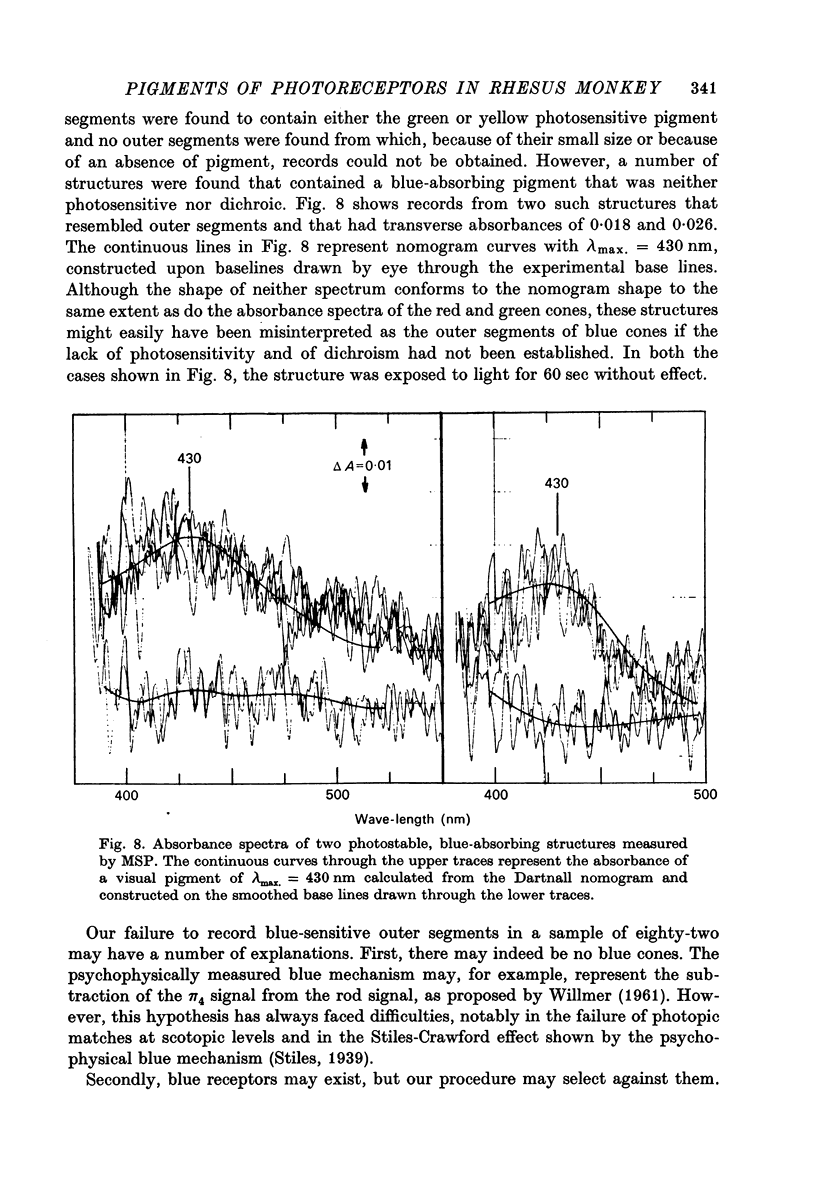
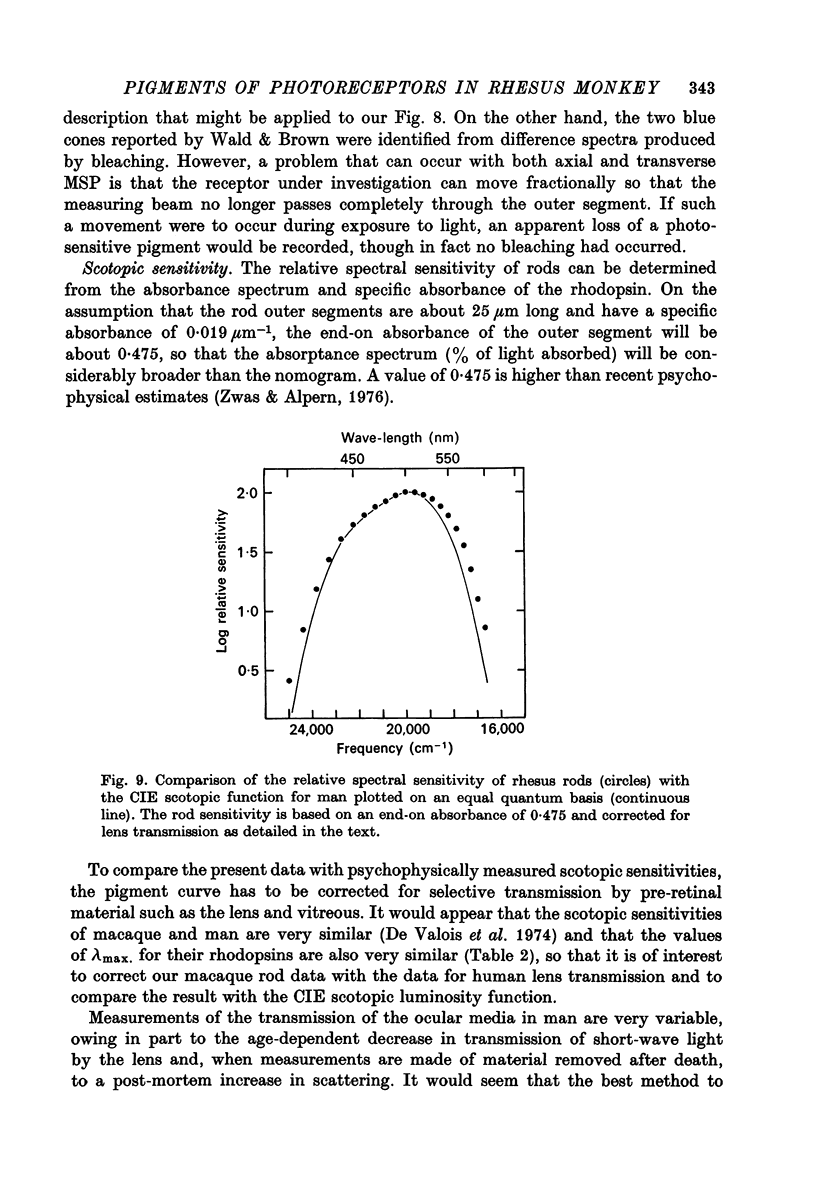
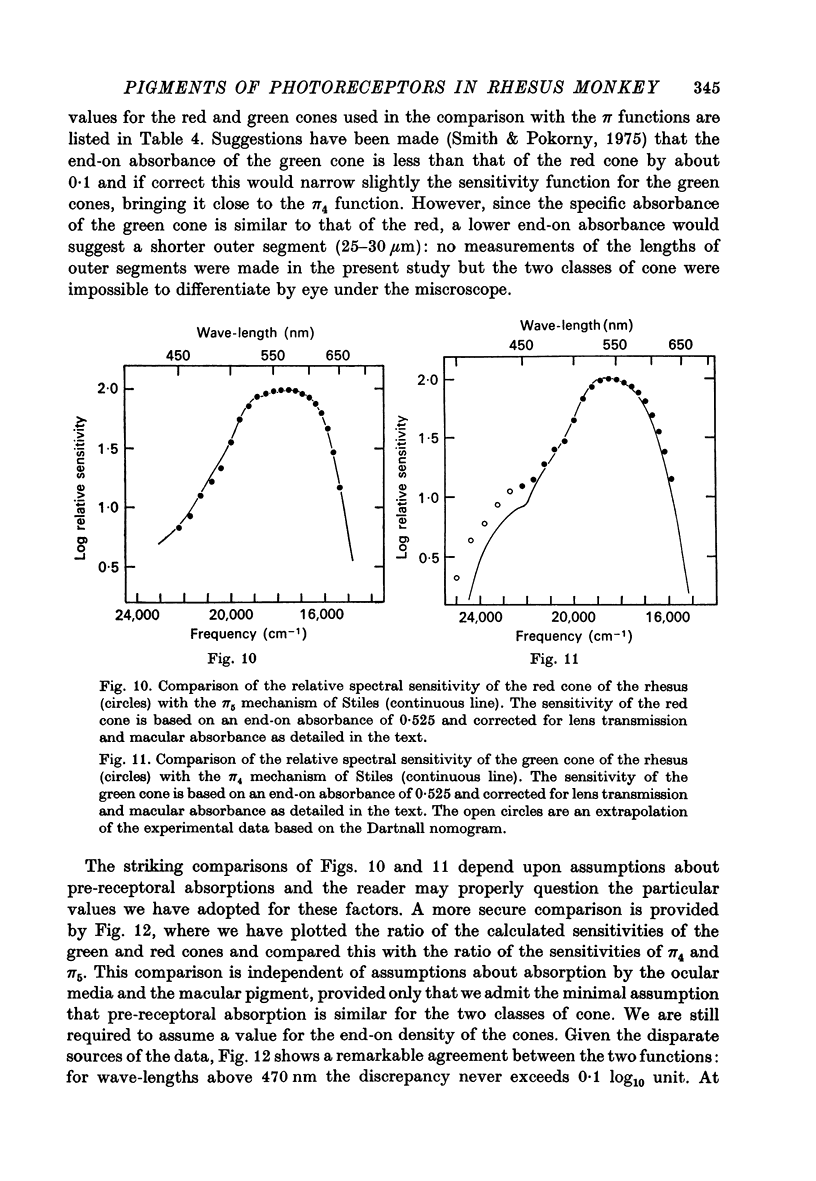
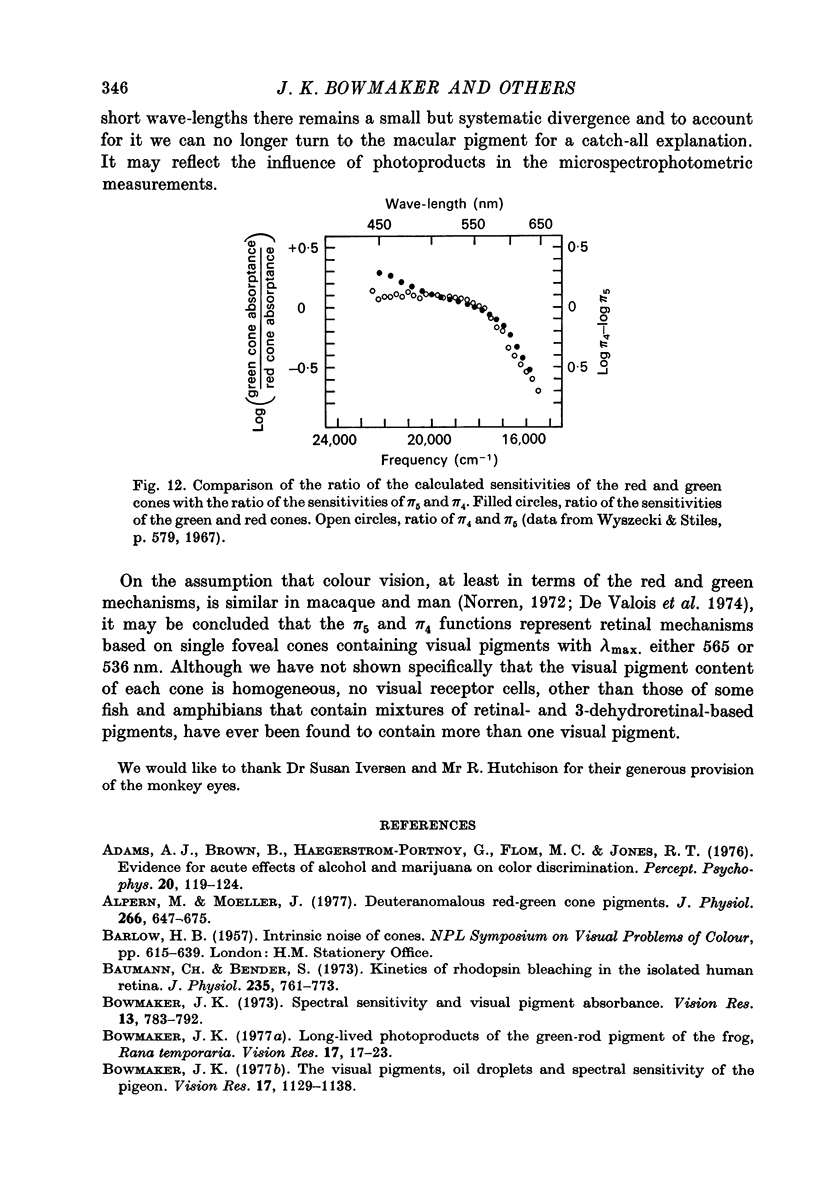
Abstract
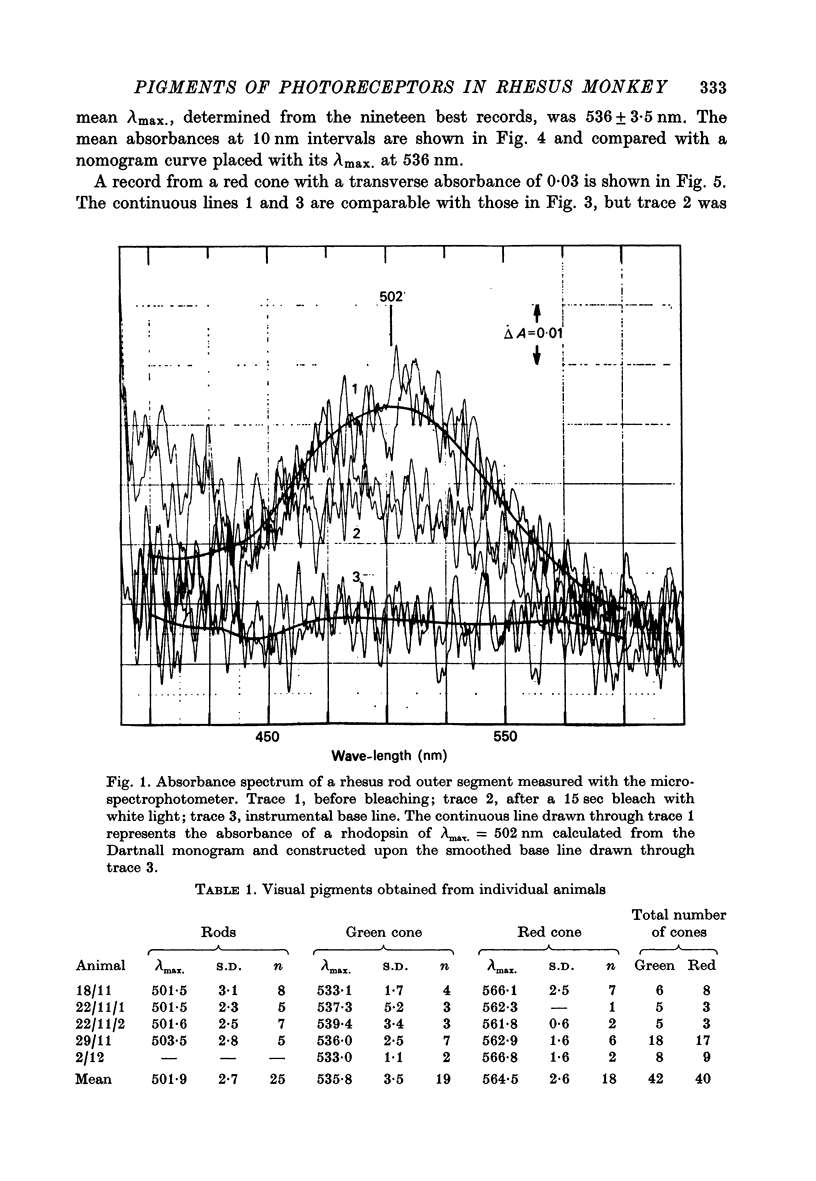
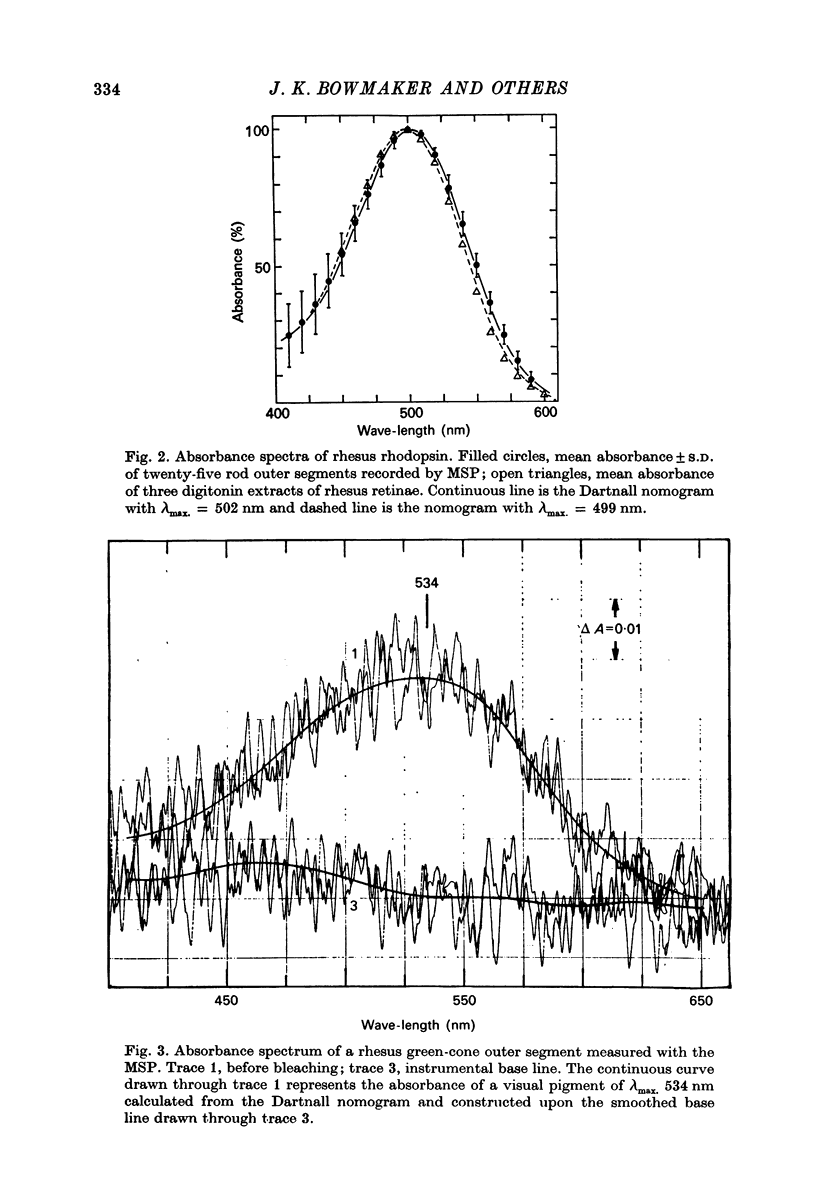
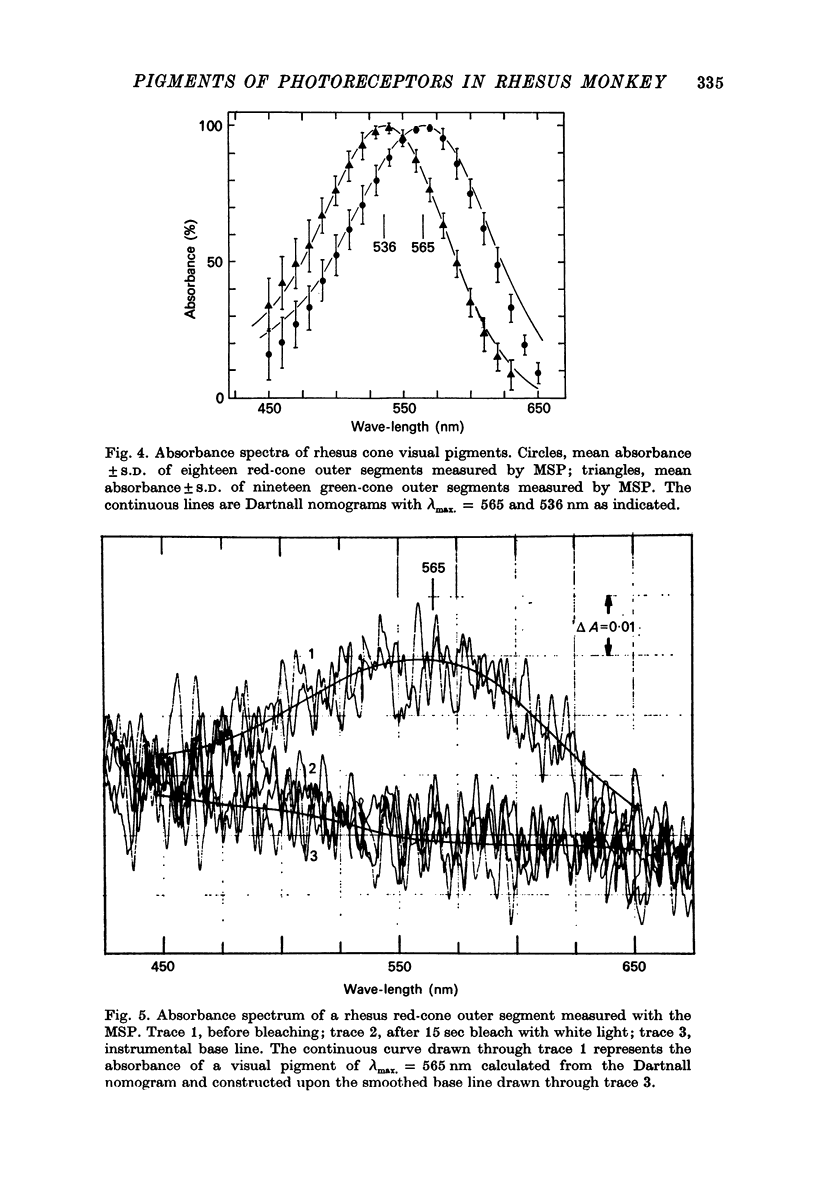
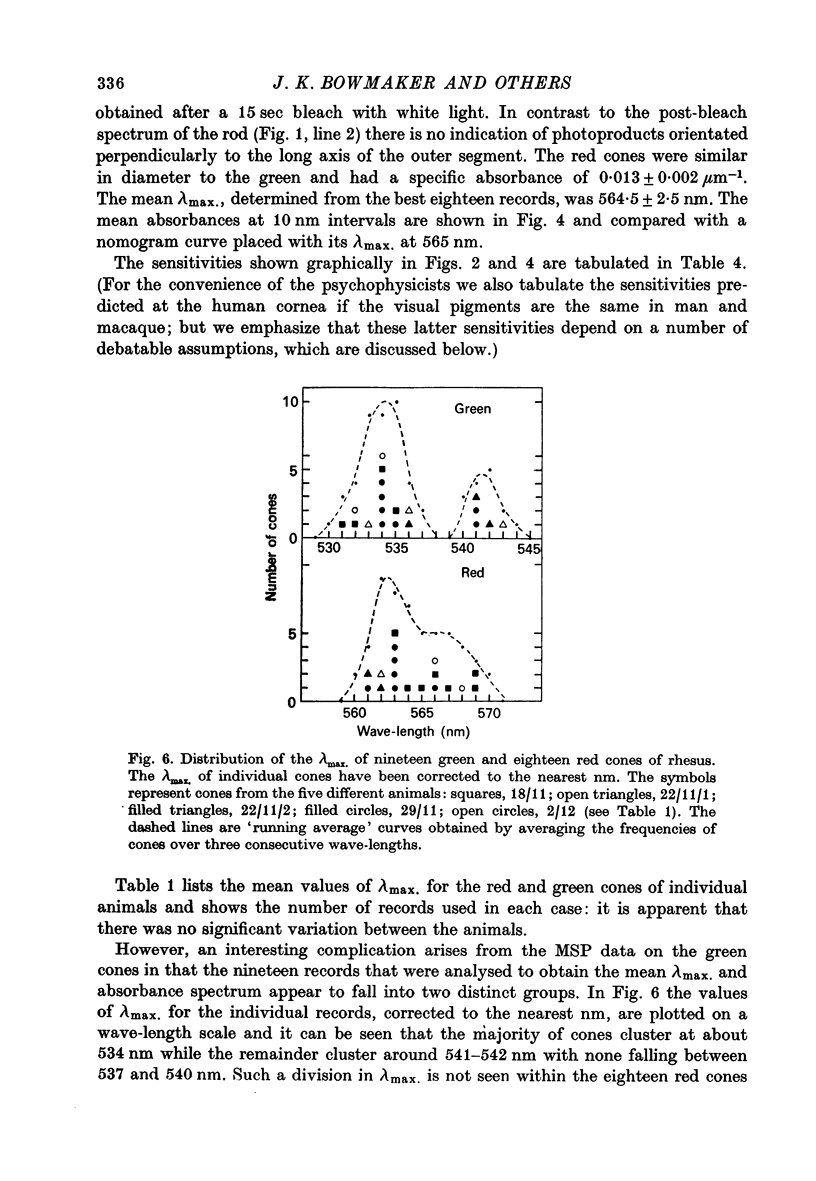
1. New microspectrophotometric measurements have been made of the photo-pigments of individual rods and cones from the retina of the rhesus monkey (Macaca mulatta). The measuring beam was passed transversely through isolated outer segments. 2. The transverse absorbance for rods ranged from 0.02 to 0.04 and that for cones from 0.01 to 0.03. 3. The mean absorbance spectrum for rods (n = 25) had a peak of 502 +/- 2.7 nm. A digitonin extract from the same group of eyes gave a lambda-max. of 499 +/- 1 nm. 4. Of a sample of 82 cones, 40 were 'red' (P565 nm) and 42 were 'green' (P536 nm). The mean absorbance spectrum for the green cones is very similar to the Dartnall nomogram, but that for the red cones is narrower. 5. No bleachable, blue-sensitive outer segments were recorded, although structures were found that absorbed at short wave-lengths and were neither photosensitive nor dichroic. 6. If the long wave-length and middle wave-length cone pigments of the rhesus monkey are assumed to be identical to those of man and if additional assumptions are made about the lengths of human outer segments and about prereceptoral absorption, it is possible to derive psychophysical sensitivities that closely resemble the pi5 and pi4 mechanisms of W. S. Stiles.
Full text
PDF



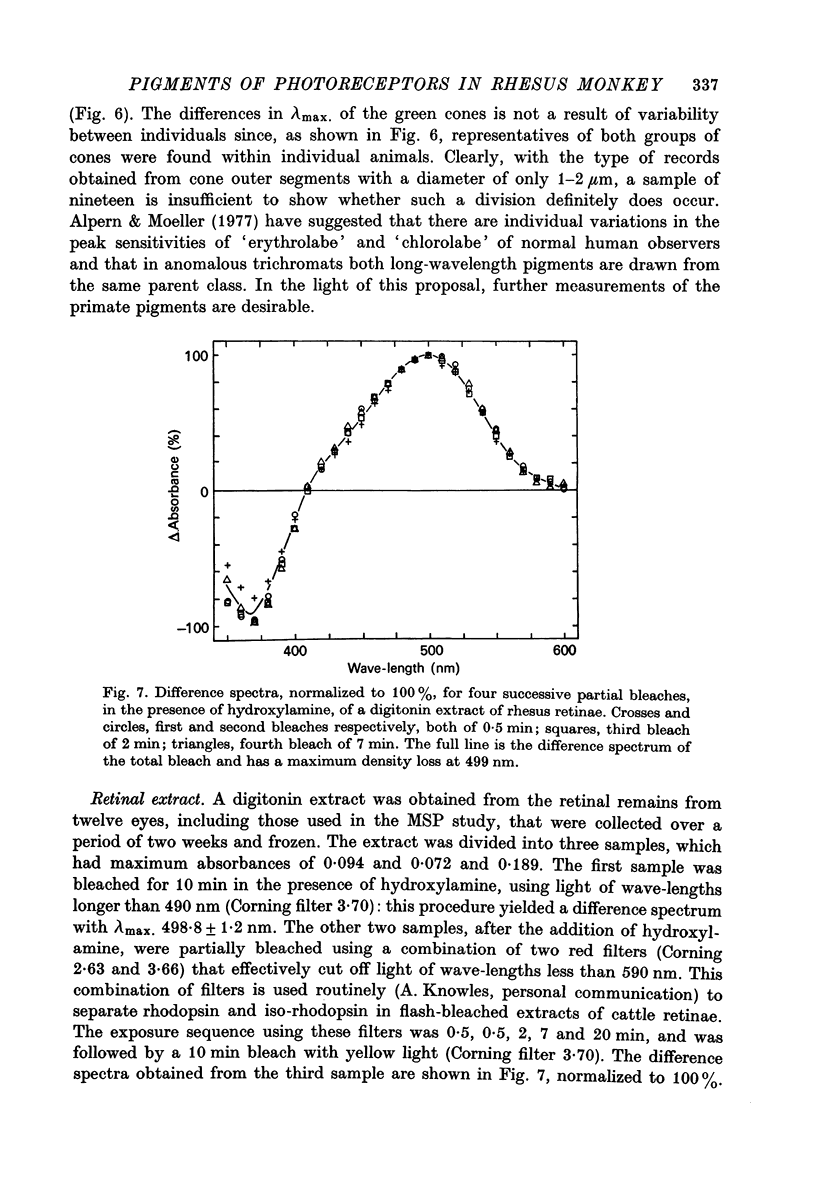
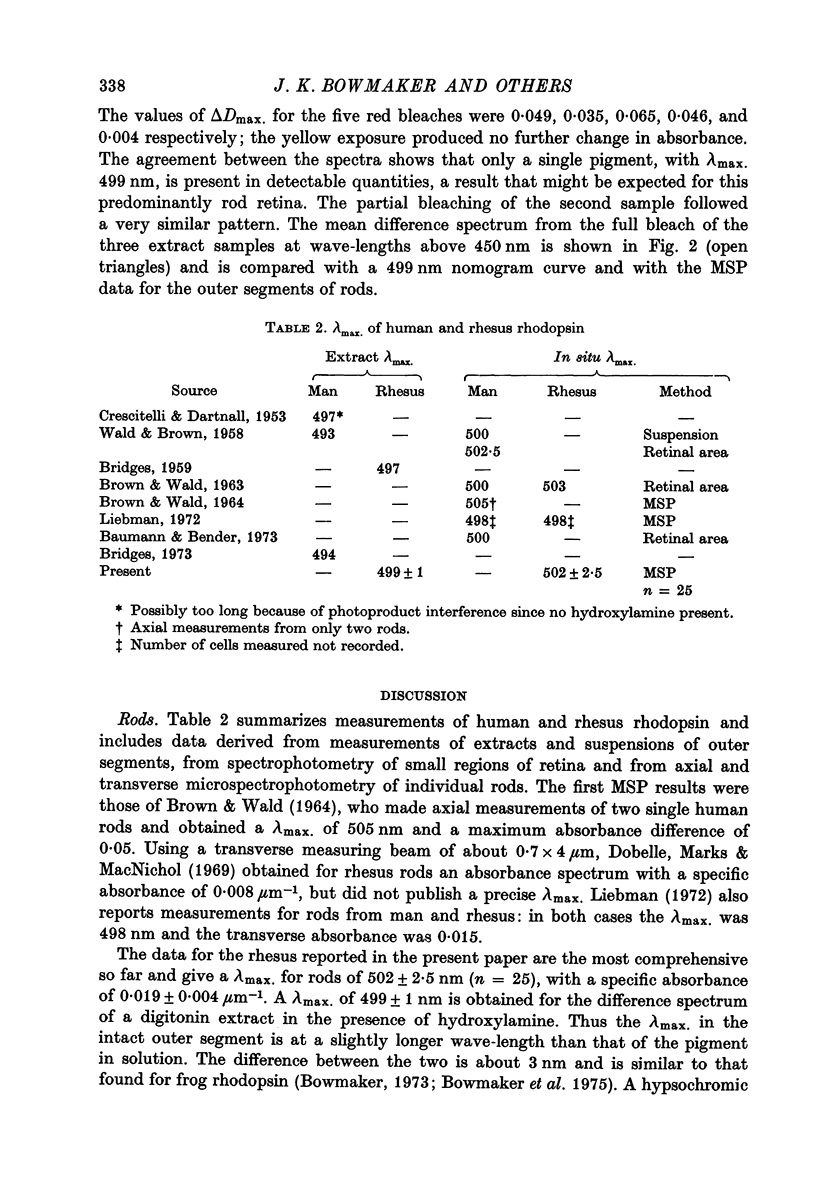
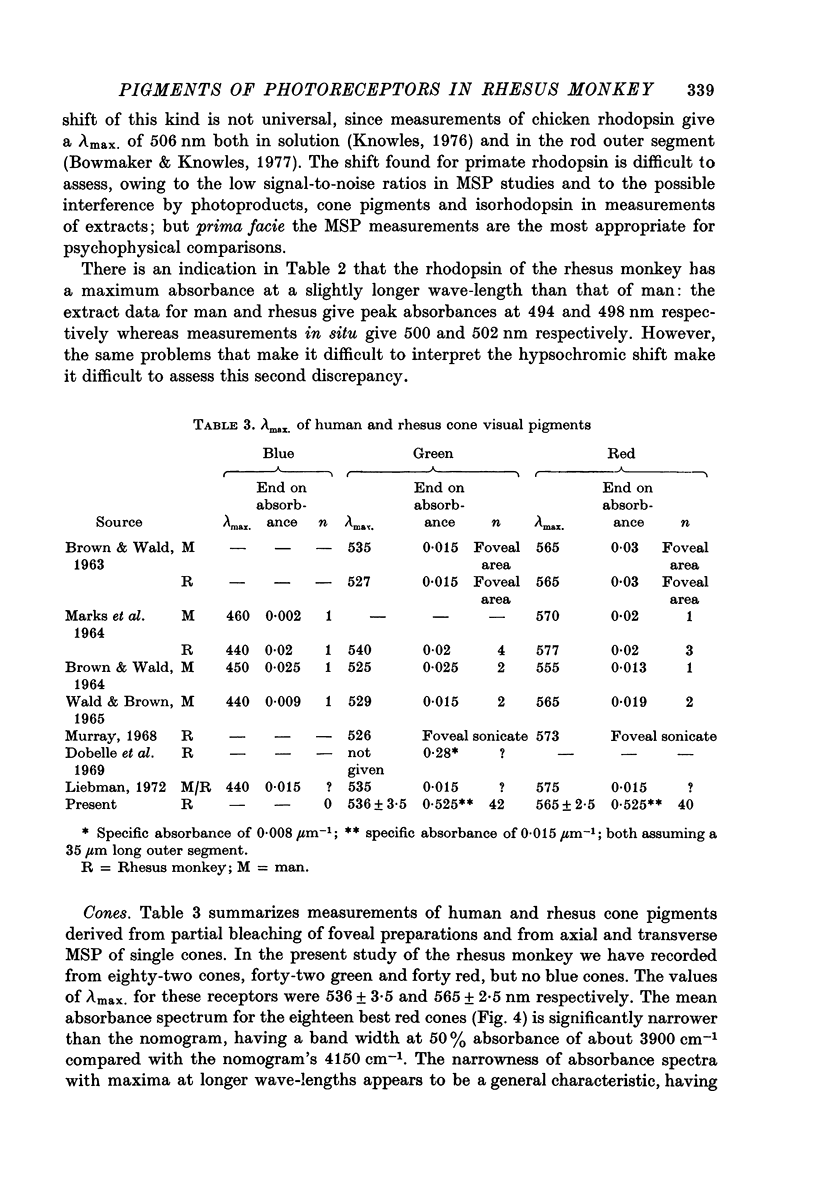
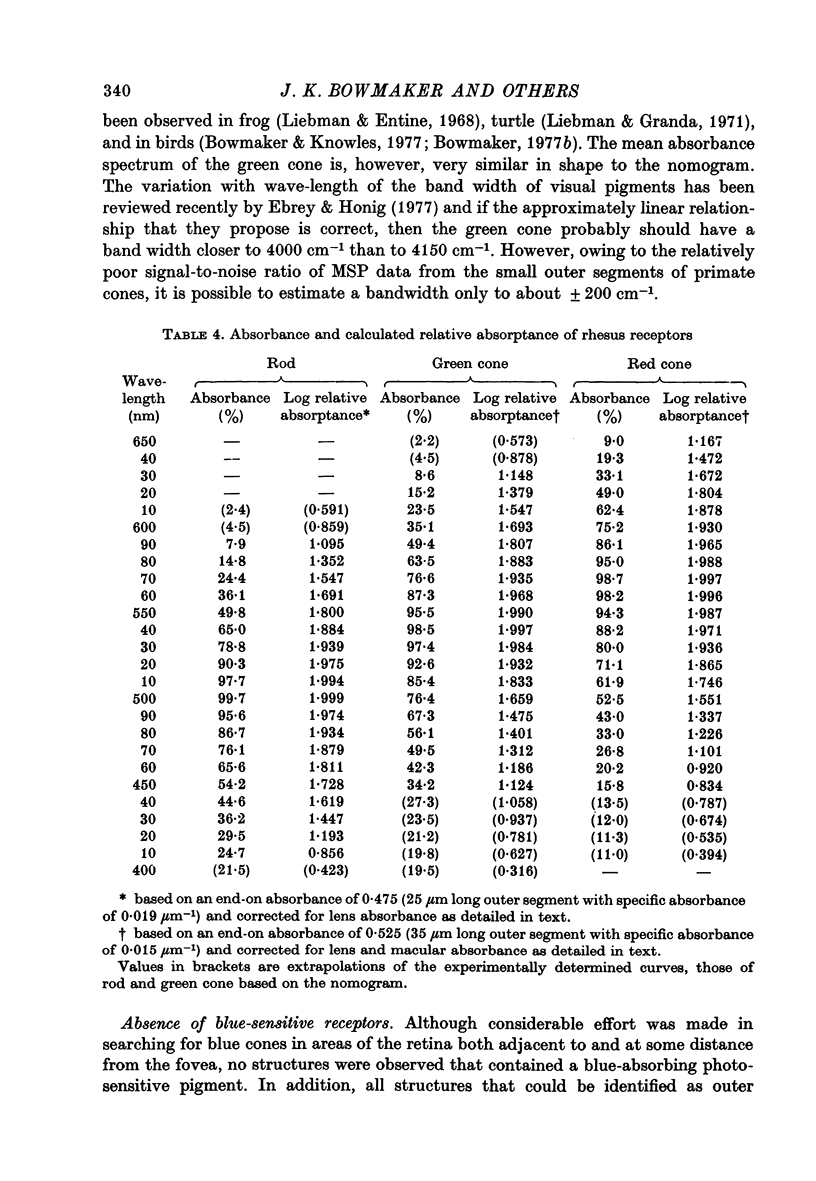




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alpern M., Moeller J. The red and green cone visual pigments of deuternomalous trichromacy. J Physiol. 1977 Apr;266(3):647–675. doi: 10.1113/jphysiol.1977.sp011786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRIDGES C. D. Visual pigments of some common laboratory mammals. Nature. 1959 Nov 28;184(Suppl 22):1727–1728. doi: 10.1038/1841727a0. [DOI] [PubMed] [Google Scholar]
- BROWN P. K., WALD G. VISUAL PIGMENTS IN HUMAN AND MONKEY RETINAS. Nature. 1963 Oct 5;200:37–43. doi: 10.1038/200037a0. [DOI] [PubMed] [Google Scholar]
- BROWN P. K., WALD G. VISUAL PIGMENTS IN SINGLE RODS AND CONES OF THE HUMAN RETINA. DIRECT MEASUREMENTS REVEAL MECHANISMS OF HUMAN NIGHT AND COLOR VISION. Science. 1964 Apr 3;144(3614):45–52. doi: 10.1126/science.144.3614.45. [DOI] [PubMed] [Google Scholar]
- Baumann C., Bender S. Kinetics of rhodopsin bleaching in the isolated human retina. J Physiol. 1973 Dec;235(3):761–773. [PMC free article] [PubMed] [Google Scholar]
- Bowmaker J. K., Knowles A. The visual pigments and oil droplets of the chicken retina. Vision Res. 1977;17(7):755–764. doi: 10.1016/0042-6989(77)90117-1. [DOI] [PubMed] [Google Scholar]
- Bowmaker J. K., Loew E. R., Liebman P. A. Variation in the lambdamax of rhodopsin from individual frogs. Vision Res. 1975 Aug-Sep;15:997–1003. doi: 10.1016/0042-6989(75)90242-4. [DOI] [PubMed] [Google Scholar]
- Bowmaker J. K. Long-lived photoproducts of the green-rod pigment of the frog, Rana temporaria. Vision Res. 1977;17(1):17–23. doi: 10.1016/0042-6989(77)90195-x. [DOI] [PubMed] [Google Scholar]
- Bowmaker J. K. Spectral sensitivity and visual pigment absorbance. Vision Res. 1973 Apr;13(4):783–792. doi: 10.1016/0042-6989(73)90042-4. [DOI] [PubMed] [Google Scholar]
- Bowmaker J. K. The visual pigments, oil droplets and spectral sensitivity of the pigeon. Vision Res. 1977;17(10):1129–1138. doi: 10.1016/0042-6989(77)90147-x. [DOI] [PubMed] [Google Scholar]
- Bridges C. D., Quilliam T. A. Visual pigments of men, moles and hedgehogs. Vision Res. 1973 Dec;13(12):2417–2421. doi: 10.1016/0042-6989(73)90239-3. [DOI] [PubMed] [Google Scholar]
- Bridges C. D. Spectroscopic properties of porphyropsins. Vision Res. 1967 May;7(5):349–369. doi: 10.1016/0042-6989(67)90044-2. [DOI] [PubMed] [Google Scholar]
- CRESCITELLI F., DARTNALL H. J. Human visual purple. Nature. 1953 Aug 1;172(4370):195–197. doi: 10.1038/172195a0. [DOI] [PubMed] [Google Scholar]
- De Valois R. L., Morgan H. C., Polson M. C., Mead W. R., Hull E. M. Psychophysical studies of monkey vision. I. Macaque luminosity and color vision tests. Vision Res. 1974 Jan;14(1):53–67. doi: 10.1016/0042-6989(74)90116-3. [DOI] [PubMed] [Google Scholar]
- Dobelle W. H., Marks W. B., MacNichol E. F., Jr Visual pigment density in single primate foveal cones. Science. 1969 Dec 19;166(3912):1508–1510. doi: 10.1126/science.166.3912.1508. [DOI] [PubMed] [Google Scholar]
- Ebrey T. G., Honig B. New wavelength dependent visual pigment nomograms. Vision Res. 1977;17(1):147–151. doi: 10.1016/0042-6989(77)90213-9. [DOI] [PubMed] [Google Scholar]
- Hárosi F. I. Absorption spectra and linear dichroism of some amphibian photoreceptors. J Gen Physiol. 1975 Sep;66(3):357–382. doi: 10.1085/jgp.66.3.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowles A. The effects of chloride ion upon chicken visual pigments. Biochem Biophys Res Commun. 1976 Nov 8;73(1):56–62. doi: 10.1016/0006-291x(76)90496-4. [DOI] [PubMed] [Google Scholar]
- Liebman P. A., Entine G. Sensitive low-light-level microspectrophotometer: detection of photosensitive pigments of retinal cones. J Opt Soc Am. 1964 Dec;54(12):1451–1459. doi: 10.1364/josa.54.001451. [DOI] [PubMed] [Google Scholar]
- Liebman P. A., Entine G. Visual pigments of frog and tadpole (Rana pipiens). Vision Res. 1968 Jul;8(7):761–775. doi: 10.1016/0042-6989(68)90128-4. [DOI] [PubMed] [Google Scholar]
- Liebman P. A., Granda A. M. Microspectrophotometric measurements of visual pigments in two species of turtle, Pseudemys scripta and Chelonia mydas. Vision Res. 1971 Feb;11(2):105–114. doi: 10.1016/0042-6989(71)90227-6. [DOI] [PubMed] [Google Scholar]
- MARKS W. B., DOBELLE W. H., MACNICHOL E. F., Jr VISUAL PIGMENTS OF SINGLE PRIMATE CONES. Science. 1964 Mar 13;143(3611):1181–1183. doi: 10.1126/science.143.3611.1181. [DOI] [PubMed] [Google Scholar]
- Norren D. V., Vos J. J. Spectral transmission of the human ocular media. Vision Res. 1974 Nov;14(11):1237–1244. doi: 10.1016/0042-6989(74)90222-3. [DOI] [PubMed] [Google Scholar]
- Smith V. C., Pokorny J. Spectral sensitivity of the foveal cone photopigments between 400 and 500 nm. Vision Res. 1975 Feb;15(2):161–171. doi: 10.1016/0042-6989(75)90203-5. [DOI] [PubMed] [Google Scholar]
- Sperling H. G., Sidley N. A., Dockens W. S., Jolliffe C. L. Increment-threshold spectral sensitivity of the rhesus monkey as a function of the spectral composition of the background field. J Opt Soc Am. 1968 Feb;58(2):263–268. doi: 10.1364/josa.58.000263. [DOI] [PubMed] [Google Scholar]
- WALD G., BROWN P. K. Human rhodopsin. Science. 1958 Jan 31;127(3292):222–226. doi: 10.1126/science.127.3292.222. [DOI] [PubMed] [Google Scholar]
- WILLMER E. N. Human colour vision and the perception of blue. J Theor Biol. 1961 Apr;1:141–179. doi: 10.1016/0022-5193(61)90043-1. [DOI] [PubMed] [Google Scholar]
- Wald G., Brown P. K. Human color vision and color blindness. Cold Spring Harb Symp Quant Biol. 1965;30:345–361. doi: 10.1101/sqb.1965.030.01.035. [DOI] [PubMed] [Google Scholar]
- Wald G. HUMAN VISION AND THE SPECTRUM. Science. 1945 Jun 29;101(2635):653–658. doi: 10.1126/science.101.2635.653. [DOI] [PubMed] [Google Scholar]
- Yazulla S., Granda A. M. Opponent-color units in the thalamus of the pigeon (Columba livia). Vision Res. 1973 Aug;13(8):1555–1563. doi: 10.1016/0042-6989(73)90014-x. [DOI] [PubMed] [Google Scholar]
- Zwas F., Alpern M. The density of human rhodopsin in the rods. Vision Res. 1976;16(2):121–127. doi: 10.1016/0042-6989(76)90088-2. [DOI] [PubMed] [Google Scholar]


