Abstract
Nontypeable Haemophilus influenzae (NTHI) lipooligosaccharide htrB mutants exhibited greater than 45-fold-increased sensitivity to human β-defensin 2 (HBD-2) compared to the wild type. Complementation by htrB in trans to acylation competence reversed this increased sensitivity. In contrast, NTHI was more susceptible to HBD-3 and showed no changes in sensitivity as a result of lipooligosaccharide mutations in oligosaccharide and lipid A biosynthesis genes.
β-Defensins are cationic, salt-sensitive, antimicrobial peptides with broad-spectrum activity that contribute to pulmonary mucosal immunity (4, 5, 18, 19). Endotoxin, lipooligosaccharide (LOS), lipopolysaccharide (LPS), and outer membrane proteins are major components of the gram-negative bacterial outer membrane and are the candidate structures to mediate interactions between bacteria and cationic antimicrobial peptides. For example, lipid A composition affects the sensitivity of Proteus mirabilis to polymyxin B and protegrins (14). Phosphorylcholine (ChoP) expression on the LOS of nontypeable Haemophilus influenzae (NTHI) decreases its sensitivity to the human cathelicidin LL37 (13) and promotes colonization and development of otitis media in a chinchilla model (21). ChoP also contributes to H. influenzae airway cell invasion (20) as well as persistence in the airways (24). Furthermore, structural rearrangements of ChoP can affect the sensitivity of H. influenzae to C-reactive protein (24). In another example, the Galα1-3Gal moiety on the nonreducing terminus of LOS on H. influenzae confers resistance to killing by antibody and complement (23). To date, the susceptibility of NTHI to the β-defensins and the possible role of LOS have not been studied in this common respiratory pathogen. We hypothesized that NTHI is susceptible to β-defensins and that changes in the LOS would alter this sensitivity.
NTHI lipid A acylation mutants have increased susceptibility to HBD-2, and NTHI strains are uniformly sensitive to HBD-3.
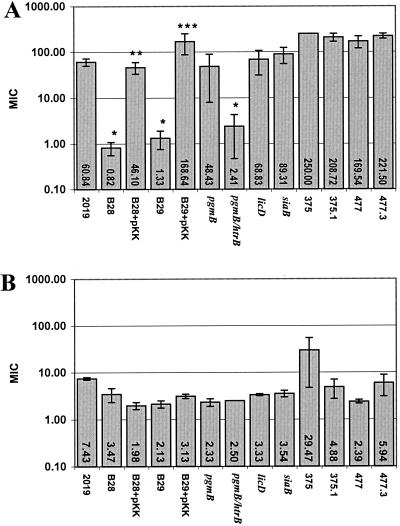
We used a modified radial diffusion assay to investigate bacterial sensitivity to the β-defensins (11). The broth and agarose plates were changed to brain heart infusion supplemented with 10 μg of both hemin and NAD per ml to optimize the survival and growth of H. influenzae. We performed killing assays on strains of NTHI with different LOS modifications (Table 1). The parent H. influenzae strain for all the mutants was NTHI 2019. Exceptions to this are the parents of 477.3 and 375.1 which are mutants of the strains 477 and 375, respectively. 477.3 and 375.1 lack CMP-NANA synthase and cannot sialylate their LOS. Recombinant HBD-2 and HBD-3 (Peprotech, Rocky Hill, N.J.) were rigorously analyzed for purity and concentration by mass spectrometry, amino acid composition, and gel electrophoresis. Tobramycin was used as a positive control for bacterial killing. Statistical significance of the sensitivity of the bacteria to the β-defensins was determined using Student's two-tailed t test. The results of the antimicrobial assays are shown in Fig. 1. The tobramycin-positive control killed all NTHI strains, with similar MICs between 0.13 and 0.54 μg/ml (±0.01 to 0.14 [standard error of the mean]) Wild-type NTHI and mutants involved in LOS biosynthesis (2019 pgmB, licD, siaB, 375.1, and 477.3) were relatively resistant to killing by HBD-2 (Fig. 1A). NTHI 2019 htrB mutants (B28 and B29) have a predominantly tetraacyl lipid A compared to the hexaacylated parent strain (10). These htrB mutants exhibited a more than 45-fold increase in sensitivity to HBD-2 (P = 0.008 and 0.0001, respectively). The two NTHI htrB mutants complemented with a pKK plasmid containing htrB reverted to a resistant phenotype to HBD-2 (P = 0.026 and 0.009, respectively). This complementation experiment demonstrates that a hexaacyl lipid A is required for HBD-2 resistance. Similarly, the double mutant for htrB and pgmB was significantly more susceptible to HBD-2 (P = 0.0002). In contrast, HBD-3 killed all strains of NTHI, regardless of LOS mutation, in low microgram-per-milliliter concentrations (Fig. 1B). It should be pointed out that by the nature of the methods used for this radial diffusion assay, MICs above 79 may indicate a high degree of variability and should be considered as equivalently resistant.
TABLE 1.
NTHI LOS mutants
| NTHI strain | Structure with mutation | Reference |
|---|---|---|
| 2019 | Wild type | 16 |
| B28 | Acylation of lipid A | 10 |
| B28+pKK | Complemented acylation | 10 |
| B29 | Acylation of lipid A | 10 |
| B29+pKK | Complemented acylation | 10 |
| pgmB | Oligosaccharide chain | 20 |
| htrB/pgmB | Acylation + oligosaccharide | This study |
| licD | ChoP | 21 |
| siaB | Sialic acid | W. E. Swords, P. Jones, and M. A. Apicella, unpublished data |
| 375 | Wild type | 6 |
| 375.1 | Sialic acid | 6 |
| 477 | Wild type | 6 |
| 477.3 | Sialic acid | 6 |
FIG. 1.
Sensitivity of NTHI LOS mutants to HBD-2 (A) and HBD-3 (B). All data are the mean MIC (in micrograms per milliliter) as determined by the radial-diffusion assay (n = 3 to 7). Symbols: ∗, P < 0.01 compared to 2019; ∗∗, P = 0.026 compared to uncomplemented htrB mutant B28, ∗∗∗, P = 0.01 compared to uncomplemented htrB mutant B29. Error bars represent the standard error of the mean.
These results are reminiscent of those of Guo et al., who demonstrated that hyperacylation of Salmonella LPS increases resistance to cationic antimicrobial peptides (3). Both results indicate that factors controlling the hydrophobicity of the membrane are important determinants of β-defensin sensitivity. Similarly, Pseudomonas aeruginosa from cystic fibrosis patients synthesize LPS containing palmitate, aminoarabinose, and a variety of penta- and hexaacylated lipid A structures that are also associated with resistance to cationic antimicrobial peptides (2). In a xenograft model, htrB transcription increased when NTHI was in an airway environment (20a). This increased htrB transcription might ensure the hexaacylation of lipid A, leading to decreased β-defensin sensitivity and increased persistence in the airway.
Given that ChoP expression results in decreased susceptibility to LL37 (13), it was somewhat surprising that there were no significant differences in β-defensin sensitivity with the ChoP mutants or with the pgmB mutant which is missing not only the terminal group but also all of the oligosaccharide chains extending from the triheptose structure. We anticipated that the cationic β-defensins might have increased activity against mutants without the positively charged ChoP because of increased electrostatic attraction. This result emphasizes that different classes of cationic antimicrobial peptides may have unique interactions with bacteria.
Another surprising result was the striking difference in the antimicrobial activities of the two β-defensins. β-Defensins are cationic peptides of 33 to 47 amino acids with a conserved 6-cysteine motif forming a specific pattern of three disulfide bonds that confers an amphipathic tertiary structure (17). Their initial interactions with negatively charged bacterial membranes are thought to be electrostatic. Defensins permeabilize bacterial membranes by forming pores (25), perhaps with oligomeric structures (7, 17), but their mechanisms of action are incompletely understood. If the human β-defensins have similar mechanisms of action, one might expect to see similar patterns of antimicrobial sensitivity, possibly with different potencies. These studies, however, demonstrate significant differences in the antimicrobial activities of the β-defensins against the NTHI LOS mutants. The greater activity of HBD-3 may reflect its higher net cationic charge density (4, 9), its ability to form oligomers (17), a different mechanism of action, or interaction with different binding sites on bacteria.
These are the first data to show that the human β-defensins exhibit antimicrobial activity against H. influenzae. NTHI is a common commensal in the human upper respiratory tract (12) and is also a significant disease-associated pathogen (15). Because β-defensins are widely expressed in the respiratory tract mucosa (4, 9, 18, 19) these results have implications for understanding innate host defense against this common organism.
The virulence of NTHI and its ability to persist in the airway may be influenced by β-defensin sensitivity. NTHI htrB mutants have a decreased ability to multiply and cause infection in a chinchilla model of otitis media (1). Recent data also indicate that NTHI htrB mutants have a significantly reduced ability to persist in airway epithelia in vitro and that they elicit lesser degrees of cytoskeletal rearrangements and stimulate less host cell signaling in vivo (Swords et al., submitted). One possible explanation for this decreased virulence is that the NTHI htrB mutants are more sensitive to inducible defensins such as HBD-2. Since the acylation state of lipid A influences the sensitivity of NTHI to killing by human β-defensins, we speculate that steps in this process may provide targets for future protection and intervention strategies. Deacetylins have been developed that reduce lipid A acylation in Salmonella enterica serovar Typhimurium (22) and competitively inhibit lipid A biosynthesis enzymes and have antimicrobial activity against Escherichia coli (8). Furthermore, the single-copy genes involved in lipid A biosynthesis are highly conserved, and homologues of these genes are present in most sequenced gram-negative bacteria. Development of pharmacotherapeutic agents that inhibit the acylation of lipid A may increase the sensitivity of NTHI to β-defensins and thereby enable innate immune factors to more readily clear infections by this common pathogen.
Acknowledgments
These studies were supported in part by grants from the National Institutes of Health (HL61234 to P.B.M., AI24616 and AI65298 to M.A.A., AI 07511 to W.E.S., and HL07638 to T.D.S) and the Cystic Fibrosis Foundation (Clinical Fellowship Grant to T.D.S).
We thank Derek W. Hood for his generous donation of NTHi strains 477, 477.3, 375, and 375.1. We also thank Jerrod Julius, Stacy Coffman, and Paul Rhomberg for their technical assistance.
Editor: E. I. Tuomanen
REFERENCES
- 1.DeMaria, T. F., M. A. Apicella, W. A. Nichols, and E. R. Leake. 1997. Evaluation of the virulence of nontypeable Haemophilus influenzae lipooligosaccharide htrB and rfaD mutants in the chinchilla model of otitis media. Infect. Immun. 65:4431-4435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ernst, R. K., E. C. Yi, L. Guo, K. B. Lim, J. L. Burns, M. Hackett, and S. I. Miller. 1999. Specific lipopolysaccharide found in cystic fibrosis airwayPseudomonas aeruginosa. Science 286:1561-1565. [DOI] [PubMed] [Google Scholar]
- 3.Guo, L., K. B. Lim, C. M. Poduje, M. Daniel, J. S. Gunn, M. Hackett, and S. I. Miller. 1998. Lipid A acylation and bacterial resistance against vertebrate antimicrobial peptides. Cell 95:189-198. [DOI] [PubMed] [Google Scholar]
- 4.Harder, J., J. Bartels, E. Christophers, and J. M. Schroder. 2001. Isolation and characterization of human β-defensin-3, a novel human inducible peptide antibiotic. J. Biol. Chem. 276:5707-5713. [DOI] [PubMed] [Google Scholar]
- 5.Harder, J., J. Bartels, E. Christophers, and J. M. Schroder. 1997. A peptide antibiotic from human skin. Nature 387:861.. [DOI] [PubMed] [Google Scholar]
- 6.Hood, D. W., K. Makepeace, M. E. Deadman, R. F. Rest, P. Thibault, A. Martin, J. C. Richards, and E. R. Moxon. 1999. Sialic acid in the lipopolysaccharide of Haemophilus influenzae: strain distribution, influence on serum resistance and structural characterization. Mol. Microbiol. 33:679-692. [DOI] [PubMed] [Google Scholar]
- 7.Hoover, D. M., K. R. Rajashankar, R. Blumenthal, A. Puri, J. J. Oppenheim, O. Chertov, and J. Lubkowski. 2000. The structure of human beta-defensin-2 shows evidence of higher order oligomerization. J. Biol. Chem. 275:32911-32918. [DOI] [PubMed] [Google Scholar]
- 8.Jackman, J. E., C. A. Fierke, L. N. Tumey, M. Pirrung, T. Uchiyama, S. H. Tahir, O. Hindsgaul, and C. R. Raetz. 2000. Antibacterial agents that target lipid A biosynthesis in gram-negative bacteria. Inhibition of diverse UDP-3-O-(r- 3-hydroxymyristoyl)-n-acetylglucosamine deacetylases by substrate analogs containing zinc binding motifs. J. Biol. Chem. 275:11002-11009. [DOI] [PubMed] [Google Scholar]
- 9.Jia, H. P., B. C. Schutte, A. Schudy, R. Linzmeier, J. M. Guthmiller, G. K. Johnson, B. F. Tack, J. P. Mitros, A. Rosenthal, T. Ganz, and P. B. McCray, Jr. 2001. Discovery of new human beta-defensins using a genomics-based approach. Gene 263:211-218. [DOI] [PubMed] [Google Scholar]
- 10.Lee, N. G., M. G. Sunshine, J. J. Engstrom, B. W. Gibson, and M. A. Apicella. 1995. Mutation of the htrB locus of Haemophilus influenzae nontypable strain 2019 is associated with modifications of lipid A and phosphorylation of the lipo-oligosaccharide. J. Biol. Chem. 270:27151-27159. [PubMed] [Google Scholar]
- 11.Lehrer, R. I., M. Rosenman, S. S. Harwig, R. Jackson, and P. Eisenhauer. 1991. Ultrasensitive assays for endogenous antimicrobial polypeptides. J. Immunol. Methods 137:167-173. [DOI] [PubMed] [Google Scholar]
- 12.Lerman, S. J., J. C. Kucera, and J. M. Brunken. 1979. Nasopharyngeal carriage of antibiotic-resistant Haemophilus influenzae in healthy children. Pediatrics 64:287-291. [PubMed] [Google Scholar]
- 13.Lysenko, E. S., J. Gould, R. Bals, J. M. Wilson, and J. N. Weiser. 2000. Bacterial phosphorylcholine decreases susceptibility to the antimicrobial peptide LL-37/hCAP18 expressed in the upper respiratory tract. Infect. Immun. 68:1664-1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McCoy, A. J., H. Liu, T. J. Falla, and J. S. Gunn. 2001. Identification of Proteus mirabilis mutants with increased sensitivity to antimicrobial peptides. Antimicrob. Agents Chemother. 45:2030-2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Murphy, T. F., and M. A. Apicella. 1987. Nontypable Haemophilus influenzae: a review of clinical aspects, surface antigens, and the human immune response to infection. Rev. Infect. Dis. 9:1-15. [DOI] [PubMed] [Google Scholar]
- 16.Murphy, T. F., K. C. Dudas, J. M. Mylotte, and M. A. Apicella. 1983. A subtyping system for nontypable Haemophilus influenzae based on outer- membrane proteins. J. Infect. Dis. 147:838-846. [DOI] [PubMed] [Google Scholar]
- 17.Schibli, D. J., H. N. Hunter, V. Aseyev, T. D. Starner, J. M. Wiencek, P. B. McCray, Jr., B. F. Tack, and H. J. Vogel. 2002. The solution structures of the human beta-defensins lead to a better understanding of the potent bactericidal activity of HBD-3 against Staphylococcus aureus. J. Biol. Chem. 277:8279-8289. [DOI] [PubMed] [Google Scholar]
- 18.Schutte, B. C., and P. B. McCray, Jr. 2002. β-Defensins in lung host defense. Annu. Rev. Physiol. 64:709-748. [DOI] [PubMed] [Google Scholar]
- 19.Singh, P. K., H. P. Jia, K. Wiles, J. Hesselberth, L. Liu, B. A. Conway, E. P. Greenberg, E. V. Valore, M. J. Welsh, T. Ganz, B. F. Tack, and P. B. McCray, Jr. 1998. Production of beta-defensins by human airway epithelia. Proc. Natl. Acad. Sci. USA 95:14961-14966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Swords, W. E., B. A. Buscher, K. Ver Steeg Ii, A. Preston, W. A. Nichols, J. N. Weiser, B. W. Gibson, and M. A. Apicella. 2000. Non-typeable Haemophilus influenzae adhere to and invade human bronchial epithelial cells via an interaction of lipooligosaccharide with the PAF receptor. Mol. Microbiol. 37:13-27. [DOI] [PubMed] [Google Scholar]
- 20a.Swords, W. E., D. L. Chance, L. A. Cohn, J. Shao, M. A. Apicella, and A. L. Smith. 2002. Acylation of the lipooligosaccharide of Haemophilus influenzae and colonization: an htrB mutation diminishes the colonization of human airway epithelial cells. Infect. Immun. 70:4661-4668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tong, H. H., L. E. Blue, M. A. James, Y. P. Chen, and T. F. DeMaria. 2000. Evaluation of phase variation of nontypeable Haemophilus influenzae lipooligosaccharide during nasopharyngeal colonization and development of otitis media in the chinchilla model. Infect. Immun. 68:4593-4597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Trent, M. S., W. Pabich, C. R. Raetz, and S. I. Miller. 2001. A PhoP/PhoQ- induced lipase (PagL) that catalyzes 3-O-deacylation of lipid A precursors in membranes of Salmonella typhimurium. J. Biol. Chem. 276:9083-9092. [DOI] [PubMed] [Google Scholar]
- 23.Weiser, J. N., and N. Pan. 1998. Adaptation of Haemophilus influenzae to acquired and innate humoral immunity based on phase variation of lipopolysaccharide. Mol. Microbiol. 30:767-775. [DOI] [PubMed] [Google Scholar]
- 24.Weiser, J. N., N. Pan, K. L. McGowan, D. Musher, A. Martin, and J. Richards. 1998. Phosphorylcholine on the lipopolysaccharide of Haemophilus influenzae contributes to persistence in the respiratory tract and sensitivity to serum killing mediated by C-reactive protein. J. Exp. Med. 187:631-640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wimley, W. C., M. E. Selsted, and S. H. White. 1994. Interactions between human defensins and lipid bilayers: evidence for formation of multimeric pores. Protein Sci. 3:1362-1373. [DOI] [PMC free article] [PubMed] [Google Scholar]