization with the CRM-MenC vaccine induced bactericidal antibodies at titers comparable to those induced by the vaccine given s.c. Figure 3 clearly shows that the alum-adjuvanted vaccine given s.c. was faster in inducing detectable titers of serum bactericidal antibodies than the i.n. vaccines were. However, after three immunizations, the vaccines formulated with both the LTK63 mutant and the SMBV were equivalent to the s.c. vaccine in inducing bactericidal antibodies.
FIG. 3.
Bactericidal activity in serum samples and nasal washes of mice immunized three times with the different formulations shown in Table 1, containing the conjugated CRM-MenC vaccine (2.5 μg of oligosaccharide per dose) and the LTK63 mutant (1 μg per dose) with or without the SMBV (100 μg per dose). Bactericidal antibody titers from pooled serum samples taken before (pre) and after (post-1, -2, and -3) each immunization are shown. Bactericidal titers from individual serum samples did not differ from the data obtained with pooled samples (not shown). Results are expressed as the last dilution of the serum samples giving 50% bacterial killing. Bactericidal activity was also tested in nasal washes taken after the third immunization and pooled. Each pool was tested, starting from a 1:2 dilution. Considering the dilution factor inherent in the nasal wash, results are expressed as the actual numbers of surviving bacterial colonies.
These data suggested that i.n. immunization with conjugated vaccines appropriately formulated can induce protective efficacy, at least comparable to that induced by parenteral vaccines. Considering that parenteral vaccines do not induce significant mucosal responses, we asked whether bactericidal activity was also detected in the nasal washes taken after the three immunizations. Figure 3 shows the results of these experiments. Surprisingly, nasal washes from groups of mice immunized i.n. with the CRM-MenC vaccine formulated with the SMBV and the nontoxic LTK63 mutant exhibited clearly detectable bactericidal antibody titers. Since each nasal wash contained 1 ml of washing buffer, the activities shown in the figure should be considered quite substantial, since they do not take into consideration (in the abscissa) the starting dilution factor of the nasal washing. It is interesting that the groups of mice with bactericidal activity in the nasal washes were the same groups of animals with the highest anti-MenC-specific IgA (but not IgG) antibodies in their nasal washes (Fig. 1). It is thus logical to propose that MenC-specific IgA antibodies produced locally at the level of the nasal mucosa were mediating the bactericidal activity detected in our samples. We tend to exclude the possibility that the effect we observed was due to opsonophagocytosis, which has been shown to be mediated by mucosal IgA directed against different encapsulated bacteria (16). In fact, the storage conditions of the nasal washes before the assay did probably destroy most of the phagocytic cells possibly present at the moment of the harvesting of the washes. The bactericidal effect observed at the nasal level could instead have been directly mediated by complement activation. Indeed, IgA can activate the complement through the alternative pathway (15) or through the newly described third pathway of complement activation driven by the mannan-binding lectin (27). In this manner, mucosal IgA represents an efficacious effector system against invading microorganisms (5). In agreement with this hypothesis, it is known that individuals with genetic deficiencies of the alternative (properdin) pathway exhibit an increased susceptibility to meningococcal diseases (11). Furthermore, an increased susceptibility to meningococcal infections has also been reported in adults (29) and children (30) with heterozygous and homozygous mutations in the MBL gene.
The data presented in this paper clearly show that i.n. delivery of conjugated MenC vaccines in the presence of LTK63 mutant and SMBV significantly enhances the immunogenicity and the protective efficacy of the vaccine. The best results were obtained when both the LTK63 and the SMBV were given together with the conjugated vaccine, in terms of antibody titers, and, more importantly, in terms of induction of protective bactericidal antibodies at the mucosal level. Formulations combining the nontoxic LT mutant and this delivery system clearly deserve further testing in humans. Some concerns have been expressed about the potential risks of localization in the central nervous system (CNS) of LT, CT, and their derivatives given as i.n. adjuvants (32). This may limit the use of these molecules in humans. However, the concomitant use of appropriate delivery systems, such as the SMBV, may contribute to reducing the amount of LT mutant necessary for good adjuvanticity. Furthermore, additional studies are required to determine whether fully nontoxic LT mutants, such as the LTK63 used in our study, may have side effects at the level of the CNS when delivered i.n. Comprehensive studies specifically addressing these issues are in progress.
Importantly, we have shown that this immunization strategy induces IgA-mediated bactericidal activity at the mucosal level, which can be particularly effective in fighting the infection at its portal of entry and in reducing the colonization by group C N. meningitidis. It is tempting to hypothesize that such an i.n. vaccine would show superior efficacy compared to the conventional parenteral vaccines not only in protecting against the meningogoccal disease, but also in protecting against bacterial carriage, thus reducing the spread of the meningococci from humans to humans.
Acknowledgments
We thank Silvia Mancianti for superb technical assistance and Marco Tortoli for technical help with mice.
Editor: J. T. Barbieri
REFERENCES
- 1.Bartlett, G. R. J. 1959. Phosphorus assay in column chromatography. J. Biol. Chem. 234:466-468. [PubMed] [Google Scholar]
- 2.Betbeder, D., A. Etienne, I. De Miguel, R. Kravtzoff, and M. Major. January 1995. Mucosal administration of substances to mammals. U.S. patent 6017513.
- 3.Betbeder, D., C. Davrinche, J. L. Davignon, and E. Prieur. 1996. Method for enhancing immunogenicity product obtained and pharmaceutical composition. WO patent 96/06638.
- 4.Castignolles, N., S. Morgeaux, C. Gontier-Jallet, D. Samain, D. Betbeder, and P. Perrin. 1996. A new family of carriers (biovectors) enhances the immunogenicity of rabies antigens. Vaccine 14:1353-1360. [DOI] [PubMed] [Google Scholar]
- 5.Corthesy, B., and J. P. Kraehenbuhl. 1999. Antibody-mediated protection of mucosal surfaces. Curr. Top. Microbiol. Immunol. 236:93-112. [DOI] [PubMed] [Google Scholar]
- 6.Costantino, P., S. Viti, A. Podda, M. A. Velmonte, L. Nencioni, and R. Rappuoli. 1992. Development and phase I clinical testing of a conjugate vaccine against meningococcus A and C. Vaccine 10:691-698. [DOI] [PubMed] [Google Scholar]
- 7.Del Giudice, G., M. Pizza, and R. Rappuoli. 1999. Mucosal delivery of vaccines. Methods 19:148-155. [DOI] [PubMed] [Google Scholar]
- 8.De Miguel, I., L. Imertie, V. Rieumajor, M. Major, R. Kravtzoff, and D. Betbeder. 2000. Proofs of the structure of lipid coated particles (SMTV) used as drug carriers. Pharm. Res. 17:817-824. [DOI] [PubMed] [Google Scholar]
- 9.Dicko, M., A. Q. Oni, S. Ganivet, S. Kone, L. Pierre, and B. Jacquet. 2000. Safety of immunization injections in Africa: not simply a problem of logistics. Bull. W. H. O. 78:163-169. [PMC free article] [PubMed] [Google Scholar]
- 10.Dubois, K., A. Gilles, J. K. Hamilton, P. A. Rebers, and F. Smith. 1956. Colorimetric method for determination of sugar related substances. Anal. Chem. 28:350-356. [Google Scholar]
- 11.Figueroa, J. E., and P. Densen. 1991. Infectious disease associated with complement deficiencies. Clin. Microbiol. Rev. 4:359-395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Giuliani, M. M., G. Del Giudice, V. Giannelli, G. Dougan, G. Douce, R. Rappuoli, and M. Pizza. 1998. Mucosal adjuvanticity and immunogenicity of LTR72, a novel mutant of Escherichia coli heat-labile enterotoxin with partial knockout of ADP-ribosyltransferase activity. J. Exp. Med. 187:1123-1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goldschneider, I., E. C. Gotschlich, and M. S. Artenstein. 1969. Human immunity to meningococcus. I. The role of human antibodies. J. Exp. Med. 129:1307-1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Granoff, D. M., S. E. Maslanka, G. M. Carlone, B. D. Plikaytis, G. F. Santos, A. Mokatrin, and H. V. Raff. 1998. A modified enzyme-linked immunosorbent assay for measurement of antibody responses to meningococcal C polysaccharide that correlate with bactericidal responses. Clin. Diagn. Lab. Immunol. 5:479-485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hiemstra, P. S., A. Gorter, L. E. Stuurman, L. A. Van Es, and M. R. Daha. 1987. Activation of the alternative pathway of complement by human serum IgA. Eur. J. Immunol. 17:321-326. [DOI] [PubMed] [Google Scholar]
- 16.Janoff, E. N., C. Fasching, J. M. Orenstein, J. B. Rubins, N. L. Opstad, and A. P. Dalmasso. 1999. Killing of Streptococcus pneumoniae by capsular polysaccharide-specific polymeric IgA, complement, and phagocytes. J. Clin. Investig. 104:1139-1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kane, M. 1998. Unsafe injections. Bull. W. H. O. 76:99-100. [PMC free article] [PubMed] [Google Scholar]
- 18.Lamsey, M. E., N. Andrews, E. B. Kaczmarski, and E. Miller. 2001. Efficacy of meningococcal serogroup C conjugate vaccine in teenagers and toddlers in England. Lancet 357:195-196. [DOI] [PubMed] [Google Scholar]
- 19.Levine, M. M., J. B. Kaper, R. E. Black, and M. L. Clemens. 1983. New knowledge on pathogenesis of bacterial infections as applied to vaccine development. Microbiol. Rev. 47:510-550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lieberman, J. M., S. S. Chiu, V. K. Wong, S. Partidge, S. J. Chang, C. Y. Chiu, L. L. Gheesling, G. M. Carlone, and J. I. Ward. 1996. Safety and immunogenicity of a serogroups A/C Neisseria meningitidis oligosaccharide-protein conjugate vaccine in young children. A randomized controlled trial. JAMA 275:1499-1503. [PubMed] [Google Scholar]
- 21.MacDonald, N. E., S. A. Halperin, B. J. Law, B. Forrest, L. E. Danzig, and D. M. Granoff. 1998. Induction of immunologic memory by conjugated versus plain meningococcal C polysaccharide vaccine in toddlers: a randomized controlled trial. JAMA 280:1685-1689. [DOI] [PubMed] [Google Scholar]
- 22.Major, M., E. Prieur, J. F. Tocanne, D. Betbeder, and A. M. Sautereau. 1997. Characterization and phase behaviour of phospholipid bilayers adsorbed on spherical polysaccharidic nanoparticles. Biochim. Biophys. Acta 1327:32-40. [DOI] [PubMed] [Google Scholar]
- 23.Pizza, M., M. M. Giuliani, M. R. Fontana, E. Monaci, G. Douce, G. Dougan, K. H. Mills, R. Rappuoli, and G. Del Giudice. 2001. Mucosal vaccines: nontoxic derivatives of LT and CT as mucosal adjuvants. Vaccine 19:2534-2541. [DOI] [PubMed] [Google Scholar]
- 24.Pizza, M., V. Scarlato, V. Masignani, M. M. Giuliani, B. Aricò, M. Comanducci, G. T. Jennings, L. Baldi, E. Bartolini, B. Capecchi, C. L. Galeotti, E. Luzzi, R. Manetti, E. Marchetti, M. Mora, S. Nuti, G. Ratti, L. Santini, S. Savino, M. Scarselli, E. Storni, P. Zuo, M. Broeker, E. Hundt, B. Knapp, E. Blair, T. Mason, H. Tettelin, D. W. Hood, A. C. Jeffries, N. J. Saunders, D. M. Granoff, J. C. Venter, E. R. Moxon, G. Grandi, and R. Rappuoli. 2000. Identification of vaccine candidates against serogroup B meningococcus by whole-genome sequencing. Science 287:1816-1820. [DOI] [PubMed] [Google Scholar]
- 25.Prieur, E., D. Betbeder, F. Niederdang, M. Major, A. Alcover, J. L. Davignon, and C. Davrinche. 1996. Combination of human cytomegalovirus recombinant immediate-early protein (IE1) with 80 nm cationic biovectors: protection from proteolysis and potentiation of presentation to CD4+ T-cell clones in vitro. Vaccine 14:511-520. [DOI] [PubMed] [Google Scholar]
- 26.Rappuoli, R., M. Pizza, G. Douce, and G. Dougan. 1999. Structure and mucosal adjuvanticity of cholera and Escherichia coli heat-labile enterotoxins. Immunol. Today 20:493-500. [DOI] [PubMed] [Google Scholar]
- 27.Roos, A., L. H. Bouwman, D. J. van Gijlswijk-Janssen, M. C. Faber-Krol, G. L. Stahl, and M. R. Daha. 2001. Human IgA activates the complement system via the mannan-binding lectin pathway. J. Immunol. 167:2861-2868. [DOI] [PubMed] [Google Scholar]
- 28.Samir, E. M., A. Casanova, D. Betbeder, and F. Triebel. 2001. Combination of interleukin-2 with 60 nm cationic supramolecular biovectors: treatment of established tumors by subcutaneous or intranasal administration. Eur. J. Cancer 37:1053-1060. [DOI] [PubMed] [Google Scholar]
- 29.Summerfield, J. A., S. Ryder, M. Sumiya, M. Thursz, A. Gorchein, M. A. Monteil, and M. W. Turner. 1995. Mannose binding protein gene mutations associated with unusual and severe infections in adults. Lancet 345:886-889. [DOI] [PubMed] [Google Scholar]
- 30.Summerfield, J. A., M. Sumiya, M. Levin, and M. W. Turner. 1997. Association of mutations in mannose binding protein gene with childhood infection in consecutive hospital series. Br. Med. J. 314:1229-1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ugozzoli, M., G. Santos, J. Donnelly, and D. O'Hagan. 2001. Potency of genetically detoxified mucosal adjuvant derived from the heat-labile enterotoxin of Escherichia coli (LTK63) is not adversely affected by the presence of preexisting immunity to the adjuvant. J. Infect. Dis. 183:351-354. [DOI] [PubMed] [Google Scholar]
- 32.Van Ginkel, F. W., R. J. Jackson, Y. Yuki, and J. R. McGhee. 2000. The mucosal adjuvant cholera toxin redirects vaccine proteins into olfactory tissues. J. Immunol. 165:4778-4782. [DOI] [PubMed] [Google Scholar]