Abstract
1. Post-synaptic effects of n-octanol at concentrations of 0·1-1 mM were examined in toad sartorius muscles by use of extracellular and voltage-clamp techniques.
2. Octanol depressed the amplitude and duration of miniature end-plate currents and hence depressed neuromuscular transmission.
3. The decay of miniature end-plate currents remained exponential in octanol solutions even when the time constant of decay (τD) was decreased by 80-90%.
4. The lifetime of end-plate channels, obtained by analysis of acetylcholine noise, was also decreased by octanol. The average lifetime measured from noise spectra agreed reasonably well with the time constant of decay of miniature end-plate currents, both in control solution and in octanol solutions.
5. Octanol caused a reduction in the conductance of end-plate channels. Single channel conductance was on average about 25 pS in control solution and 20 pS in octanol.
6. In most cells the normal voltage sensitivity of the decay of miniature end-plate currents was retained in octanol solutions. The lifetime of end-plate channels measured from acetylcholine noise also remained voltage-sensitive in octanol solutions. In some experiments in which channel lifetime was exceptionally reduced the voltage sensitivity was less than normal.
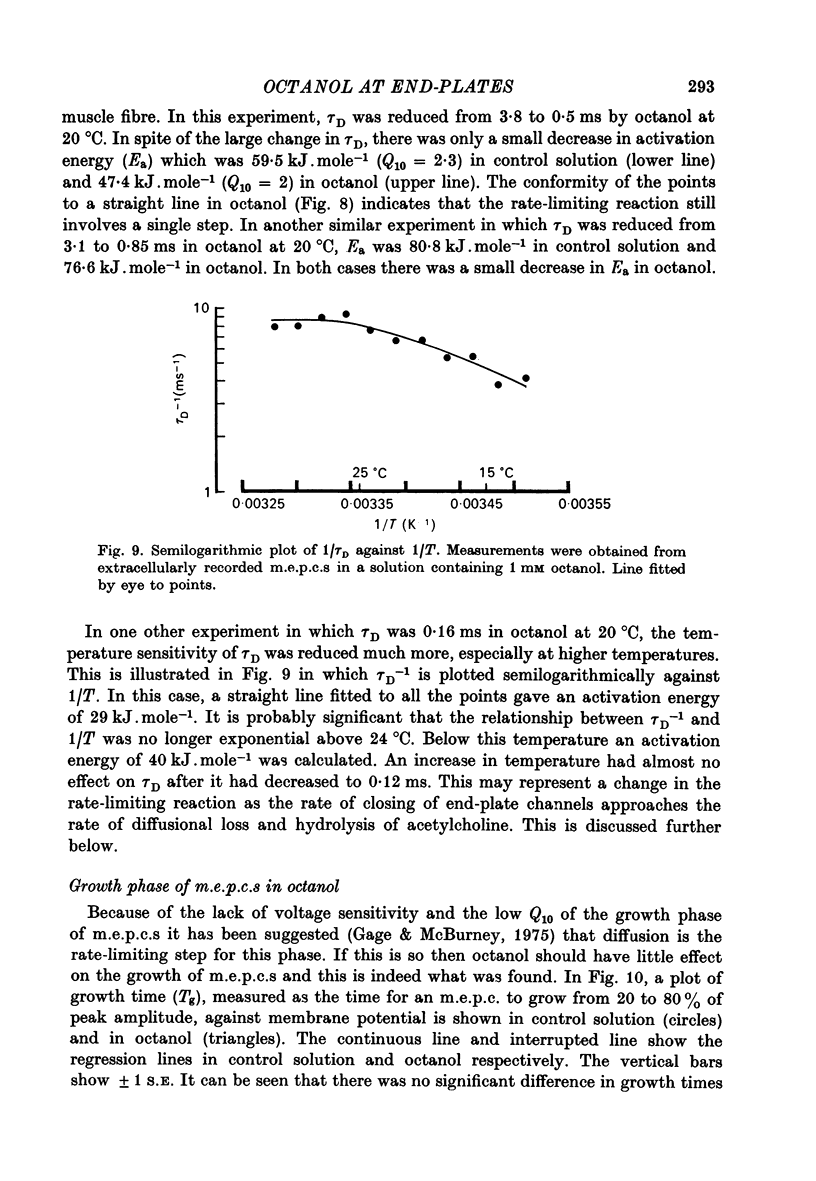
7. In octanol solutions, τD was still very sensitive to temperature changes in most cells although in some the temperature sensitivity of τD was clearly reduced. Changes in τD with temperature could generally be fitted by the Arrhenius equation suggesting that a single step reaction controlled the decay of currents both in control and in octanol solutions. In some cells in which τD became less than 0·3 ms, the relationship between τD and temperature became inconsistent with the Arrhenius equation.
8. As the decay of end-plate currents in octanol solutions remains exponential, and the voltage and temperature sensitivity can be unchanged even when τD is significantly reduced, it seems likely that octanol decreases τD by increasing the rate of the reaction which normally controls the lifetime of end-plate channels.
Full text
PDF


















Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams P. R. Drug blockade of open end-plate channels. J Physiol. 1976 Sep;260(3):531–552. doi: 10.1113/jphysiol.1976.sp011530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson C. R., Stevens C. F. Voltage clamp analysis of acetylcholine produced end-plate current fluctuations at frog neuromuscular junction. J Physiol. 1973 Dec;235(3):655–691. doi: 10.1113/jphysiol.1973.sp010410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colquhoun D., Large W. A., Rang H. P. An analysis of the action of a false transmitter at the neuromuscular junction. J Physiol. 1977 Apr;266(2):361–395. doi: 10.1113/jphysiol.1977.sp011772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FATT P., KATZ B. An analysis of the end-plate potential recorded with an intracellular electrode. J Physiol. 1951 Nov 28;115(3):320–370. doi: 10.1113/jphysiol.1951.sp004675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gage P. W., Armstrong C. M. Miniature end-plate currents in voltage-clamped muscle fibre. Nature. 1968 Apr 27;218(5139):363–365. doi: 10.1038/218363b0. [DOI] [PubMed] [Google Scholar]
- Gage P. W., Eisenberg R. S. Capacitance of the surface and transverse tubular membrane of frog sartorius muscle fibers. J Gen Physiol. 1969 Mar;53(3):265–278. doi: 10.1085/jgp.53.3.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gage P. W., Hamill O. P. Effects of several inhalation anaesthetics on the kinetics of postsynaptic conductance changes in mouse diaphragm. Br J Pharmacol. 1976 Jun;57(2):263–272. doi: 10.1111/j.1476-5381.1976.tb07476.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gage P. W., McBurney R. N. An analysis of the relationship between the current and potential generated by a quantum of acetylcholine in muscle fibers without transverse tubules. J Membr Biol. 1973;12(3):247–272. doi: 10.1007/BF01870004. [DOI] [PubMed] [Google Scholar]
- Gage P. W., McBurney R. N. Effects of membrane potential, temperature and neostigmine on the conductance change caused by a quantum or acetylcholine at the toad neuromuscular junction. J Physiol. 1975 Jan;244(2):385–407. doi: 10.1113/jphysiol.1975.sp010805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gage P. W., McBurney R. N. Miniature end-plate currents and potentials generated by quanta of acetylcholine in glycerol-treated toad sartorius fibres. J Physiol. 1972 Oct;226(1):79–94. doi: 10.1113/jphysiol.1972.sp009974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gage P. W., McBurney R. N., Schneider G. T. Effects of some aliphatic alcohols on the conductance change caused by a quantum of acetylcholine at the toad end-plate. J Physiol. 1975 Jan;244(2):409–429. doi: 10.1113/jphysiol.1975.sp010806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gage P. W., McBurney R. N., Van Helden D. Endplate currents are shortened by octanol: possible role of membrane lipid. Life Sci. 1974 Jun 1;14(11):2277–2283. doi: 10.1016/0024-3205(74)90109-x. [DOI] [PubMed] [Google Scholar]
- Gage P. W. The effect of methyl, ethyl and n-propyl alcohol on neuromuscular transmission in the rat. J Pharmacol Exp Ther. 1965 Nov;150(2):236–243. [PubMed] [Google Scholar]
- Grisham C. M., Barnett R. E. The effects of long-chain alcohols on membrane lipids and the (Na++K+)-ATPase. Biochim Biophys Acta. 1973 Jul 6;311(3):417–422. doi: 10.1016/0005-2736(73)90322-2. [DOI] [PubMed] [Google Scholar]
- Inoue F., Frank G. B. Effects of ethyl alcohol on excitability and on neuromuscular transmission in frog skeletal muscle. Br J Pharmacol Chemother. 1967 May;30(1):186–193. doi: 10.1111/j.1476-5381.1967.tb02124.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kordas M. An attempt at an analysis of the factors determining the time course of the end-plate current. I. The effects of prostigmine and of the ratio of Mg 2+ to Ca 2+ . J Physiol. 1972 Jul;224(2):317–332. doi: 10.1113/jphysiol.1972.sp009897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kordas M. The effect of membrane polarization on the time course of the end-plate current in frog sartorius muscle. J Physiol. 1969 Oct;204(2):493–502. doi: 10.1113/jphysiol.1969.sp008926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magleby K. L., Stevens C. F. A quantitative description of end-plate currents. J Physiol. 1972 May;223(1):173–197. doi: 10.1113/jphysiol.1972.sp009840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magleby K. L., Stevens C. F. The effect of voltage on the time course of end-plate currents. J Physiol. 1972 May;223(1):151–171. doi: 10.1113/jphysiol.1972.sp009839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeman P. The membrane actions of anesthetics and tranquilizers. Pharmacol Rev. 1972 Dec;24(4):583–655. [PubMed] [Google Scholar]
- Seyama I., Narahashi T. Mechanism of blockade of neuromuscular transmission by pentobarbital. J Pharmacol Exp Ther. 1975 Jan;192(1):95–104. [PubMed] [Google Scholar]
- TAKEUCHI A., TAKEUCHI N. Active phase of frog's end-plate potential. J Neurophysiol. 1959 Jul;22(4):395–411. doi: 10.1152/jn.1959.22.4.395. [DOI] [PubMed] [Google Scholar]
- Torda T. A., Gage P. W. Effect of barbiturates on synaptic currents. Anaesth Intensive Care. 1976 Aug;4(3):199–202. doi: 10.1177/0310057X7600400305. [DOI] [PubMed] [Google Scholar]


