Abstract
1. Stimulation of fluid secretion from fly salivary glands by 5-hydroxytryptamine (5-HT) is known to involve calcium and cyclic AMP. Isolated salivary glands were used to investigate the role of these second messengers in the control of enzyme (sucrase) secretion.
2. The protein component of secretion from isolated glands treated with 5-HT appears to be identical to that of saliva secreted by flies during feeding.
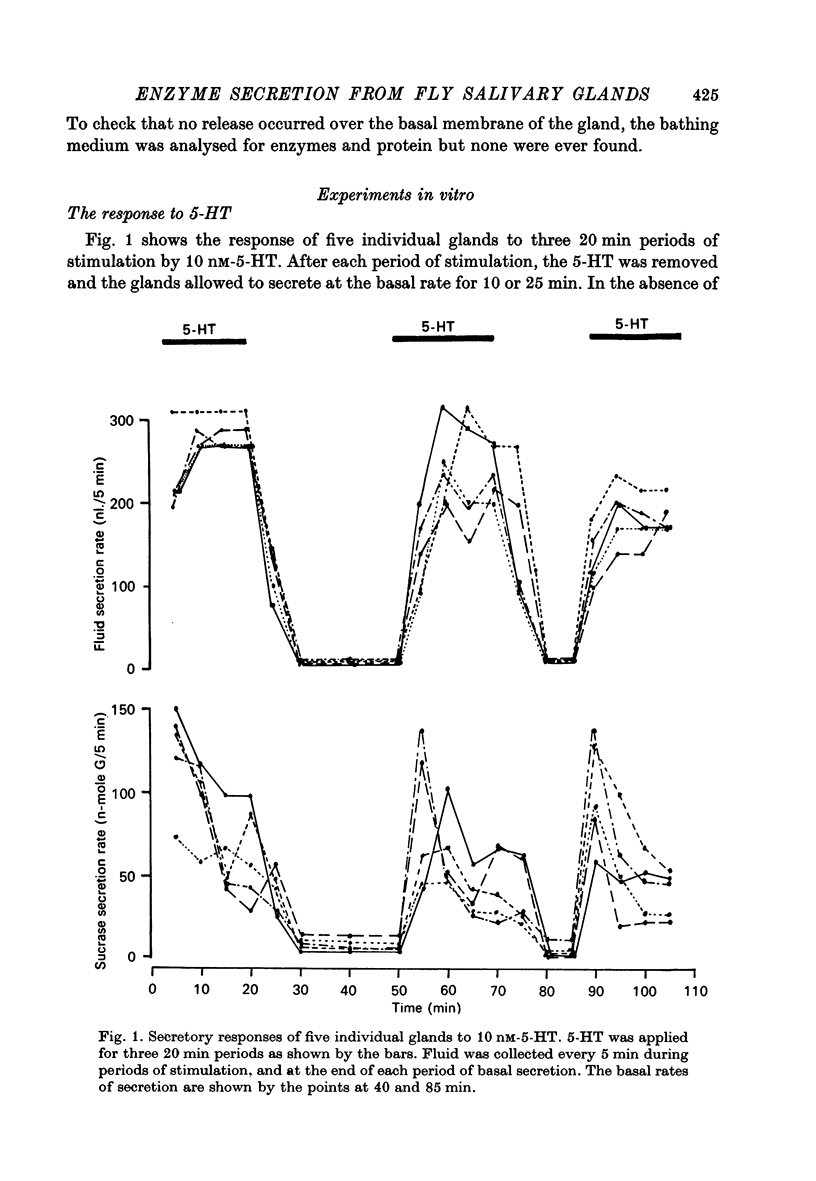
3. Stimulation of fluid secretion by 5-HT follows a definite dose-response curve, but there is no consistent relationship between the rate of enzyme secretion and the stimulating concentration of 5-HT.
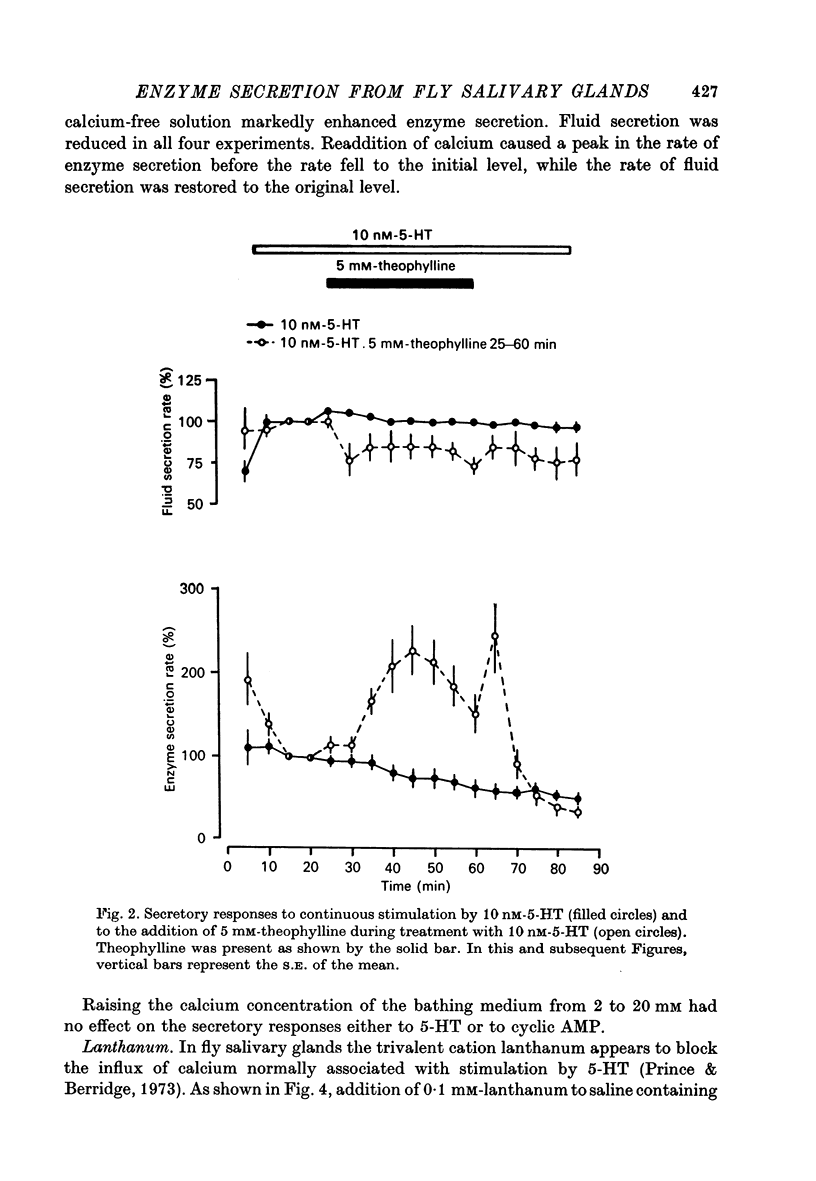
4. Exogenous cyclic AMP causes secretion of enzymes as well as of fluid, thus mimicking the action of 5-HT. The phosphodiesterase inhibitor theophylline enhances the rate of 5-HT-stimulated enzyme secretion.
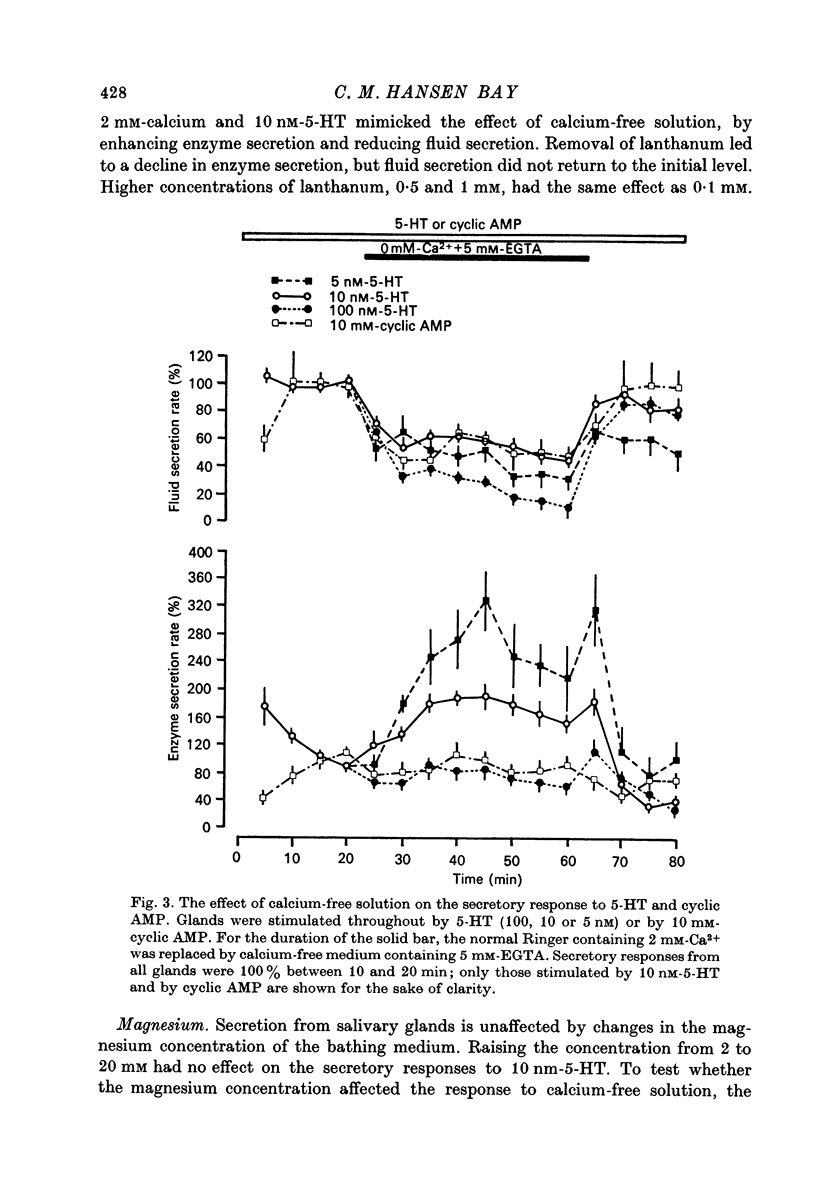
5. Removal of calcium from the bathing medium enhances enzyme secretion in response to 5 or 10 nM-5-HT but has no effect on enzyme secretion stimulated by 100 nM-5-HT or by cyclic AMP.
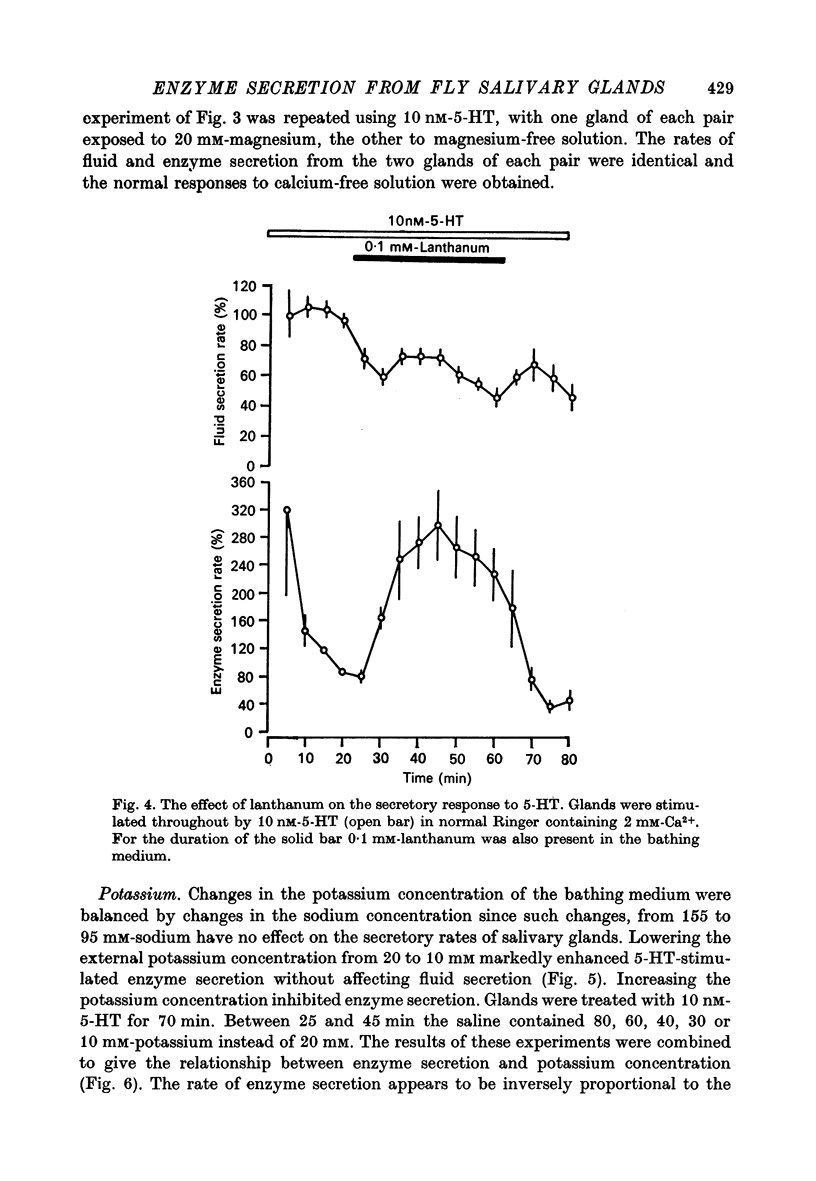
6. Addition of 0·1 mM-lanthanum to medium containing 2 mM-calcium mimics the effect of calcium-free solution on 5-HT-stimulated enzyme secretion.
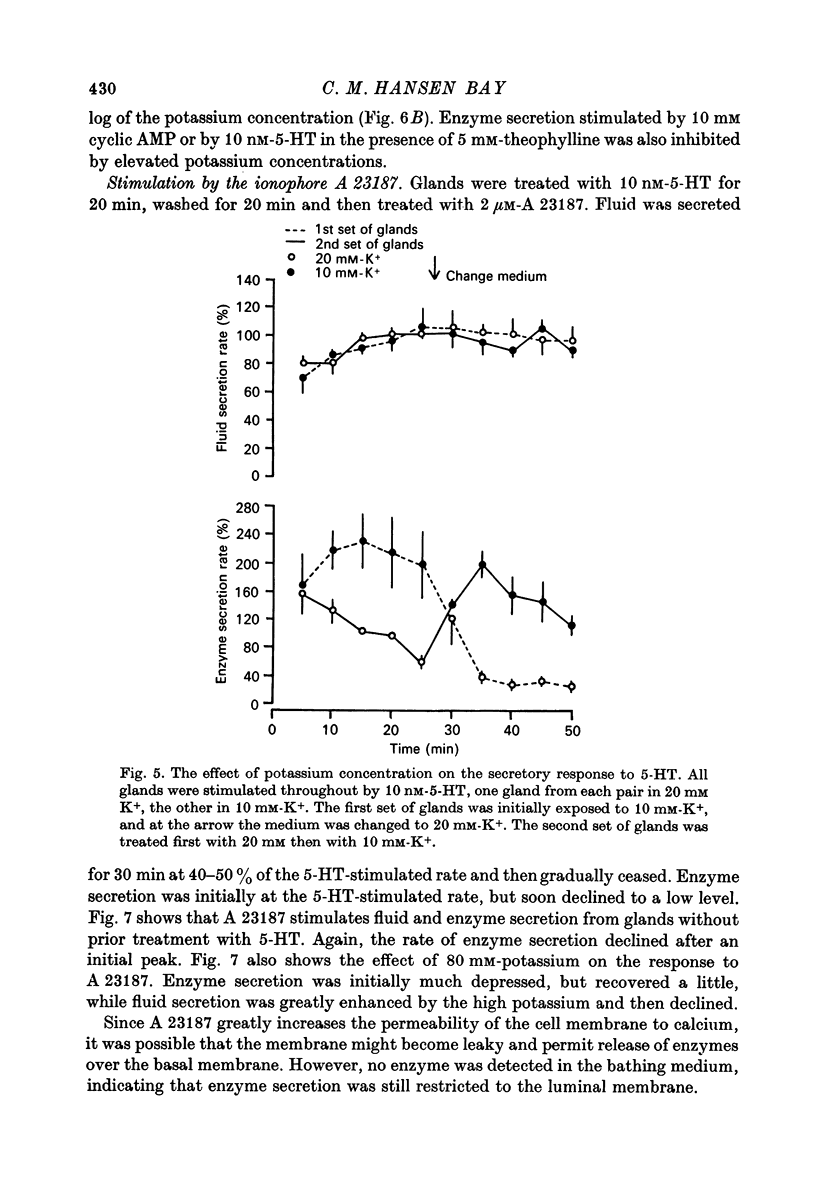
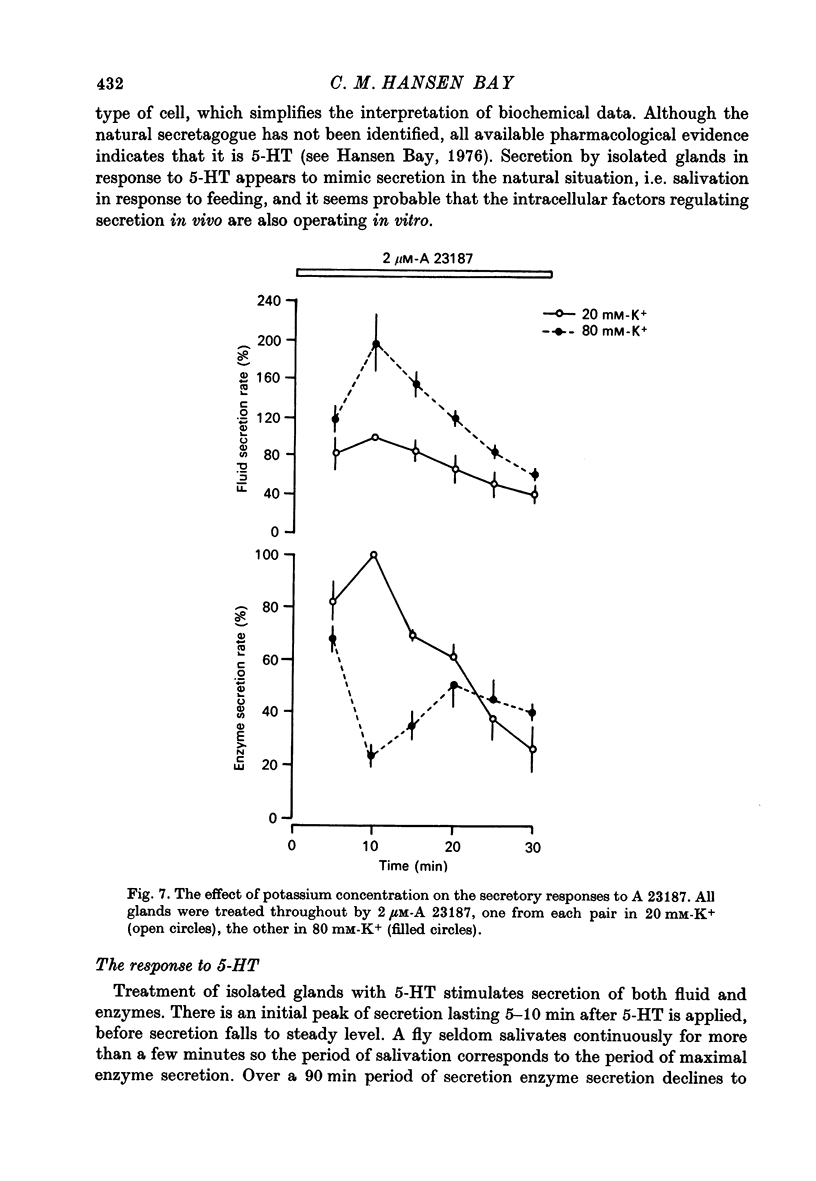
7. The ionophore A 23187 causes secretion of both fluid and enzyme. The secretory rate is initially high but soon declines and ceases after about 40 min.
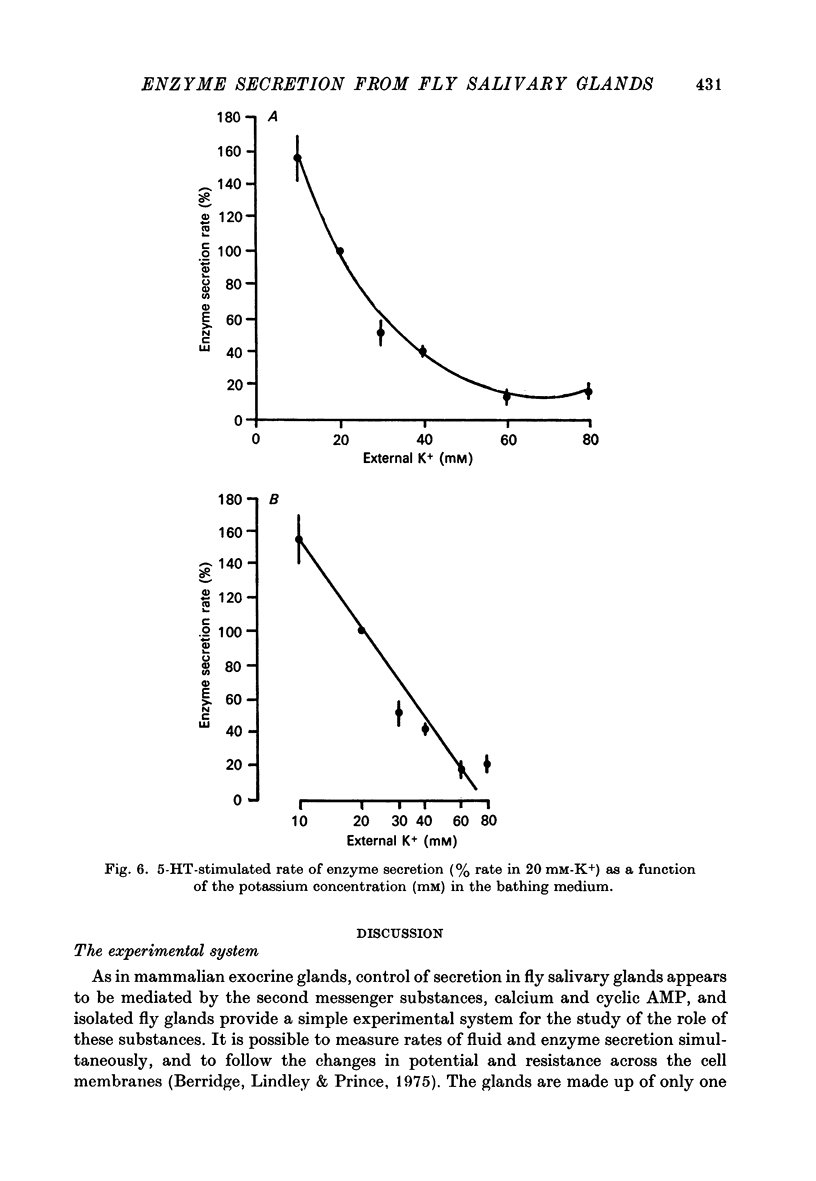
8. Enzyme secretion in response to 5-HT or to cyclic AMP is progressively inhibited as the concentration of potassium is increased from 10 to 80 mM. Secretion in response to A 23187 is initially inhibited by 80 mM-potassium but then partially recovers.
9. The rate of enzyme secretion appears to be affected by the intracellular concentrations of both calcium and cyclic AMP. It is possible that the rate of enzyme secretion increases as the intracellular calcium concentration rises, until the optimal calcium concentration is reached when further increase in the level of calcium progressively inhibits secretion. The optimal calcium concentration for enzyme secretion is lower than that for fluid secretion, and 5-HT normally causes maximal fluid secretion and submaximal enzyme secretion.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amos W. B. An apparatus for microelectrophoresis in polyacrylamide slab-gels. Anal Biochem. 1976 Feb;70(2):612–615. doi: 10.1016/0003-2697(76)90487-5. [DOI] [PubMed] [Google Scholar]
- Argent B. E., Case R. M., Scratcherd T. Amylase secretion by the perfused cat pancreas in relation to the secretion of calcium and other electrolytes and as influenced by the external ionic environment. J Physiol. 1973 May;230(3):575–593. doi: 10.1113/jphysiol.1973.sp010205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M. J., Lindley B. D., Prince W. T. Membrane permeability changes during stimulation of isolated salivary glands of Calliphora by 5-hydroxytryptamine. J Physiol. 1975 Jan;244(3):549–567. doi: 10.1113/jphysiol.1975.sp010812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M. J., Lindley B. D., Prince W. T. Studies on the mechanism of fluid secretion by isolated salivary glands of Calliphora. J Exp Biol. 1976 Apr;64(2):311–322. doi: 10.1242/jeb.64.2.311. [DOI] [PubMed] [Google Scholar]
- Berridge M. J., Prince W. T. Transepithelial potential changes during stimulation of isolated salivary glands with 5-hydroxytryptamine and cyclic AMP. J Exp Biol. 1972 Feb;56(1):139–153. doi: 10.1242/jeb.56.1.139. [DOI] [PubMed] [Google Scholar]
- Berridge M. J. The interaction of cyclic nucleotides and calcium in the control of cellular activity. Adv Cyclic Nucleotide Res. 1975;6:1–98. [PubMed] [Google Scholar]
- Berridge M. J. The role of 5-hydroxytryptamine and cyclic AMP in the control of fluid secretion by isolated salivary glands. J Exp Biol. 1970 Aug;53(1):171–186. doi: 10.1242/jeb.53.1.171. [DOI] [PubMed] [Google Scholar]
- Blinks J. R., Olson C. B., Jewell B. R., Bravený P. Influence of caffeine and other methylxanthines on mechanical properties of isolated mammalian heart muscle. Evidence for a dual mechanism of action. Circ Res. 1972 Apr;30(4):367–392. doi: 10.1161/01.res.30.4.367. [DOI] [PubMed] [Google Scholar]
- Butcher F. R. The role of calcium and cyclic nucleotides in alpha-amylase release from slices of rat parotid: studies with the divalent cation ionophore A-23187. Metabolism. 1975 Mar;24(3):409–418. doi: 10.1016/0026-0495(75)90120-1. [DOI] [PubMed] [Google Scholar]
- Cochrane D. E., Douglas W. W. Histamine release by exocytosis from rat mast cells on reduction of extracellular sodium: a secretory response inhibited by calcium, strontium, barium or magnesium. J Physiol. 1976 May;257(2):433–448. doi: 10.1113/jphysiol.1976.sp011377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leslie B. A., Putney J. W., Jr, Sherman J. M. alpha-Adrenergic, beta-adrenergic and cholinergic mechanisms for amylase secretion by rat parotid gland in vitro. J Physiol. 1976 Sep;260(2):351–370. doi: 10.1113/jphysiol.1976.sp011519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oschman J. L., Berridge M. J. The structural basis of fluid secretion. Fed Proc. 1971 Jan-Feb;30(1):49–56. [PubMed] [Google Scholar]
- Prince W. T., Berridge M. J., Rasmussen H. Role of calcium and adenosine-3':5'-cyclic monophosphate in controlling fly salivary gland secretion. Proc Natl Acad Sci U S A. 1972 Mar;69(3):553–557. doi: 10.1073/pnas.69.3.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prince W. T., Rasmussen H., Berridge M. J. The role of calcium in fly salivary gland secretion analyzed with the ionophore A-23187. Biochim Biophys Acta. 1973 Nov 2;329(1):98–107. doi: 10.1016/0304-4165(73)90012-3. [DOI] [PubMed] [Google Scholar]
- Robyt J. F., Ackerman R. J., Keng J. G. Reducing value methods for maltodextrins. II. Automated methods and chain-length independence of alkaline ferricyanide. Anal Biochem. 1972 Feb;45(2):517–524. doi: 10.1016/0003-2697(72)90214-x. [DOI] [PubMed] [Google Scholar]
- van Breemen C., Farinas B. R., Casteels R., Gerba P., Wuytack F., Deth R. Factors controlling cytoplasmic Ca 2+ concentration. Philos Trans R Soc Lond B Biol Sci. 1973 Mar 15;265(867):57–71. doi: 10.1098/rstb.1973.0009. [DOI] [PubMed] [Google Scholar]



